Hybrid Vitis Cultivars with American or Asian Ancestries Show Higher Tolerance towards Grapevine Trunk Diseases
Abstract
:1. Introduction
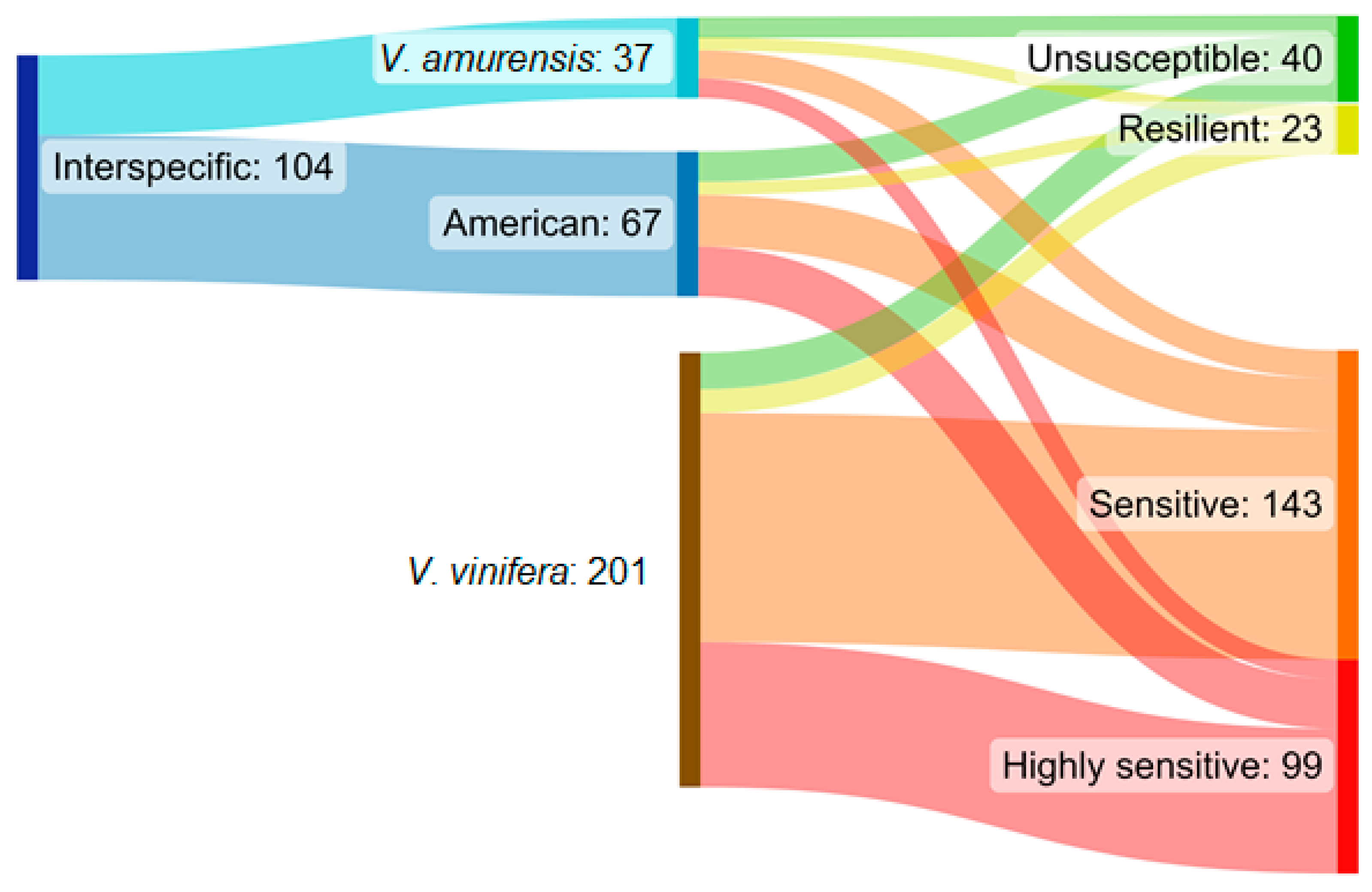
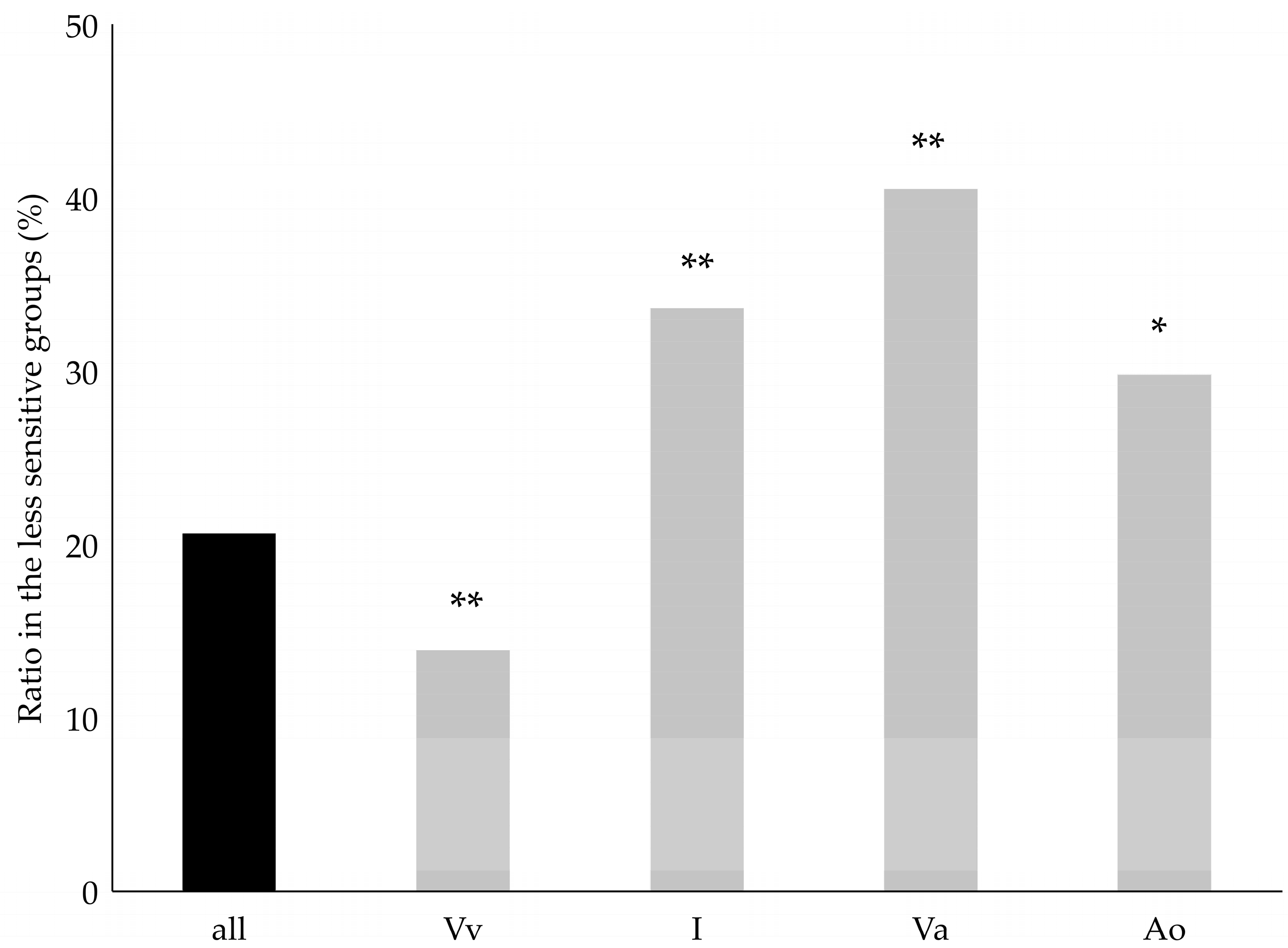
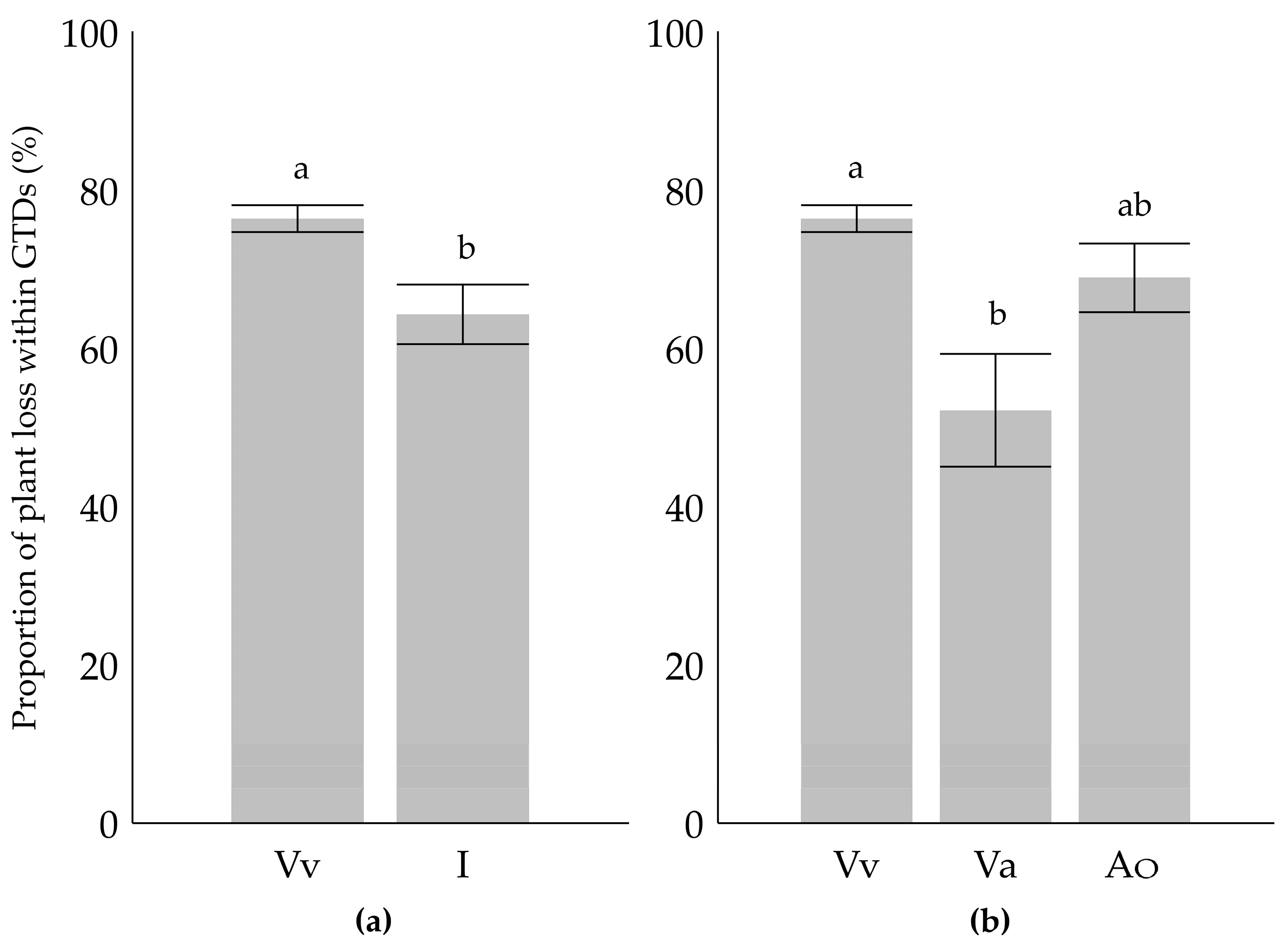
2. Results
3. Discussion
4. Materials and Methods
4.1. Survey Sites and Cultivars
| Badacsonytomaj | Kecskemét | Pallag | Pécs | |
|---|---|---|---|---|
| Climate | Submediterranean with dry, warm summer | Continental | Continental | Submediterranean with dry, warm summer |
| Soil 1 | Erubase soil/Eutric Histosol | Sand/Haplic Arenosoil | Sand/ Haplic Arenosoil | Brown earth/Chromic Cambisol |
| Relief | Mountain slope (top-valley row direction, terrace cultivation) | Lowland | Lowland | Mountain slope (terrace cultivation) |
| Cultivation type | Grafted | Own rooted | Own rooted | Grafted |
| Relative climate sector 2 | IIIc | Ib | Ia | IIIb |
| Average temperature fluctuation (°C) | 21–22 | 23–24.5 | 23–24 | 21–22 |
| Annual precipitation (mm) | 600–800 | 500–550 | 550–700 | 600–800 |
| Annual sunshine duration (h) | 1950–2050 | 2000–2150 | 1900–2050 | 2000–2100 |
4.2. Data Analysis
4.2.1. Susceptibility Analysis
4.2.2. Sensitivity Categories and Analysis
4.3. Statistical Analysis and Software Background
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hofstetter, V.; Buyck, B.; Croll, D.; Viret, O.; Couloux, A.; Gindro, K. What if esca disease of grapevine were not a fungal disease? Fungal Divers. 2012, 54, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Corio-Costet, M.-F. Grapevine Trunk Diseases. A Review; OIV Publications: Paris, France, 2016; ISBN 979-10-91799-60-7. Available online: https://hal.science/hal-01604038/ (accessed on 28 March 2023).
- Pollastro, S.; Dongiovanni, C.; Abbatecola, A.; Faretra, F. Observations on the Fungi Associated with Esca and on Spatial Distribution of Esca-Symptomatic Plants in Apulian (Italy) Vineyards. Phytopathol. Mediterr. 2000, 39, 206–210. [Google Scholar]
- Kovács, C.; Balling, P.; Bihari, Z.; Nagy, A.; Sándor, E. Incidence of grapevine trunk diseases is influenced by soil, topology and vineyard age, but not by Diplodia seriata infection rate in the Tokaj Wine Region, Hungary. Phytoparasitica 2017, 45, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.T.; Cobos, R. Identification of fungi associated with grapevine decline in Castilla y León (Spain). Phytopathol. Mediterr. 2007, 46, 18–25. [Google Scholar]
- Úrbez-Torres, J.R.; Haag, P.; Bowen, P.; O’Gorman, D.T. Grapevine Trunk Diseases in British Columbia: Incidence and Characterization of the Fungal Pathogens Associated with Esca and Petri Diseases of Grapevine. Plant Dis. 2014, 98, 469–482. [Google Scholar] [CrossRef] [Green Version]
- Surico, G.; Marchi, G.; Braccini, P.; Mugnai, L. Epidemiology of Esca in Some Vineyards in Tuscany (Italy). Phytopathol. Mediterr. 2000, 39, 190–205. [Google Scholar] [CrossRef]
- Calzarano, F.; Di Marco, S. Wood discoloration and decay in grapevines with esca proper and their relationship with foliar symptoms. Phytopathol. Mediterr. 2007, 46, 96–101. [Google Scholar] [CrossRef]
- Lehoczky, J. Black dead-arm disease of grapevine caused by Botryosphaeria stevensii infection. Acta Phytopathol. Acad. Sci. Hung. 1974, 9, 319–327. [Google Scholar]
- Marchi, G.; Peduto, F.; Mugnai, L.; Di Marco, S.; Calzarano, F.; Surico, G. Some Observations on the Relationship of Manifest and Hidden Esca to Rainfall. Phytopathol. Mediterr. 2006, 45, S117–S126. [Google Scholar]
- Lecomte, P.; Darrieutort, G.; Laveau, C.; Blancard, D.; Louvet, G.; Goutouly, J.-P.; Rey, P.; Guérin-Dubrana, L. Impact of biotic and abiotic factors on the development of Esca decline disease. Integr. Prot. Prod. Vitic. IOBC/wprs Bull. 2011, 67, 171–180. [Google Scholar]
- Jakab, M.K.; Werner, J.; Csikászné Krizsics, A. Az évjáratok hatása a faszöveti betegségek tüneteinek jelentkezésére különböző szőlőfajtákon. Georg. Agric. 2016, 20, 39–43. [Google Scholar]
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing Grapevine Trunk Diseases with Respect to Etiology and Epidemiology: Current Strategies and Future Prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [Green Version]
- Surico, G.; Mugnai, L.; Marchi, G. Older and more recent observations on esca: A critical overview. Phytopathol. Mediterr. 2006, 45, S68–S86. [Google Scholar]
- Dubos, B. Le syndrome de l’Esca. In Maladies Cryptogamiques de Lavigne, 2nd ed.; Editions Féret: Bordeaux, France, 2002; pp. 127–136. [Google Scholar]
- Fussler, L.; Kobes, N.; Bertrand, F.; Maumy, M.; Grosman, J.; Savary, S. A Characterization of Grapevine Trunk Diseases in France from Data Generated by the National Grapevine Wood Diseases Survey. Phytopathology 2008, 98, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Songy, A.; Fernandez, O.; Clément, C.; Larignon, P.; Fontaine, F. Grapevine trunk diseases under thermal and water stresses. Planta 2019, 249, 1655–1679. [Google Scholar] [CrossRef]
- Dewasme, C.; Mary, S.; Darrieutort, G.; Roby, J.-P.; Gambetta, G.A. Long-Term Esca Monitoring Reveals Disease Impacts on Fruit Yield and Wine Quality. Plant Dis. 2022, 106, 3076–3082. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A Review of Fifteen Years of Trials for Their Control with Chemicals and Biocontrol Agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosnowski, M.; Ayres, M.; Wicks, T.; McCarthy, M. In search of resistance to grapevine trunk diseases. Wine Vitic. J. 2013, 28, 55–58. [Google Scholar]
- Guan, X.; Essakhi, S.; Laloue, H.; Nick, P.; Bertsch, C.; Chong, J. Mining new resources for grape resistance against Botryosphaeriaceae: A focus on Vitis vinifera subsp. Sylvestris. Plant Pathol. 2016, 65, 273–284. [Google Scholar] [CrossRef]
- del Pilar Martínez-Diz, M.; Díaz-Losada, E.; Barajas, E.; Ruano-Rosa, D.; Andrés-Sodupe, M.; Gramaje, D. Screening of Spanish Vitis vinifera germplasm for resistance to Phaeomoniella chlamydospora. Sci. Hortic. 2019, 246, 104–109. [Google Scholar] [CrossRef]
- Reynolds, A.G. (Ed.) Grapevine breeding in France–A historical perspective. In Grapevine Breeding Programs for the Wine Industry; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2015; pp. 65–76. [Google Scholar] [CrossRef]
- Kozma, P. A Szőlő és Termesztése I; Akadémiai Kiadó: Budapest, Hungary, 2000; Volume 91, p. 319. ISBN 9630577208. [Google Scholar]
- Buonassisi, D.; Colombo, M.; Migliaro, D.; Dolzani, C.; Peressotti, E.; Mizzotti, C.; Velasco, R.; Masiero, S.; Perazzolli, M.; Vezzulli, S. Breeding for grapevine downy mildew resistance: A review of “omics” approaches. Euphytica 2017, 213, 103. [Google Scholar] [CrossRef]
- Armijo, G.; Schlechter, R.; Agurto, M.; Muñoz, D.; Muñez, C.; Arce-Johnson, P. Grapevine Pathogenic Microorganisms: Understanding Infection Strategies and Host Response Scenarios. Front. Plant Sci. 2016, 7, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merdinoglu, D.; Schneider, C.; Prado, E.; Wiedemann-Merdinoglu, S.; Mestre, P. Breeding for durable resistance to downy and powdery mildew in grapevine. OENO One 2018, 52, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Villano, C.; Aversano, R. Towards grapevine (Vitis vinifera L.) mildews resistance: Molecular defence mechanisms and New Breeding Technologies. Italus Hortus 2020, 27, 1–17. [Google Scholar] [CrossRef]
- Blasi, P.; Blanc, S.; Wiedemann-Merdinoglu, S.; Prado, E.; Rühl, E.H.; Mestre, P.; Merdinoglu, D. Construction of a reference linkage map of Vitis amurensis and genetic mapping of Rpv8, a locus conferring resistance to grapevine downy mildew. Theor. Appl. Genet. 2011, 123, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Kuczmog, A.; Galambos, A.; Horváth, S.; Mátai, A.; Kozma, P.; Szegedi, E.; Putnoky, P. Mapping of crown gall resistance locus Rcg1 in grapevine. Theor. Appl. Genet. 2012, 125, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Schwander, F.; Eibach, R.; Fechter, I.; Hausmann, L.; Zyprian, E.; Töpfer, R. Rpv10: A new locus from the Asian Vitis gene pool for pyramiding downy mildew resistance loci in grapevine. Theor. Appl. Genet. 2012, 124, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H. Review: Research progress in amur grape, Vitis amurensis Rupr. Can. J. Plant Sci. 2013, 93, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Venuti, S.; Copetti, D.; Foria, S.; Falginella, L.; Hoffmann, S.; Bellin, D.; Cindrić, P.; Kozma, P.; Scalabrin, S.; Morgante, M.; et al. Historical introgression of the downy mildew resistance gene Rpv12 from the Asian species Vitis amurensis into grapevine varieties. PLoS ONE 2013, 8, e6122. [Google Scholar] [CrossRef]
- Fu, P.; Wu, W.; Lai, G.; Li, R.; Peng, Y.; Yang, B.; Wang, B.; Yin, L.; Qu, J.; Song, S.; et al. Identifying Plasmopara viticola resistance Loci in grapevine (Vitis amurensis) via genotyping-by-sequencing-based QTL mapping. Plant Physiol. Biochem. 2020, 154, 75–84. [Google Scholar] [CrossRef]
- Kozma, P.; Dula, T. Inheritance of Resistance to Downy Mildew and Powdery Mildew of Hybrid Family Muscadinia x v. Vinifera x v. Amurensis x Franco-American Hybrid. Acta Hortic. 2003, 603, 457–463. [Google Scholar] [CrossRef]
- Foria, S.; Magris, G.; Jurman, I.; Schwope, R.; De Candido, M.; De Luca, E.; Ivanišević, D.; Morgante, M.; Di Gaspero, G. Extent of wild–to–crop interspecific introgression in grapevine (Vitis vinifera) as a consequence of resistance breeding and implications for the crop species definition. Hortic. Res. 2022, 9, uhab010. [Google Scholar] [CrossRef] [PubMed]
- Myles, S.; Boyko, A.R.; Owens, C.L.; Brown, P.J.; Grassi, F.; Aradhya, M.K.; Prins, B.; Reynolds, A.; Chia, J.-M.; Ware, D.; et al. Genetic structure and domestication history of the grape. Proc. Natl. Acad. Sci. USA 2011, 108, 3530–3535. [Google Scholar] [CrossRef] [Green Version]
- Grassi, F.; De Lorenzis, G. Back to the Origins: Background and Perspectives of Grapevine Domestication. Int. J. Mol. Sci. 2021, 22, 4518. [Google Scholar] [CrossRef] [PubMed]
- Dubos, B. Mise au point sur les maladies de dépéréssiment dans la vignoble francais. Progrés Agric. Vitic. 1987, 104, 135–140. [Google Scholar]
- Carter, M.V. The Status of Eutypa lata as a Pathogen; Monograph-Phytopathological Paper No. 32; International Mycological Institute: Surrey, UK, 1991. [Google Scholar]
- Borgo, M.; Pegoraro, G.; Sartori, E. Susceptibility of grape varieties to esca disease. BIO Web Conf. 2016, 7, 01041. [Google Scholar] [CrossRef] [Green Version]
- Murolo, S.; Romanazzi, G. Effects of grapevine cultivar, rootstock and clone on esca disease. Australas. Plant Pathol. 2014, 43, 215–221. [Google Scholar] [CrossRef]
- Sosnowski, M.; Ayres, M.; McCarthy, M.; Wicks, T.; Scott, E. Pests and diseases: Investigating the potential for resistance to grapevine trunk diseases. Wine Vitic. J. 2016, 31, 41. [Google Scholar] [CrossRef]
- Travadon, R.; Rolshausen, P.E.; Gubler, W.D.; Cadle-Davidson, L.; Baumgartner, K. Susceptibility of Cultivated and Wild Vitis spp. to Wood Infection by Fungal Trunk Pathogens. Plant Dis. 2013, 97, 1529–1536. [Google Scholar] [CrossRef] [Green Version]
- Billones-Baaijens, R.; Jones, E.; Ridgway, H.; Jaspers, M.V. Susceptiblity of common rootstock and scion varieties of grapevines to Botryosphaeriaceae species. Australas. Plant Pathol. 2014, 43, 25–31. [Google Scholar] [CrossRef]
- Foglia, R.; Landi, L.; Romanazzi, G. Analyses of Xylem Vessel Size on Grapevine Cultivars and Relationship with Incidence of Esca Disease, a Threat to Grape Quality. Appl. Sci. 2022, 12, 1177. [Google Scholar] [CrossRef]
- Feliciano, A.; Eskalen, A.; Gubler, W.D. Differential susceptibility of three grapevine cultivars to Phaeomoniella chlamydospora in California. Phytopathol. Mediterr. 2004, 43, 66–69. [Google Scholar] [CrossRef]
- Cardot, C.; Mappa, G.; La Camera, S.; Gaillard, C.; Vriet, C.; Lecomte, P.; Ferrari, G.; Coutos-Thévenot, P. Comparison of the Molecular Responses of Tolerant, Susceptible and Highly Susceptible Grapevine Cultivars during Interaction with the Pathogenic Fungus Eutypa lata. Front. Plant Sci. 2019, 10, 991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Péros, J.P.; Berger, G. A rapid method to assess the aggressiveness of Eutypa lata isolates and the susceptibility of grapevine cultivars to Eutypa Dieback. Agronomie 1994, 14, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Quaglia, M.; Covarelli, L.; Zazzerini, A. Epidemiological survey on esca disease in Umbria, central Italy. Phytopathol. Mediterr. 2009, 48, 84–91. [Google Scholar] [CrossRef]
- Maul, E.; Töpfer, R. Vitis International Variety Catalogue. 2023. Available online: www.vivc.de (accessed on 2 April 2023).
- Sankeymatic Online Diagram Builder. Available online: https://sankeymatic.com (accessed on 7 January 2023).
- Claverie, M.; Notaro, M.; Fontaine, F.; Wery, J. Current knowledge on Grapevine Trunk Diseases with complex etiology: A systemic approach. Phytopathol. Mediterr. 2020, 59, 29–53. [Google Scholar] [CrossRef]
- Moret, F.; Lemaître-Guillier, C.; Grosjean, C.; Clément, G.; Coelho, C.; Negrel, J.; Jacquens, L.; Morvan, G.; Mouille, G.; Trouvelot, S.; et al. Clone-dependent expression of esca disease revealed by leaf metabolite analysis. Front. Plant Sci. 2019, 9, 1960. [Google Scholar] [CrossRef]
- Serra, S.; Ligios, V.; Schianchi, N.; Prota, V.A.; Deidda, A.; Scanu, B. Incidence of grapevine trunk diseases on four cultivars in Sardinia, Southern Italy. Vitis 2021, 60, 35–42. [Google Scholar] [CrossRef]
- Bruez, E.; Lecomte, P.; Grosman, J.; Doublet, B.; Bertsch, C.; Fontaine, F.; Ugaglia, A.; Teissedre, P.-L.; Da Costa, J.-P.; Guerin-Dubrana, L.; et al. Overview of grapevine trunk diseases in France in the 2000s. Phytopathol. Mediterr. 2013, 52, 262–275. [Google Scholar]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins Produced by Fungi Associated with Grapevine Trunk Diseases. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef] [Green Version]
- Bortolami, G.; Gambetta, G.A.; Cassan, C.; Dayer, S.; Farolfi, E.; Ferrer, N.; Gibon, Y.; Jolivet, J.; Lecomte, P.; Delmas, C.E.L. Grapevines under drought do not express esca leaf symptoms. Proc. Natl. Acad. Sci. USA 2021, 118, e2112825118. [Google Scholar] [CrossRef] [PubMed]
- Pouzoulet, J.; Scudiero, E.; Schiavon, M.; Rolshausen, P.E. Xylem Vessel Diameter Affects the Compartmentalization of the Vascular Pathogen Phaeomoniella chlamydospora in Grapevine. Front. Plant Sci. 2017, 8, 1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouzoulet, J.; Pivovaroff, A.L.; Santiago, L.; Rolshausen, P.E. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Front. Plant Sci. 2014, 5, 253. [Google Scholar] [CrossRef] [PubMed]
- Csótó, A.; Balling, P.; Nagy, A.; Sándor, E. The role of cultivar susceptibility and vineyard age in GTD: Examples from the Carpathian Basin. Acta Agrar. Debr. 2020, 2, 57–63. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Greve, L.C.; Labavitch, J.M.; Mahoney, N.E.; Molyneux, R.J.; Gubler, W.D. Pathogenesis of Eutypa lata in Grapevine: Identification of Virulence Factors and Biochemical Characterization of Cordon Dieback. Phytopathology 2008, 98, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.S.; Centinari, M. Young grapevines exhibit interspecific differences in hydraulic response to freeze stress but not in recovery. Planta 2019, 250, 495–505. [Google Scholar] [CrossRef]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (Black Measles) and Brown Wood-Streaking: Two Old and Elusive Diseases of Grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, A.L.; Rodriguez-Zaccaro, F.D.; Lee, T.F.; Valdovinos, J.; Toschi, H.S.; Martinez, J.A.; Pratt, R.B. Grapevine xylem development, architecture, and function. In Functional and Ecological Xylem Anatomy; Hacke, U., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 133–162. [Google Scholar]
- Guo, X.W.; Fu, W.H.; Wang, G.J. Studies on cold hardiness of grapevine roots. Vitis 1987, 26, 161–171. [Google Scholar] [CrossRef]
- DeKrey, D.H.; Klodd, A.E.; Clark, M.D.; Blanchette, R.A. Grapevine trunk diseases of cold-hardy varieties grown in Northern Midwest vineyards coincide with canker fungi and winter injury. PLoS ONE 2022, 17, e0269555. [Google Scholar] [CrossRef]
- Csótó, A.; Balling, P.; Rakonczás, N.; Kovács, C.; Nagy, A.; Sándor, E. The Effect of Extreme Weather Conditions on the Incidence and Spreading of Grapevine Trunk Diseases. In Proceedings of the 16th Congress of the Mediterranean Phytopathological Union, Limassol, Cyprus, 4–8 April 2022; p. 60. Available online: https://cyprusconferences.org/mpu2022/wp-content/uploads/2022/04/MPU-2022_ABSTRACTS-ALL-13_04_2022-2.pdf (accessed on 2 April 2023).
- Zhao, Y.; Wang, Z.-X.; Yang, Y.-M.; Liu, H.-S.; Shi, G.-L.; Ai, J. Analysis of the cold tolerance and physiological response differences of amur grape (Vitis amurensis) germplasms during overwintering. Sci. Hortic. 2020, 259, 108760. [Google Scholar] [CrossRef]
- Xin, H.; Zhu, W.; Wang, L.; Xiang, Y.; Fang, L.; Li, J.; Sun, X.; Wang, N.; Londo, J.; Li, S. Genome Wide Transcriptional Profile Analysis of Vitis amurensis and Vitis vinifera in Response to Cold Stress. PLoS ONE 2013, 8, e58740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Wu, X.C.; Niu, R.X.; Liu, Y.; Liu, N.; Xu, W.; Wang, Y. Cold-resistance evaluation in 25 wild grape species. Vitis 2012, 51, 153–160. [Google Scholar] [CrossRef]
- Wang, Y.; Xin, H.; Fan, P.; Zhang, J.; Liu, Y.; Dong, Y.; Wang, Z.; Yang, Y.; Zhang, Q.; Ming, R.; et al. The genome of Shanputao (Vitis amurensis) provides a new insight into cold tolerance of grapevine. Plant J. 2021, 105, 1495–1506. [Google Scholar] [CrossRef]
- Chai, F.; Liu, W.; Xiang, Y.; Meng, X.; Sun, X.; Cheng, C.; Liu, G.; Duan, L.; Xin, H.; Li, S. Comparative metabolic profiling of and Vitis vinifera during cold acclimation. Hortic. Res. 2019, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Gamboa, G.; Liu, S.-Y.; Sun, X.; Fang, Y. Oenological potential and health benefits of Chinese non-Vitis vinifera species: An opportunity to the revalorization and to breed new varieties. Food Res. Int. 2020, 137, 109443. [Google Scholar] [CrossRef] [PubMed]
- Ciaffi, M.; Paolacci, A.R.; Paolocci, M.; Alicandri, E.; Bigini, V.; Badiani, M.; Muganu, M. Transcriptional regulation of stilbene synthases in grapevine germplasm differentially susceptible to downy mildew. BMC Plant Biol. 2019, 19, 404. [Google Scholar] [CrossRef]
- Clark, M.D. Development of Cold Climate Grapes in the Upper Midwestern U.S.: The Pioneering Work of Elmer Swenson. In Plant Breeding Reviews; Goldman, I., Ed.; Wiley: Hoboken, NJ, USA, 2020; Volume 43, pp. 31–60. [Google Scholar] [CrossRef]
- Viret, O.; Spring, J.-L.; Gindro, K. Stilbenes: Biomarkers of grapevine resistance to fungal diseases. OENO One 2018, 52, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Leng, H.; Guo, Y.; Kondo, S.; Zhao, Y.; Shi, G.; Guo, X. QTLs and candidate genes for downy mildew resistance conferred by interspecific grape (V. vinifera L. × V. amurensis Rupr.) crossing. Sci. Hortic. 2019, 244, 200–207. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Yin, L.; Lu, J. The Mode of Host Resistance to Plasmopara viticola Infection of Grapevines. Phytopathology 2012, 102, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, J.; Yin, L.; Zhang, Y.; Qu, J.; Lu, J. Comparative transcriptome analysis reveals defense-related genes and pathways against grapevine. Plant Physiol. Biochem. 2015, 95, 1–14. [Google Scholar] [CrossRef]
- Pretorius, I.S.; Høj, P.B. Grape and wine biotechnology: Challenges, opportunities and potential benefits. Aust. J. Grape Wine Res. 2005, 11, 83–108. [Google Scholar] [CrossRef]
- Barba, P.; Lillis, J.; Luce, R.S.; Travadon, R.; Osier, M.; Baumgartner, K.; Wilcox, W.F.; Reisch, B.I.; Cadle-Davidson, L. Two dominant loci determine resistance to Phomopsis cane lesions in F1 families of hybrid grapevines. Theor. Appl. Genet. 2018, 131, 1173–1189. [Google Scholar] [CrossRef] [Green Version]
- Travadon, R.; Baumgartner, K.; Rolshausen, P.E.; Gubler, W.D.; Sosnowski, M.R.; Lecomte, P.; Halleen, F.; Péros, J.-P. Genetic structure of the fungal grapevine pathogen Eutypa lata from four continents. Plant Pathol. 2012, 61, 85–95. [Google Scholar] [CrossRef]
- Töpfer, R.; Hausmann, L.; Harst, M.; Maul, E.; Zyprian, E.; Eibach, R. New Horizons for Grapevine Breeding. In Fruits, Vegetable and Cereal Science and Biotechnology; Flachowsky, H., Hanke, M.V., Eds.; Methods in Temperate Fruit Breeding; Global Science Books Ltd.: London, UK; Kagawa, Japan, 2011; pp. 79–100. ISBN 978-4-903313-75-7. Available online: http://www.globalsciencebooks.info/Online/GSBOnline/images/2011/FVCSB_5(SI1)/FVCSB_5(SI1)79-100o.pdf (accessed on 7 April 2023).
- Bartholy, J.; Weidinger, T. Magyarország éghajlati képe. In Pannon Enciklopédia–Magyarország Földje; Karátson, D., Ed.; Urbis: Budapest, Hungary, 2010; pp. 240–241. ISBN 978-963-9706-68-2. [Google Scholar]
- Rakonczás, N. Production data of wine grape gene bank (Vitis spp.) of University of Debrecen, east Hungary. Int. J. Hortic. Sci. 2019, 25, 32–36. [Google Scholar] [CrossRef]
- KSH: Borvidékek. Available online: https://www.ksh.hu/docs/hun/teruleti/egyeb_egysegek/borvidekek.pdf (accessed on 8 April 2023).
- Patocskai, Z.; Vidéki, R.; Szépligeti, M.; Bidlo, A.; Kovács, G. Talajviszonyok a Szent György-Hegyen. Talajvédelem Special Issue; Talajvédelmi Alapítvány Bessenyei György Könyvkiadó Nyíregyháza: Nyíregyháza, Hungary, 2008; pp. 639–644. [Google Scholar]
- Fehér, O.; Füleky, G.; Madarasz, B.; Kertész, Á. Hét vulkáni kőzeten kialakult talajszelvény morfológiai és diagnosztikai jellemzői a hazai genetikai talajosztályozás és a WRB (World Reference Base for Soil Resources, 1998) szerint. Agrokémia Talajt. 2006, 55, 347–366. [Google Scholar] [CrossRef] [Green Version]
- National Land Centre Genetic Soil Type. Available online: https://nfi.nfk.gov.hu/genetic_soil_type (accessed on 25 May 2023).
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 Internation Soil Classifcation System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; ISBN 978-92-5-108370-3. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 25 May 2023).
- European Commission; Joint Research Centre; Soil Atlas of Europe; European Soil Bureau Network European Commission; Office for Official Publications of the European. L-2995 Luxembourg. 2005, pp. 62–63. Available online: https://esdac.jrc.ec.europa.eu/Projects/Soil_Atlas/Download/40-79.pdf (accessed on 6 April 2023).
- Díaz, G.A.; Latorre, B.A. Efficacy of paste and liquid fungicide formulations to protect pruning wounds against pathogens associated with grapevine trunk diseases in Chile. Crop. Prot. 2013, 46, 106–112. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Leavitt, G.M.; Guerrero, J.C.; Guevara, J.; Gubler, W.D. Identification and Pathogenicity of Lasiodiplodia theobromae and Diplodia seriata, the Causal Agents of Bot Canker Disease of Grapevines in Mexico. Plant Dis. 2008, 92, 519–529. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.B. Statistics and Probability. Available online: https://stattrek.com/ (accessed on 7 April 2023).



| Cultivars 1 | GTDs | Inoculation Test/Disease Incidence Survey 3 | References | ||
|---|---|---|---|---|---|
| Tolerance | Disease 2 | ||||
| White | Chardonnay | high | BD, Eutypa | Test | [44] |
| medium | BD | Survey | [43] | ||
| medium | Esca | Survey | [41] | ||
| Pinot Gris | high | BD, Eutypa | Survey | [43] | |
| medium | Esca | Survey | [10] | ||
| Riesling | high | Eutypa | Survey | [43] | |
| medium | BD | Test | [45] | ||
| medium/low | Esca | Test | [44] | ||
| Sauvignon Blanc | high | BD | Test | [45] | |
| medium | Eutypa | Test and Survey | [43] | ||
| low | BD | Test and Survey | [43] | ||
| low | Esca | Survey | [42,46] | ||
| Semillon | high | BD, Eutypa | Test and Survey | [43] | |
| low | Esca | Survey | [10] | ||
| Thompson seedless | high | Esca | Test | [44,47] | |
| medium/low | Eutypa | Test | [44] | ||
| low | BD, Eutypa | Test | [44,47] | ||
| Ugni Blanc | medium/high | BD | Survey | [43] | |
| low | Eutypa | Test | [48] | ||
| low | Esca, Eutypa | Test | [49] | ||
| Survey | [41,43] | ||||
| Welshriesling | high | BD, Eutypa | Test and Survey | [43] | |
| Survey | [41] | ||||
| low | Esca | Survey | [7] | ||
| Red | Cabernet Franc | medium/high | Eutypa | Test | [44] |
| medium | BD | Test | [44] | ||
| low | Esca | Test and Survey | [43] | ||
| Cabernet Sauvignon | high | BD | Test | [45] | |
| low | Eutypa | Test | [48] | ||
| low | Esca, Eutypa | Survey | [41,46,50] | ||
| BD | Survey | [43] | |||
| Grenache | high | Esca, Eutypa | Survey | [43] | |
| high | Esca | Test | [47] | ||
| BD | medium/high | Survey | [43] | ||
| Merlot | high | Eutypa | Test | [44,48] | |
| medium/high | BD | Test | [44] | ||
| medium | Esca | Survey | [42,50] | ||
| Pinot Noir | high | Esca | Survey | [41] | |
| Eutypa, Esca | Test and Survey | [43] | |||
| medium | BD | Test and Survey | [43] | ||
| Sangiovese | high | BD, Esca, Eutypa | Test and Survey | [43] | |
| medium | Esca | Survey | [41] | ||
| Syrah | high | Esca | Survey | [41] | |
| low | BD, Eutypa | Test | [21,44] | ||
| Test and Survey | [43] | ||||
| Hybrid (V. labrusca hybrid) | Concord (Vitis labrusca hybrid) | high | BD, Esca, Eutypa | Test | [44] |
| Location | No. Samples * | GTDs | |
|---|---|---|---|
| DI% (±SE) | Proportion of Plant Loss (% ± SE) | ||
| Badacsonytomaj | 90 | 44.58 (±2.62) c | 74.63 (±3.14) a |
| Kecskemét | 130 | 28.05 (±2.19) a | 76.49 (±3.11) a |
| Pallag | 166 | 37.05 (±2.16) b | 73.78 (±3.10) a |
| Pécs | 151 | 28.41 (±1.92) a | 69.94 (±3.23) a |
| Total | 537 | 33.70 (±1.13) | 73.56 (±1.59) |
| Ancestors in Parent or Grandparent Level | Categorization I. | Categorization II. |
|---|---|---|
| Vitis vinifera | Vitis vinifera (Vv) | Vitis vinifera (Vv) |
| Occurrence of American species 1 | Interspecific (I) | American origin (Ao) |
| Occurrence of Vitis amurensis | Interspecific (I) | Vitis amurensis origin (Va) |
| Sensitivity Categories | GTD Symptoms | ||
|---|---|---|---|
| Two Groups | Four Groups | Apoplexy (Dead Plant) | Leaf Symptoms and Fresh Dieback |
| More sensitive | Highly sensitive (HS) | Exclusively | - |
| Sensitive (S) | Present | Present | |
| Less sensitive | Resilient (R) | - | Exclusively |
| Unsusceptible (U) | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csótó, A.; Nagy, A.; Laurinyecz, N.; Nagy, Z.A.; Németh, C.; Németh, E.K.; Csikász-Krizsics, A.; Rakonczás, N.; Fontaine, F.; Fekete, E.; et al. Hybrid Vitis Cultivars with American or Asian Ancestries Show Higher Tolerance towards Grapevine Trunk Diseases. Plants 2023, 12, 2328. https://doi.org/10.3390/plants12122328
Csótó A, Nagy A, Laurinyecz N, Nagy ZA, Németh C, Németh EK, Csikász-Krizsics A, Rakonczás N, Fontaine F, Fekete E, et al. Hybrid Vitis Cultivars with American or Asian Ancestries Show Higher Tolerance towards Grapevine Trunk Diseases. Plants. 2023; 12(12):2328. https://doi.org/10.3390/plants12122328
Chicago/Turabian StyleCsótó, András, Antal Nagy, Nóra Laurinyecz, Zóra Annamária Nagy, Csaba Németh, Erzsébet Krisztina Németh, Anna Csikász-Krizsics, Nándor Rakonczás, Florence Fontaine, Erzsébet Fekete, and et al. 2023. "Hybrid Vitis Cultivars with American or Asian Ancestries Show Higher Tolerance towards Grapevine Trunk Diseases" Plants 12, no. 12: 2328. https://doi.org/10.3390/plants12122328
APA StyleCsótó, A., Nagy, A., Laurinyecz, N., Nagy, Z. A., Németh, C., Németh, E. K., Csikász-Krizsics, A., Rakonczás, N., Fontaine, F., Fekete, E., Flipphi, M., Karaffa, L., & Sándor, E. (2023). Hybrid Vitis Cultivars with American or Asian Ancestries Show Higher Tolerance towards Grapevine Trunk Diseases. Plants, 12(12), 2328. https://doi.org/10.3390/plants12122328








