Effects of Drip Irrigation-Fertilization on Growth, Flowering, Photosynthesis and Nutrient Absorption of Containerized Seedlings of Hibiscus syriacus L. (Haeoreum)
Abstract
:1. Introduction
2. Results
2.1. Growth Characteristics According to Fertilization and Irrigation
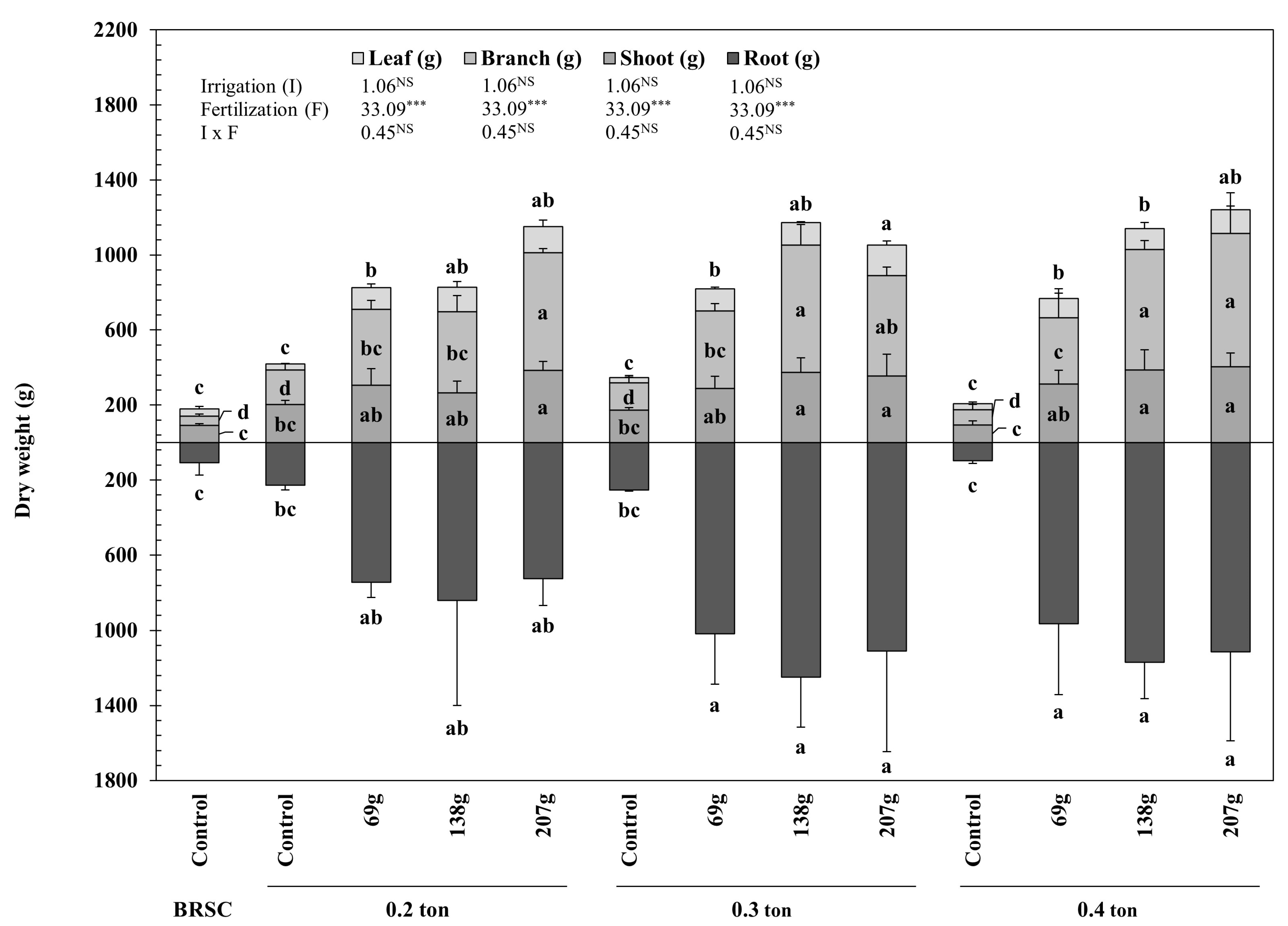
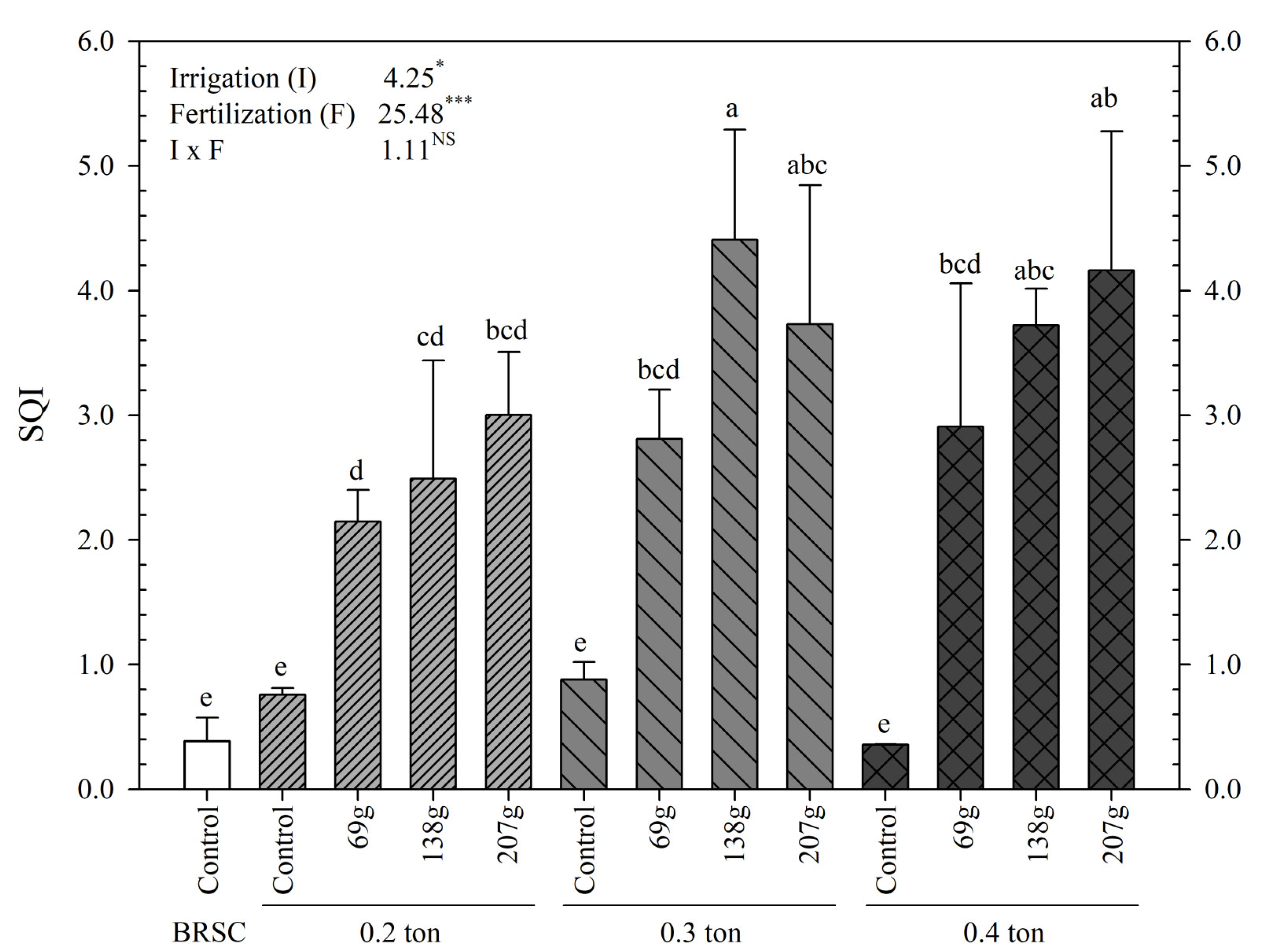
2.2. Biomass Amount, T/R Rate and Quality Index According to Fertilization and Irrigation
2.3. Flowering Characteristics According to Fertilization and Irrigation
2.4. Changes in Photosynthesis Parameters According to Fertilization and Irrigation
2.5. Chlorophyll Fluorescence Change According to Fertilization and Irrigation
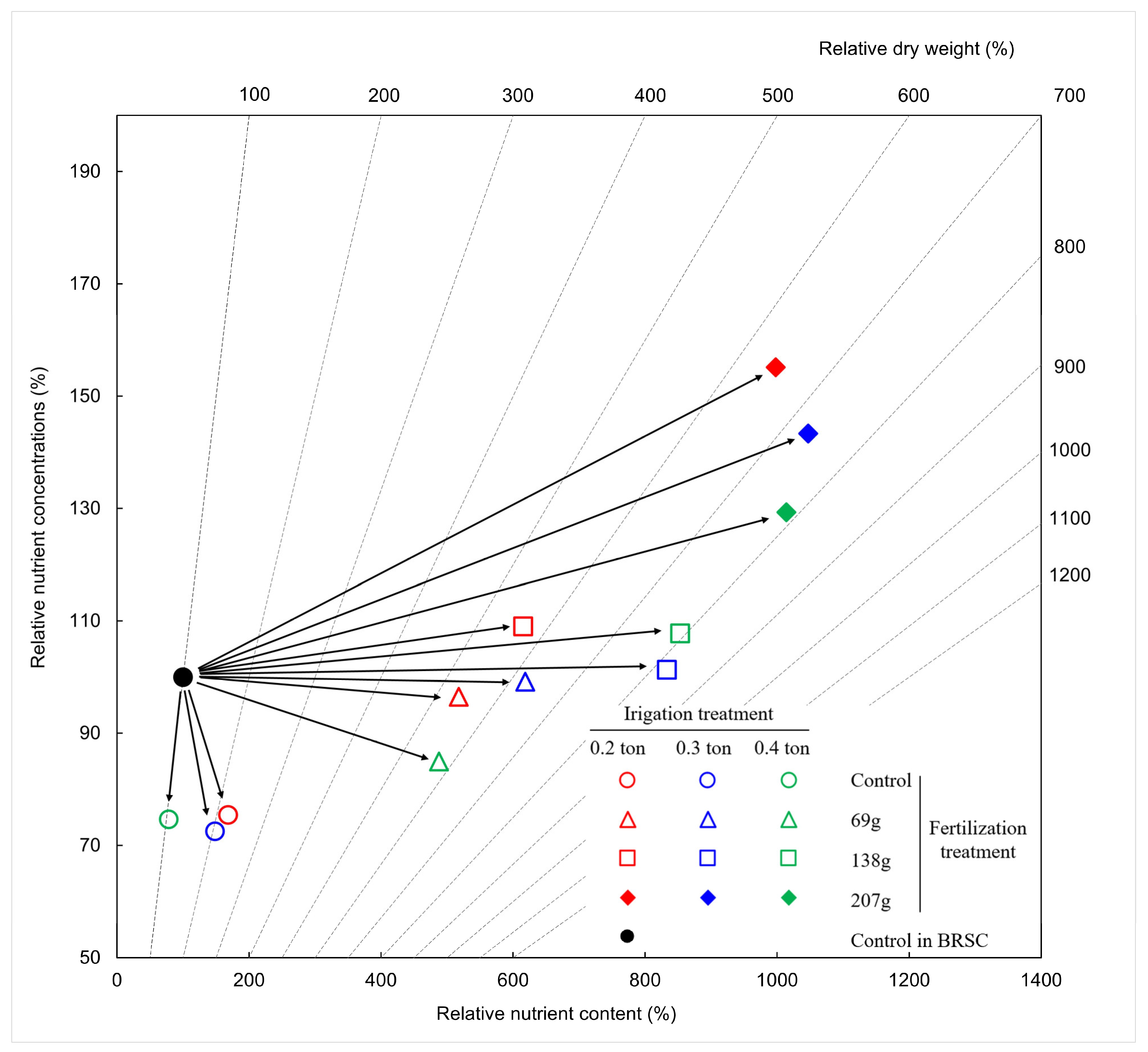
2.6. Nitrogen Nutrient Characteristics According to Fertilization and Irrigation
3. Discussion
3.1. Effect of Different Irrigation-Fertilization on Growth Performances
3.2. Changes in Biomass Production and Quality of Seedlings Due to Fertilization and Irrigation
3.3. Changes in Flowering Characteristics According to Fertilization and Irrigation
3.4. Photophysiological Changes According to Fertilization and Irrigation
3.5. Chlorophyll Fluorescence Change According to Fertilization and Irrigation
3.6. Nitrogen Nutrient Changes According to Fertilization and Irrigation
4. Materials and Methods
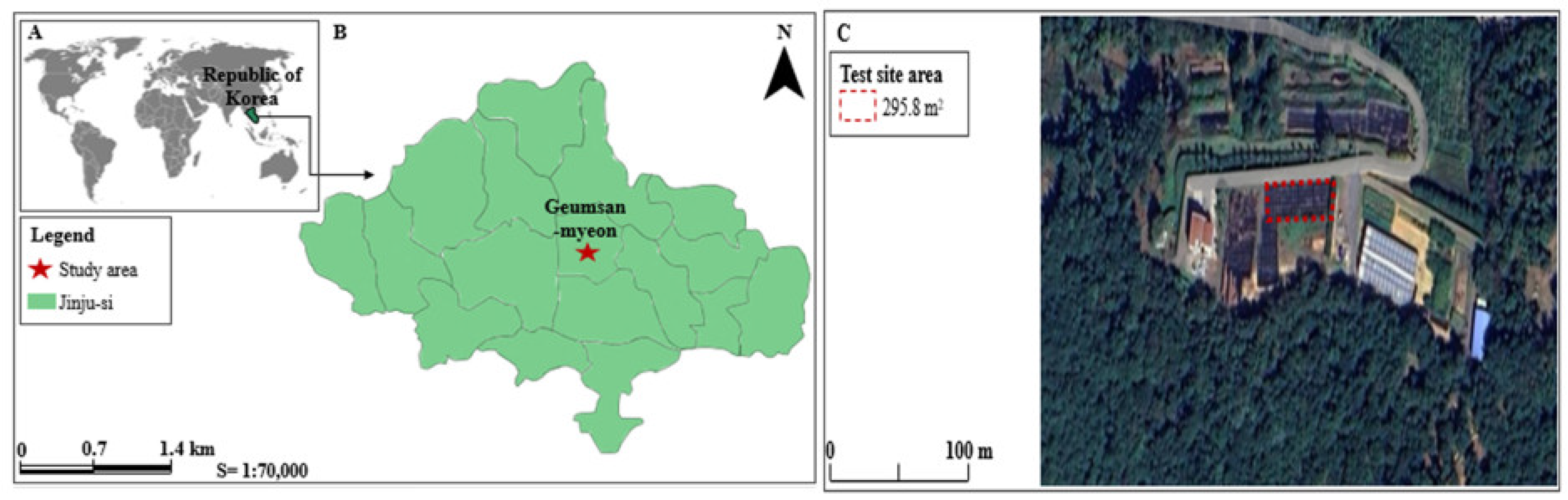
4.1. Experimental Site Description
4.2. Irrigation-Fertilization
4.3. Growth Performances Investigation
4.4. Flowering Characteristics Investigation
4.5. Photosynthetic Characteristics Investigation
4.6. Chlorophyll Fluorescence Investigation
4.7. Investigation of Nitrogen Nutrient Characteristics
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cho, Y.J.; Park, H.S.; Chang, Y.S.; Shin, M.Y.; Chung, D.J. Characteristics of photosynthesis of dwarf and street tree cultivars of Hibiscus syriacus L. Korean J. Agric. For. Meteorol. 2007, 9, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Ha, Y.M.; Kim, D.Y.; Shim, K.K. A new cultivar Hibiscus syriacus ‘Red Bohanjae’ with small violet-pink flowers for a abst plant. Hortic. Sci. Technol. 2015, 33, 292–297. [Google Scholar]
- Ji, N.S.; An, Y.J.; Lee, S.Y. Basic Research for the Creation of the Sejong-Type Mugunghwa City; Daejeon Sejong Institute: Daejeon, Republic of Korea, 2017; p. 100. [Google Scholar]
- Joon, R.J.; Yoon, S.C.; Ji, H.C.; Je, J.A.; Young, H.K. Development of container for standardized production for woody plant. J. Korean Soc. People Plants Environ. 2016, 19, 497–503. [Google Scholar]
- Cho, Y.H. A Comparison of the Growth of Woody Landscape Plants in Cultivation Method Using Container. Master’s Thesis, Honam University, Gwangju, Republic of Korea, 2014. [Google Scholar]
- Kang, H.C.; Kim, D.Y.; Ha, Y.M. Selection of cultivars with vigorous growth habit for street Tree, dwarf tree form for pot plants, and spreading branches for groundcovers in the recently developed cultivars of rose of Sharon (Hibiscus spp.) for landscape uses. J. Korean Inst. Tradit. Landsc. Archit. 2016, 34, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.Y.; Yeam, D.Y. Korean National Flower, Hibiscus Syriacus; Hakwon Co., Ltd.: Seoul, Republic of Korea, 1987; 424p. [Google Scholar]
- Shim, K.K.; Ha, Y.M. Planting and Maintenance of New Cultivars of Hibiscus spp. as Korean National Flower; New Industrial Strategy Research: Seoul, Republic of Korea, 2010; p. 69. [Google Scholar]
- Oh, Y.N.; Yoo, E.H.; Kim, W.H.; Yoo, B.S.; Lee, E.K.; Kim, K.S.; Lee, D.W. Newly bred Hibiscus syriacus ‘Hangyeore’, ‘Hanbora’, and ‘Hansomi’. Korean J. Breed. Sci. 2003, 35, 136. [Google Scholar]
- Kwon, K.W.; Cho, M.S.; Kim, G.N.; Lee, S.W.; Jang, K.H. Photosynthetic characteristics and growth performances of containerized seedling and bare root seedling of Quercus acutissima growing at different fertilizing schemes. J. Korean Soc. For. Sci. 2009, 98, 331–338. [Google Scholar]
- Lee, E.; Suh, J. Effect of watering control on growth and bulb size of plug seedling in onion (Allium cepa L.) set production. Korean J. Hortic. Sci. Technol. 2009, 27, 167–173. [Google Scholar]
- Lee, S.W.; Choi, J.H.; Yoo, S.K.; Kim, S.K.; Bae, J.H.; Kyo, H.S. Effect of raw material properties on growth characteristics of broad-leaved container seedlings. J. Bio-Environ. Control 2006, 15, 244–249. [Google Scholar]
- Kramer, P.J. Water Relations of Plants; Academic Press: New York, NY, USA, 1983; p. 489. [Google Scholar]
- Alghamdi, S.A.; Alharby, H.F.; Bamagoos, A.A.; Zaki, S.N.S.; Abu El-Hassan, A.M.; Desoky, E.S.M.; Mohamed, I.A.A.; Rady, M.M. Rebalancing nutrients, reinforcing antioxidant and osmoregulatory capacity, and improving yield quality in drought-stressed Phaseolus vulgaris by foliar application of a bee-honey solution. Plants 2022, 12, 63. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Alharbi, K.; Rady, M.M.; Elnahal, A.S.; Selem, E.; Arnaout, S.M.; Mansour, E. Physiological, biochemical, anatomical, and agronomic responses of sesame to exogenously applied polyamines under different irrigation regimes. Agronomy 2023, 13, 875. [Google Scholar] [CrossRef]
- Beeson, R.C.; Knox, G.W. Analysis of efficiency of overhead irrigation in container production. HortScience 1991, 26, 848–850. [Google Scholar] [CrossRef] [Green Version]
- Briercliffe, T.; Hewson, A.; Brough, W. Independent water audits for container grown nursery stock producers. In Summary Report for MAFF Water Audits; Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2000. [Google Scholar]
- Olga, M.G.; Michael, J.D.; Helen, L.; Christopher, J.A. Irrigation scheduling and irrigation systems: Optimising irrigation efficiency for container ornamental shrubs. Irrig. Sci. 2009, 27, 139–153. [Google Scholar]
- Cha, Y.G.; Choi, K.S.; Song, K.S.; Sung, H.I.; Kim, J.J. Growth characteristics of one-year-old container seedlings of Pinus densiflora by irrigation level. J. Bio-Environ. Control 2017, 26, 167–174. [Google Scholar] [CrossRef]
- Broschat, T.K. Nitrate, phosphate, and potassium leaching from container-grown plants fertilized by several methods. HortScience 1995, 30, 74–77. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.C.; Noh, H.S.; Kim, J.W.; Han, S.S. Physiological responses of Cirsium setidens and Pleurospermum camtschaticum under different shading treatments. J. Bio-Environ. Control 2012, 21, 153–161. [Google Scholar]
- Scagel, C.F.; Bi, G.; Fuchigami, L.H.; Regan, R.P. Nitrogen availability alters mineral nutrient uptake and demand in container-grown deciduous and evergreen Rhododendron. J. Environ. Hortic. 2008, 26, 177–187. [Google Scholar]
- Jang, S.H.; Cho, J.G.; Han, J.H.; Jeong, J.H.; Lee, S.K.; Lee, D.Y.; Lee, K.S. Diagnosis of nitrogen content in the leaves of apple tree using spectral imagery. J. Bio-Environ. Control 2022, 31, 384–392. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr.; Arnold, M.A.; Bilderback, T.E.; Bolusky, B.; Chandler, S.; Gramling, H.M.; Lea-Cox, J.D.; Harris, J.R.; Klinger, P.J.; Mathers, H.M.; et al. Strategic vision of container nursery irrigation in the next ten years. J. Environ. Hortic. 2004, 22, 113–115. [Google Scholar] [CrossRef]
- Lambers, H.A.N.S.; Poorter, H. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. Adv. Ecol. Res. 1992, 23, 187–261. [Google Scholar]
- Hernández, E.I.; Vilagrosa, A.; Luis, V.C.; Llorca, M.; Chirino, E.; Vallejo, V.R. Root hydraulic conductance, gas exchange and leaf water potential in seedlings of Pistacia lentiscus L. and Quercus suber L. grown under different fertilization and light regimes. Environ. Exp. Bot. 2009, 67, 269–276. [Google Scholar] [CrossRef]
- Juntunen, M.L.; Hammar, T.; Rikala, R. Nitrogen and phosphorus leaching and uptake by container birch seedlings (Betula pendula Roth) grown in three different fertilizations. New For. 2003, 25, 133–147. [Google Scholar] [CrossRef]
- Compton, J.E.; Watrud, L.S.; Porteous, L.A.; DeGrood, S. Response of soil microbial biomass and community composition to chronic nitrogen additions at Harvard forest. For. Ecol. Manag. 2004, 196, 143–158. [Google Scholar] [CrossRef]
- Frey, S.D.; Knorr, M.; Parrent, J.L.; Simpson, R.T. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For. Ecol. Manag. 2004, 196, 159–171. [Google Scholar] [CrossRef]
- Thirukkumaran, C.M.; Parkinson, D. Microbial activity, nutrient dynamics and litter decomposition in a Canadian Rocky Mountain pine forest as affected by N and P fertilizers. For. Ecol. Manag. 2002, 159, 187–201. [Google Scholar] [CrossRef]
- Carlson, W.C.; Preisig, C.L. Effects of controlled-release fertilizers on the shoot and root development of Douglas-fir seedlings. Can. J. For. Res. 1981, 11, 231–243. [Google Scholar] [CrossRef]
- Imo, M.; Timmer, V.R. Vector competition analysis of black spruce seedling responses to nutrient loading and vegetation control. Can. J. For. Res. 1999, 29, 474–486. [Google Scholar] [CrossRef]
- Quoreshi, M.; Timmer, V.R. Growth, nutrient dynamics, and ectomycorrhizal development of container-grown Picea mariana seedlings in response to exponential nutrient loading. Can. J. For. Res. 2000, 30, 191–201. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Sung, S.J.S.; Pinto, J.R.; Ross-Davis, A.; Scott, D.A. Morphology, gas exchange, and chlorophyll content of longleaf pine seedlings in response to rooting volume, copper root pruning, and nitrogen supply in a container nursery. New For. 2013, 44, 881–897. [Google Scholar] [CrossRef] [Green Version]
- Landis, T.D.; Tinus, R.W.; McDonald, S.E.; Barnett, J.P. Seedling Nutrition and Irrigation; The Container Tree Nursery Manual; USDA Forest Service: Washington, DC, USA, 1989; Volume 4, 674p.
- Cha, Y.G.; Choi, K.S.; Song, K.S.; Sung, H.I.; Lee, H.N.; Sung, H.I.; Kim, J.J. Growth characteristics and visible injury of container seedling of Pinus densiflora by fertilization level. J. Bio-Environ. Control 2019, 28, 66–77. [Google Scholar] [CrossRef]
- Ingestad, T. Mineral nutrient requirements of Pinus silvestris and Picea abies seedlings. Physiol. Plant. 1979, 45, 373–380. [Google Scholar] [CrossRef]
- Deans, J.D.; Mason, W.L.; Cannell, M.G.R.; Sharpe, A.L.; Sheppard, L.J. Growing regimes for bare-root stock of sitka spruce, douglas-fir and scots pine. 1. Morphology at the end of the nursery phase. Forestry 1989, 62, 53–60. [Google Scholar]
- Burdett, A.N. Physiological processes in plantation establishment and the development of specifications for forest planting stock. Can. J. For. Res. 1990, 20, 415–427. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Why seedlings survive: Influence of plant attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Johnson, F. Artificial Regeneration of Ontario’s Forests: Species and Stock Selection Manual No. 131; Ontario Ministry of Natural Resources: Sault Ste. Marie, ON, Canada, 1996; p. 52.
- Mackensen, J.; Hölscher, D.; Klinge, R.; Fölster, H. Nutrient transfer to the atmosphere by burning of debris in eastern Amazonia. For. Ecol. Manag. 1996, 86, 121–128. [Google Scholar] [CrossRef]
- Cho, M.S.; Lee, S.W.; Bae, J.H.; Park, G.S. Effect of different fertilization on physiological characteristics and growth performances of Eucalyptus pellita and Acacia mangium in a container nursery system. J. Bio-Environ. Control 2011, 20, 123–133. [Google Scholar]
- Han, Q.; Chiba, Y. Leaf photosynthetic responses and related nitrogen changes associated with crown reclosure after thinning in a young Chamaecyparis obtusa stand. J. For. Res. 2009, 14, 349–357. [Google Scholar] [CrossRef]
- Richards, N.A.; Leaf, A.L.; Bickelhaupt, D.H. Growth and nutrient uptake of coniferous seedlings: Comparison among 10 species at various seedbed densities. Plant Soil 1973, 38, 125–143. [Google Scholar] [CrossRef]
- Inagaki, M.; Inagaki, Y.; Kamo, K.; Titin, J. Fine-root production in response to nutrient application at three forest plantations in Sabah, Malaysia: Higher nitrogen and phosphorus demand by Acacia mangium. J. For. Res. 2009, 14, 178–182. [Google Scholar] [CrossRef]
- Tsakaldimi, M.; Zagas, T.; Tsitsoni, T.; Ganatsas, P. Root morphology, stem growth and field performance of seedlings of two Mediterranean evergreen oak species raised in different container types. Plant Soil 2005, 278, 85–93. [Google Scholar] [CrossRef]
- Aranda, I.; Gil, L.; Pardos, J.A. Physiological responses of Fagus sylvatica L. seedlings under Pinus sylvestris L. and Quercus pyrenaica Willd. overstories. For. Ecol. Manag. 2002, 162, 153–164. [Google Scholar] [CrossRef]
- Leiva, M.J.; Fernández-Alés, R. Variability in seedling water status during drought within a Quercus ilex subsp. ballota population, and its relation to seedling morphology. For. Ecol. Manag. 1998, 111, 147–156. [Google Scholar] [CrossRef]
- Oliet, J.A.; Planelles, R.; Artero, F.; Valverde, R.; Jacobs, D.F.; Segura, M.L. Field performance of Pinus halepensis planted in Mediterranean arid conditions: Relative influence of seedling morphology and mineral nutrition. New For. 2009, 37, 313–331. [Google Scholar] [CrossRef]
- Trubat, R.; Cortina, J.; Vilagrosa, A. Short-term nitrogen deprivation increases field performance in nursery seedlings of Mediterranean woody species. J. Arid Environ. 2008, 72, 879–890. [Google Scholar] [CrossRef]
- Chirino, E.; Vilagrosa, A.; Hernández, E.I.; Matos, A.; Vallejo, V.R. Effects of a deep container on morpho-functional characteristics and root colonization in Quercus suber L. seedlings for reforestation in Mediterranean climate. For. Ecol. Manag. 2008, 256, 779–785. [Google Scholar] [CrossRef]
- Brunkhorst, M.J. Investigation into the flowering of sugarcane variety N29 grown under different nutrient regimes. S. Afr. Sugar Technol. Assoc. 2003, 77, 306–312. [Google Scholar]
- Berding, N.; Skinner, J.C. Improvement of sugarcane fertility by modification of cross-pollination environment. Crop Sci. 1980, 20, 463–467. [Google Scholar] [CrossRef]
- Gosnell, J.M. Some factors affecting flowering in sugarcane. S. Afr. Sugar Technol. Assoc. 1973, 47, 144–147. [Google Scholar]
- Brunkhorst, M.J. A preliminary investigation into the effect of plant nutrient levels on sugarcane flowering. S. Afr. Sugar Technol. Assoc. 2001, 75, 143–150. [Google Scholar]
- Van Dillewijn, C. Botany of Sugarcane; Chronica Botanica: Waltham, MA, USA; Stechert-Hafner: New York, NY, USA, 1952; 371p. [Google Scholar]
- Hale, A.L.; White, P.M.; Webber III, C.L.; Todd, J.R. Effect of growing media and fertilization on sugarcane flowering under artificial photoperiod. PLoS ONE 2017, 12, e0181639. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.G.; Lee, E.J. Ecophysiology of photosynthesis 1: Effects of light intensity and intercellular CO2 pressure on photosynthesis. Korean J. Agric. For. Meteorol. 2001, 3, 126–133. [Google Scholar]
- Hikosaka, K.; Terashima, I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 1995, 18, 605–618. [Google Scholar] [CrossRef]
- Yang, H.R.; Park, Y.J.; Kim, M.J.; Yeon, J.Y.; Kim, W.S. Growth responses of Korean endemic Hosta minor under sub-optimal artificial lighting. Hortic. Sci. Technol. 2022, 40, 286–295. [Google Scholar]
- Sung, J.H.; Je, S.M.; Kim, S.H.; Kim, Y.K. Effect of calcium chloride CaCl2 on chlorophyll fluorescence image and photosynthetic apparatus in the leaves of Prunus sargentii. J. Korean Soc. For. Sci. 2010, 99, 922–928. [Google Scholar]
- Lee, K.C.; Lee, H.B. Drought stress influences photosynthesis and water relations parameters of Synurus deltoides. J. Korean Soc. For. Sci. 2017, 106, 288–299. [Google Scholar]
- Faria, T.; García-Plazaola, J.I.; Abadia, A.; Cerasoli, S.; Pereira, J.S.; Chaves, M.M. Diurnal changes in photoprotective mechanisms in leaves of cork oak (Quercus suber) during summer. Tree Physiol. 1996, 16, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.H.; Woo, S.Y.; Kwon, M.J.; Chun, J.H.; Shin, J.H. Photosynthetic capacity and water use efficiency under different temperature regimes on healthy and declining Korean Fir in Mt. Halla. J. Korean For. Soc. 2006, 95, 705–710. [Google Scholar]
- Salisbury, F.B.; Ross, C.W. Plant Physiology, 4th ed.; Wadsworth Publishing Company: Belmont, MA, USA, 1992; 257p. [Google Scholar]
- Yu, S.O.; Bae, J.H. The effect of fertigation setting point on the growth and fruit quality of sweet pepper (Capsicum annuum L.). J. Bio-Environ. Control. 2004, 13, 102–106. [Google Scholar]
- Song, W.; Lee, B.; Cho, N.; Jung, S.; Kim, E.S.; Lim, J.H. Effects of drought stress and nitrogen fertilization on growth and physiological characteristics of Pinus densiflora seedlings under elevated temperature and CO2 concentration. Korean J. Agric. For. Meteorol. 2020, 22, 57–67. [Google Scholar]
- Abril, M.; Hanano, R. Ecophysiological responses of three evergreen woody Mediterranean species to water stress. Acta Oecologica 1998, 19, 377–387. [Google Scholar] [CrossRef]
- Singh, B.; Singh, G. Biomass partitioning and gas exchange in Dalbergia sissoo seedlings under water stress. Photosynthetica 2003, 41, 407–414. [Google Scholar] [CrossRef]
- Evans, J.R. The dependence of quantum yield on wavelength and growth irradiance. Funct. Plant Biol. 1987, 14, 69–79. [Google Scholar] [CrossRef]
- Wang, Z.X.; Chen, L.; Ai, J.; Qin, H.Y.; Liu, Y.X.; Xu, P.L.; Jiao, Z.Q.; Zhao, Y.; Zhang, Q.T. Photosynthesis and activity of photosystem II in response to drought stress in Amur Grape (Vitis amurensis Rupr.). Photosynthetica 2012, 50, 189–196. [Google Scholar] [CrossRef]
- Spoustová, P.; Synková, H.; Valcke, R.; Čeřovská, N. Chlorophyll a fluorescence as a tool for a study of the Potato virus Y effects on photosynthesis of nontransgenic and transgenic P ssu-ipt tobacco. Photosynthetica 2013, 51, 191–201. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; Yunus, M., Pathre, U., Eds.; Taylor and Francis: New York, NY, USA, 2000; pp. 445–483. [Google Scholar]
- Lee, K.C.; Kim, S.H.; Park, W.G.; Kim, Y.S. Effects of drought stress on photosynthetic capacity and photosystem II activity in Oplopanax elatus. Korean J. Med. Crop Sci. 2014, 22, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.J.; Koh, S.C. Chlorophyll fluorescence and antioxidative enzyme activity of Crinum leaves exposed to natural environmental stress in winter. Korean J. Environ. Biol. 2004, 22, 233–241. [Google Scholar]
- Falqueto, A.R.; Silva, F.S.; Cassol, D.; Magalhães Júnior, A.M.; Oliveira, A.C.; Bacarin, M.A. Chlorophyll fluorescence in rice: Probing of senescence driven changes of PSII activity on rice varieties differing in grain yield capacity. Braz. J. Plant Physiol. 2010, 22, 35–41. [Google Scholar] [CrossRef]
- Holland, V.; Koller, S.; Brüggemann, W. Insight into the photosynthetic apparatus in evergreen and deciduous European oaks during autumn senescence using OJIP fluorescence transient analysis. Plant Biol. 2014, 16, 801–808. [Google Scholar] [CrossRef]
- Boureima, S.; Oukarroum, A.; Diouf, M.; Cisse, N.; Van Damme, P. Screening for drought tolerance in mutant germplasm of sesame (Sesamum indicum) probing by chlorophyll a fluorescence. Environ. Exp. Bot. 2012, 81, 37–43. [Google Scholar] [CrossRef]
- Zushi, K.; Kajiwara, S.; Matsuzoe, N. Chlorophyll a fluorescence OJIP transient as a tool to characterize and evaluate response to heat and chilling stress in tomato leaf and fruit. Sci. Hortic. 2012, 148, 39–46. [Google Scholar] [CrossRef]
- Van Heerden, P.D.R.; Swanepoel, J.W.; Krüger, G.H.J. Modulation of photosynthesis by drought in two desert scrub species exhibiting C3-mode CO2 assimilation. Environ. Exp. Bot. 2007, 61, 124–136. [Google Scholar] [CrossRef]
- Rathod, D.P.; Brestic, M.; Shao, H.B. Chlorophyll a fluorescence determines the drought resistance capabilities in two varieties of mycorrhized and non-mycorrhized Glycine max Linn. Afr. J. Microbiol. Res. 2011, 5, 4197–4206. [Google Scholar] [CrossRef] [Green Version]
- Timmer, V.R.; Stone, E.L. Comparative foliar analysis of young balsam fir fertilized with nitrogen, phosphorus, potassium, and lime. Soil Sci. Soc. Am. J. 1978, 42, 125–130. [Google Scholar] [CrossRef]
- Salifu, K.F.; Jacobs, D.F. Characterizing fertility targets and multi-element interactions in nursery culture of Quercus rubra seedlings. Ann. For. Sci. 2006, 63, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.; Timmer, V.R. Rhizosphere phosphorus depletion induced by heavy nitrogen fertilization in forest nursery soils. Soil Sci. Soc. Am. J. 1995, 59, 227–233. [Google Scholar] [CrossRef]
- De Visser, P.H.B.; Keltjens, W.G. Growth and nutrient uptake of Douglas fir seedlings at different rates of ammonium supply, with or without additional nitrate and other nutrients. Neth. J. Agric. Sci. 1993, 41, 327–341. [Google Scholar] [CrossRef]
- Salifu, K.F.; Jacobs, D.F.; Birge, Z.K. Nursery nitrogen loading improves field performance of bareroot oak seedlings planted on abandoned mine lands. Restor. Ecol. 2009, 17, 339–349. [Google Scholar] [CrossRef]
- Benzian, B.; Brown, R.M.; Freeman, S.C.R. Effect of late-season top-dressings of N (and K) applied to conifer transplants in the nursery on their survival and growth on British forest sites. For. Int. J. For. Res. 1974, 47, 153–184. [Google Scholar] [CrossRef]
- Margolis, H.A.; Waring, R.H. Carbon and nitrogen allocation patterns of Douglas-fir seedlings fertilized with nitrogen in autumn. II. Field performance. Can. J. For. Res. 1986, 16, 903–909. [Google Scholar] [CrossRef]
- Malik, V.; Timmer, V.R. Interaction of nutrient-loaded black spruce seedlings with neighbouring vegetation in greenhouse environments. Can. J. For. Res. 1995, 25, 1017–1023. [Google Scholar] [CrossRef]
- Meteorological Data Open Portal. Available online: https://data.kma.go.kr/data/grnd/selectAsosRltmList.do;jsessionid=3Ycf47KBxOW6udOyJER6CDkh2EZWBFAS2ahQTsn0FFszA1tyxOjCTyZ1CkbSXuuJ.was02_servlet_engine5?pgmNo=36 (accessed on 27 January 2023).
- Hughes, A.P.; Freeman, P.R. Growth analysis using frequent small harvests. J. Appl. Ecol. 1967, 4, 553–560. [Google Scholar] [CrossRef]
- Bayala, J.; Dianda, M.; Wilson, J.; Ouedraogo, S.J.; Sanon, K. Predicting field performance of five irrigated tree species using seedling quality assessment in Burkina Faso, West Africa. New For. 2009, 38, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Šestak, Z.; Catsk, J.; Jarvis, P.G. Plant Photosynthetic Production. Manual of Methods; Hertogenbosch: The Hague, The Netherlands, 1971; 818p. [Google Scholar]
- Choi, E.Y.; Seo, T.C.; Lee, S.G.; Cho, I.H.; Stangoulis, J. Growth and physiological responses of Chinese cabbage and radish to long-term exposure to elevated carbon dioxide and temperature. Hortic. Environ. Biotechnol. 2011, 52, 376–386. [Google Scholar] [CrossRef]
- Von Caemmerer, S.V.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Kume, A.; Ino, Y. Comparison of ecophysiological responses to heavy snow in two varieties of Aucuba japonica with different areas of distribution. Ecol. Res. 1993, 8, 111–121. [Google Scholar] [CrossRef]
- Oh, S.J.; Koh, S.C. Chlorophyll fluorescence and antioxidative enzyme activity of Crinum leaves exposed to winter stress. Acta Physiol. Plant. 2004, 26, 174–175. [Google Scholar]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- RDA (Rural Development Administration). Methods of Soil and Plant Analysis; National Institute of Agricultural Science and Technology: Suwon, Republic of Korea, 2000; 202p. [Google Scholar]
- Haase, D.L.; Rose, R. Vector analysis and its use for interpreting plant nutrient shifts in response to silvicultural treatments. For. Sci. 1995, 41, 54–66. [Google Scholar] [CrossRef]
- Park, B.B.; Byun, J.K.; Sung, J.H.; Cho, M.S. Study of optimal fertilization with vector analysis in hardwood and softwood seedlings. J. Agric. Life Sci. 2013, 47, 95–107. [Google Scholar]
- Salifu, K.F.; Timmer, V.R. Nitrogen retranslocation response of young Picea mariana to nitrogen-15 supply. Soil Sci. Soc. Am. J. 2003, 67, 309–317. [Google Scholar] [CrossRef]
- Timmer, V.R. Exponential nutrient loading: A new fertilization technique to improve seedling performance on competitive sites. New For. 1997, 13, 279–299. [Google Scholar] [CrossRef]







| Irrigation (ton/yr/tree) | Fertilization | Height | Root Collar Diameter | H/D Ratio (cm·mm−1) | ||
|---|---|---|---|---|---|---|
| Growth (cm) | Relative Growth Rate (cm·day−1) | Growth (mm) | Relative Growth Rate (mm·day−1) | |||
| Control in BRSC z | 165.60 ± 21.02 c | 0.0767 ± 0.0207 b | 30.58 ± 6.61 d | 0.0025 ± 0.0003 de | 5.53 ± 0.69 abc | |
| 0.2 | Control | 195.80 ± 15.26 ab | 0.0895 ± 0.0276 ab | 34.82 ± 5.96 cd | 0.0026 ± 0.0002 cde | 5.79 ± 1.28 ab |
| 69 g/yr | 207.33 ± 9.00 ab | 0.1062 ± 0.0256 ab | 37.81 ± 4.77 a-d | 0.0030 ± 0.0002 abc | 5.58 ± 0.91 abc | |
| 138 g/yr | 196.33 ± 9.71 ab | 0.1230 ± 0.0199 a | 46.47 ± 1.89 ab | 0.0032 ± 0.0001 ab | 4.23 ± 0.38 c | |
| 207 g/yr | 221.67 ± 6.35 a | 0.1220 ± 0.0056 a | 46.65 ± 4.30 ab | 0.0031 ± 0.0000 ab | 4.77 ± 0.36 abc | |
| 0.3 | Control | 192.00 ± 6.25 b | 0.1142 ± 0.0262 ab | 32.84 ± 3.86 cd | 0.0024 ± 0.0004 e | 5.92 ± 0.88 a |
| 69 g/yr | 222.67 ± 24.19 a | 0.1196 ± 0.0322 a | 37.39 ± 4.93 bcd | 0.0032 ± 0.0001 ab | 6.00 ± 0.69 a | |
| 138 g/yr | 208.00 ± 11.36 ab | 0.1233 ± 0.0253 a | 42.31 ± 8.24 abc | 0.0034 ± 0.0003 a | 4.02 ± 0.80 abc | |
| 207 g/yr | 212.00 ± 6.25 ab | 0.1136 ± 0.0303 ab | 46.84 ± 7.77 ab | 0.0032 ± 0.0002 ab | 4.61 ± 0.80 abc | |
| 0.4 | Control | 161.67 ± 22.50 c | 0.0988 ± 0.0112 ab | 28.52 ± 8.24 d | 0.0029 ± 0.0003 bcd | 5.82 ± 0.86 ab |
| 69 g/yr | 207.67 ± 27.21 ab | 0.1069 ± 0.0264 ab | 42.52 ± 3.90 abc | 0.0028 ± 0.0002 b-e | 4.90 ± 0.63 abc | |
| 138 g/yr | 216.75 ± 17.25 ab | 0.1094 ± 0.0162 ab | 45.44 ± 3.99 ab | 0.0030 ± 0.0003 abc | 4.43 ± 0.64 abc | |
| 207 g/yr | 208.50 ± 5.51 ab | 0.1013 ± 0.0116 ab | 47.81 ± 6.37 a | 0.0029 ± 0.0000 a | 4.81 ± 0.67 bc | |
| Irrigation (I) | 1.58 NS | 1.10 NS | 0.29 NS | 0.03 NS | 0.78 NS | |
| Fertilization (F) | 9.90 *** | 1.03 NS | 14.49 *** | 15.66 *** | 5.50 ** | |
| I × F | 2.60 * | 0.49 NS | 0.76 NS | 2.68 * | 0.52 NS | |
| Irrigation | Fertilization | Flowering Beginning (Day) | Flowering Speed | Amount of Flowering (ea) | Flowering Periods |
|---|---|---|---|---|---|
| Control in BRSC z | 97.8 ± 0.7 b | 0.9 ± 0.3 fg | 113.0 ± 33.8 ef | 68.2 ± 4.5 d | |
| 0.2 ton/yr/tree | Control | 102.5 ± 1.1 b | 0.3 ± 0.1 g | 32.0 ± 7.0 f | 59.6 ± 2.3 e |
| 69 g/yr | 88.9 ± 9.7 c | 1.7 ± 0.8 ef | 194.3 ± 82.7 de | 78.6 ± 10.9 c | |
| 138 g/yr | 82.2 ± 5.0 cd | 3.1 ± 0.5 bcd | 345.7 ± 56.6 bc | 85.3 ± 4.5 abc | |
| 207 g/yr | 78.4 ± 0.3 d | 3.4 ± 0.2 a-d | 362.0 ± 35.8 abc | 88.3 ± 2.6 ab | |
| 0.3 ton/yr/tree | Control | 98.5 ± 0.7 b | 0.2 ± 0.0 g | 19.5 ± 5.0 f | 62.5 ± 0.7 de |
| 69 g/yr | 86.6 ± 3.1 c | 2.7 ± 0.7 cd | 325.0 ± 80.0 bc | 83.9 ± 3.3 abc | |
| 138 g/yr | 78.7 ± 0.2 d | 3.8 ± 0.5 ab | 443.3 ± 70.7 ab | 88.4 ± 1.3 ab | |
| 207 g/yr | 82.0 ± 1.7 cd | 4.1 ± 0.5 a | 468.7 ± 57.5 a | 91.8 ± 0.3 a | |
| 0.4 ton/yr/tree | Control | 123.8 ± 3.2 a | 0.1 ± 0.0 g | 2.5 ± 0.7 f | 22.3 ± 3.2 f |
| 69 g/yr | 88.4 ± 0.2 c | 2.4 ± 0.5 de | 292.3 ± 67.0 cd | 82.0 ± 0.3 bc | |
| 138 g/yr | 82.1 ± 5.2 cd | 3.1 ± 0.4 bcd | 342.0 ± 45.3 bc | 87.5 ± 4.8 abc | |
| 207 g/yr | 85.9 ± 4.0 cd | 3.5 ± 0.9 abc | 381.0 ± 102.36 abc | 83.6 ± 4.8 abc | |
| Irrigation (I) | 13.76 *** | 4.13 * | 4.87 * | 21.76 *** | |
| Fertilization (F) | 71.03 *** | 69.31 *** | 59.07 *** | 120.63 *** | |
| I × F | 5.63 ** | 0.82 NS | 0.99 NS | 12.96 *** | |
| Soil Media Mixture (v/v) | pH | EC (dS·m−1) | NO− (mg/kg−1) | P2O5 (mg/kg−1) | Ex-Cations (cmol·kg−1) | C.E.C (cmol·kg−1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peat Moss | Perlite | Vermiculite | K+ | Ca2+ | Mg2+ | |||||
| 1 | 1 | 1 | 6.0 | 0.1 | 0.1 | 123 | 4 | 10 | 7 | 17 |
| Total Fertilization Amount (g/year/tree) | Monthly Fertilization Amount (g/month/tree) | ||||||
|---|---|---|---|---|---|---|---|
| April | May | June | July | August | September | October | |
| 69.0 | - | 7.67 | 7.67 | 7.67 | 7.67 | 7.67 | - |
| 138.0 | - | 15.33 | 15.33 | 15.33 | 15.33 | 15.33 | - |
| 207.0 | - | 23.00 | 23.00 | 23.00 | 23.00 | 23.00 | - |
| Number of Drip | Total Irrigation Amount (ton/year/tree) | Monthly Irrigation Amount (L/month/tree) | ||||||
|---|---|---|---|---|---|---|---|---|
| April | May | June | July | August | September | October | ||
| 2 | 0.2 | 24.0 | 27.0 | 42.9 | 44.3 | 44.2 | 27.0 | 27.0 |
| 3 | 0.3 | 36.0 | 40.5 | 64.4 | 66.5 | 66.3 | 40.5 | 40.5 |
| 4 | 0.4 | 48.0 | 54.0 | 85.9 | 88.7 | 88.4 | 54.0 | 54.0 |
| Parameters | Description |
|---|---|
| Vj | Relative variable fluorescence at time (2 ms) |
| ΦPO | Probability that an absorbed photon leads to reduction further than QA− |
| ΦEO | Probability that an absorbed photon leads to electron transport further than QA− |
| ΨO | Probability that an absorbed photon leads to reduction of QA− |
| ABS/RC | Absorption flux per reaction center |
| TRo/RC | Trapping of electrons per reaction center |
| ETo/RC | Electron flux per reaction center beyond QA− |
| DIo/RC | Energy dissipation flux per reaction center |
| PIABS | Performance index on absorption basis. |
| Vector Shift | Change in Relative | Possible Diagnosis | Interpretation | ||
|---|---|---|---|---|---|
| Mass | Concentration | Content | |||
| A | + | − | + | Non-limiting | Dilution |
| B | + | 0 | + | Non-limiting | Sufficiency |
| C | + | + | + | Limiting | Deficiency |
| D | 0 | + | + | Non-toxic | Luxury consumption |
| E | − | ++ | ± | Toxic | Excess |
| F | − | − | − | Antagonisric | Excess |
| G | 0, − | − | − | Retranslocation | Depletion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, E.-J.; Yoon, J.-H.; Lee, H.; Kwon, H.-Y.; Shin, H.-N.; Yong, S.-H.; Choi, M.-S. Effects of Drip Irrigation-Fertilization on Growth, Flowering, Photosynthesis and Nutrient Absorption of Containerized Seedlings of Hibiscus syriacus L. (Haeoreum). Plants 2023, 12, 2293. https://doi.org/10.3390/plants12122293
Jin E-J, Yoon J-H, Lee H, Kwon H-Y, Shin H-N, Yong S-H, Choi M-S. Effects of Drip Irrigation-Fertilization on Growth, Flowering, Photosynthesis and Nutrient Absorption of Containerized Seedlings of Hibiscus syriacus L. (Haeoreum). Plants. 2023; 12(12):2293. https://doi.org/10.3390/plants12122293
Chicago/Turabian StyleJin, Eon-Ju, Jun-Hyuck Yoon, Hyeok Lee, Hae-Yun Kwon, Han-Na Shin, Seong-Hyeon Yong, and Myung-Suk Choi. 2023. "Effects of Drip Irrigation-Fertilization on Growth, Flowering, Photosynthesis and Nutrient Absorption of Containerized Seedlings of Hibiscus syriacus L. (Haeoreum)" Plants 12, no. 12: 2293. https://doi.org/10.3390/plants12122293






