The Invasion of Alien Populations of Solanum elaeagnifolium in Two Mediterranean Habitats Modifies the Soil Communities in Different Ways
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Soil Sampling
4.3. Soil Physicochemical Properties
4.4. Phospholipid Fatty Acid Analysis (PLFA) Extraction and Classification
4.5. Nematode Extraction and Identification
4.6. Nematode Functional Indices
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davie, K.W. Plant community diversity and native plant abundance decline with increasing abundance of an exotic annual grass. Oecologia 2011, 167, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Walker, L.R.; Smith, S.D. Impacts of invasive plants on community and ecosystem properties. In Assessment and Management of Plant Invasions; Luken, J.O., Thieret, J.W., Eds.; Springer: New York, NY, USA, 1997; pp. 69–86. [Google Scholar]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Eco. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Belnap, J.; Phillips, S.L. Soil biota in an ungrazed grassland: Response to annual grass (Bromus tectorum) invasion. Ecol. Appl. 2001, 11, 1261–1275. [Google Scholar] [CrossRef]
- Liao, C.; Peng, R.; Luo, Y.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2008, 177, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.M.; Lekberg, Y.; Mummey, D.L.; Sangwan, N.; Ramsey, P.W.; Gilbert, J.A. Invasive plants rapidly reshape soil properties in a grassland ecosystem. mSystems 2017, 2, e00178-16. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Knapp, L.S.P.; Yu, M.; Wang, G.G. Solidago canadensis invasion destabilizes the understory plant community and soil properties of coastal shelterbelt forests of subtropical China. Plant Soil 2023, 484, 65–77. [Google Scholar] [CrossRef]
- Callaway, R.M.; Thelen, G.C.; Rodriguez, A.; Holben, W.E. Soil biota and exotic plant invasion. Nature 2004, 427, 731–733. [Google Scholar] [CrossRef]
- Porazinska, D.L.; Pratt, P.D.; Glblin-Davis, R.M. Consequences of Melaleuca quinquenervia invasion on soil nematodes in the Florida Everglades. J. Nematol. 2007, 39, 305. [Google Scholar]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Ferris, H.; Matute, M. Structural and functional succession in the nematode fauna of a soil food web. Appl. Soil Ecol. 2003, 23, 93–110. [Google Scholar] [CrossRef]
- Ferris, H.; Venette, R.; Scow, K. Soil management to enhance bacterivore and fungivore nematode populations and their nitrogen mineralisation function. Appl. Soil Ecol. 2004, 25, 19–35. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.E.; Klironomos, J.N. Breaking new ground: Soil communities and exotic plant invasion. BioScience 2005, 55, 477–487. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Callaway, R.M. Soil biota and invasive plants. New Phytol. 2006, 170, 445–457. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A. Plant–soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Vilà, M.; Corbin, J.D.; Dukes, J.S.; Pino, J.; Smith, S.D. Linking plant invasions to global environmental change. In Terrestrial Ecosystems in a Changing World; Canadell, J.G., Pataki, D.E., Pitelka, L.F., Eds.; Global Change—The IGBP Series; Springer: Berlin/Heidelberg, Germany, 2007; pp. 93–102. [Google Scholar]
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef]
- Suding, K.N.; Stanley Harpole, W.; Fukami, T.; Kulmatiski, A.; MacDougall, A.S.; Stein, C.; van der Putten, W.H. Consequences of plant–soil feedbacks in invasion. J. Ecol. 2013, 101, 298–308. [Google Scholar] [CrossRef]
- Schittko, C.; Runge, C.; Strupp, M.; Wolff, S.; Wurst, S. No evidence that plant–soil feedback effects of native and invasive plant species under glasshouse conditions are reflected in the field. J. Ecol. 2016, 104, 1243–1249. [Google Scholar] [CrossRef]
- Yeates, G.W.; Williams, P.A. Influence of three invasive weeds and site factors on soil microfauna in New Zealand. Pedobiologia 2001, 45, 367–383. [Google Scholar] [CrossRef]
- Porazinska, D.L.; Bardgett, R.D.; Blaauw, M.B.; Hunt, H.W.; Parsons, A.N.; Seastedt, T.R.; Wall, D.H. Relationships at the aboveground–belowground interface: Plants, soil biota, and soil processes. Ecol. Monogr. 2003, 73, 377–395. [Google Scholar] [CrossRef]
- Fitoussi, N.; Pen-Mouratov, S.; Steinberger, Y. Soil free-living nematodes as bio-indicators for assaying the invasive effect of the alien plant Heterotheca subaxillaris in a coastal dune ecosystem. Appl. Soil Ecol. 2016, 102, 1–9. [Google Scholar] [CrossRef]
- Porazinska, D.L.; Fujisaki, I.; Purcell, M.F.; Giblin-Davis, R.M. Plant invasions from a belowground nematocentric perspective. Soil Biol. Biochem. 2014, 77, 213–220. [Google Scholar] [CrossRef]
- Renčo, M.; Baležentiené, L. An analysis of soil free-living and plant-parasitic nematode communities in three habitats invaded by Heracleum sosnowskyi in central Lithuania. Biol. Invas. 2015, 17, 1025–1039. [Google Scholar] [CrossRef]
- Čerevková, A.; Ivashchenko, K.; Miklisová, D.; Ananyeva, N.; Renčo, M. Influence of invasion by Sosnowsky’s hogweed on nematode communities and microbial activity in forest and grassland ecosystems. Global Ecol. Conserv. 2020, 21, e00851. [Google Scholar] [CrossRef]
- Čerevková, A.; Bobuľská, L.; Miklisová, D.; Renčo, M. A case study of soil food web components affected by Fallopia japonica (Polygonaceae) in three natural habitats in Central Europe. J. Nematol. 2019, 51, e2019-42. [Google Scholar] [CrossRef]
- Čerevková, A.; Miklisová, D.; Bobuľská, L.; Renčo, M. Impact of the invasive plant Solidago gigantea on soil nematodes in a semi-natural grassland and a temperate broadleaved mixed forest. J. Helminthol. 2019, 94, e51. [Google Scholar] [CrossRef]
- Gaggini, L.; Rusterholz, H.-P.; Baur, B. The invasive plant Impatiens glandulifera affects soil fungal diversity and the bacterial community in forests. Appl. Soil Ecol. 2018, 124, 335–343. [Google Scholar] [CrossRef]
- Boyd, J.; Murray, D.; Tyrl, R. Silverleaf nightshade, Solanum elaeagnifolium, origin, distribution, and relation to man. Econ. Bot. 1984, 38, 210–217. [Google Scholar] [CrossRef]
- Roche, C. Silverleaf Nightshade (Solanum elaeagnifolium Cav.). In PNW-Pacific Northwest Extension Publication; Cooperative Extension Service, Washington State Universitiy: Washington, DC, USA, 1991. [Google Scholar]
- Roberts, J.; Florentine, S. Biology, distribution and management of the globally invasive weed Solanum elaeagnifolium Cav. (silverleaf nightshade): A global review of current and future management challenges. Weed Res. 2022, 62, 393–403. [Google Scholar] [CrossRef]
- Stanton, R.; Heap, J.; Carter, R.; Wu, H. Solanum elaeagnifolium Cav. In The Biology of Australian Weeds; Panetta, F., Ed.; RG and FJ Richardson: Melbourne, Australia, 2009; Volume 3, pp. 1–35. [Google Scholar]
- Utah, W.; Rico, P. Datasheet on quarantine—Solanum elaeagnifolium. EPPO Bull. 2007, 37, 236–245. [Google Scholar] [CrossRef]
- Mekki, M. Biology, distribution and impacts of silverleaf nightshade (Solanum elaeagnifolium Cav.). EPPO Bull. 2007, 37, 114–118. [Google Scholar] [CrossRef]
- Krigas, N.; Tsiafouli, M.A.; Katsoulis, G.; Votsi, N.-E.; van Kleunen, M. Investigating the invasion pattern of the alien plant Solanum elaeagnifolium Cav.(silverleaf nightshade): Environmental and human-induced drivers. Plants 2021, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Arianoutsou, M.; Bazos, I.; Delipetrou, P.; Kokkoris, Y. The alien flora of Greece: Taxonomy, life traits and habitat preferences. Biol. Invas. 2010, 12, 3525–3549. [Google Scholar] [CrossRef]
- Krigas, N.; Votsi, N.-E.; Samartza, I.; Katsoulis, G.; Tsiafouli, M.A. Solanum elaeagnifolium (Solanaceae) invading one in five Natura 2000 protected areas of Greece and one in four habitat types: What is next? Diversity 2023, 15, 143. [Google Scholar] [CrossRef]
- Christodoulakis, N.S.; Lampri, P.-N.; Fasseas, C. Structural and cytochemical investigation of the leaf of silverleaf nightshade (Solanum elaeagnifolium), a drought-resistant alien weed of the Greek flora. Aust. J. Bot. 2009, 57, 432–438. [Google Scholar] [CrossRef]
- Ben-Ghabrit, S.; Bouhache, M.; Birouk, A.; Bon, M.C. Macromorphological variation of the invasive silverleaf nightshade (Solanum elaeagnifolium Cav.) and its relation to climate and altitude in Morocco. Rev. Marocaine Sci. Agrono. Vét. 2019, 7, 243–251. [Google Scholar]
- Singleton, J.J.; Mangat, P.K.; Shim, J.; Vavra, C.; Coldren, C.; Angeles-Shim, R.B. Cross-species transferability of Solanum spp. DNA markers and their application in assessing genetic variation in silverleaf nightshade (Solanum elaeagnifolium) populations from Texas, USA. Weed Sci. 2020, 68, 396–404. [Google Scholar] [CrossRef]
- Green, J.; Murray, D.S.; Verhalen, L.M. Full-season interference of silverleaf nightshade (Solanum elaeagnifolium) with cotton (Gossypium hirsutum). Weed Sci. 1987, 35, 813–818. Available online: www.jstor.org/stable/4044576 (accessed on 30 April 2023). [CrossRef]
- Green, J.; Murray, D.S.; Stone, J.F. Soil water relations of silverleaf nightshade (Solanum elaeagnifolium) with cotton (Gossypium hirsutum). Weed Sci. 1988, 36, 740–746. Available online: www.jstor.org/stable/4044780 (accessed on 30 April 2023). [CrossRef]
- Smith, B.S.; Pawlak, J.A.; Murray, D.S.; Verhalen, L.M.; Green, J. Interference from established stands of silverleaf nightshade (Solanum elaeagnifolium) on cotton (Gossypium hirsutum) lint yield. Weed Sci. 1990, 38, 129–133. Available online: www.jstor.org/stable/4045040 (accessed on 30 April 2023). [CrossRef]
- Tscheulin, T.; Petanidou, T.; Potts, S.G.; Settele, J. The impact of Solanum elaeagnifolium, an invasive plant in the Mediterranean, on the flower visitation and seed set of the native co-flowering species Glaucium flavum. Plant Ecol. 2009, 205, 77–85. [Google Scholar] [CrossRef]
- Tscheulin, T.; Petanidou, T. The presence of the invasive plant Solanum elaeagnifolium deters honeybees and increases pollen limitation in the native co-flowering species Glaucium flavum. Biol. Invas. 2013, 15, 385–393. [Google Scholar] [CrossRef]
- Tscheulin, T.; Petanidou, T.; Settele, J. Invasive weed facilitates incidence of Colorado potato beetle on potato crop. Intern. J. Pest Manag. 2009, 55, 165–173. [Google Scholar] [CrossRef]
- Kashefi, J.; Lagopodi, A. New pathogens of Solanum elaeagnifolium investigated as possible biocontrol agents of the weed in Greece. In Proceedings of the Environmental Weeds and Invasive Plants, 3rd International Symposium of Environmental Weeds and Invasive Plants, Ascona, Switzerland, 2–7 October 2011. [Google Scholar]
- Faizi, S.; Fayyaz, S.; Bano, S.; Yawar Iqbal, E.; Siddiqi, H.; Naz, A. Isolation of nematicidal compounds from Tagetes patula L. yellow flowers: Structure–activity relationship studies against cyst nematode Heterodera zeae infective stage larvae. J. Agric. Food Chem. 2011, 59, 9080–9093. [Google Scholar] [CrossRef]
- Balah, M.A.; AbdelRazek, G.M. Pesticidal activity of Solanum elaeagnifolium Cav. Leaves against nematodes and perennial weeds. Acta Ecol. Sin. 2020, 40, 373–379. [Google Scholar] [CrossRef]
- Balah, M.A.; Hassany, W.M.; Kobici, A.A. Allelopathy of invasive weed Solanum elaeagnifolium Cav.: An investigation in germination, growth and soil properties. J. Plant Prot. Res. 2022, 62, 58–70. [Google Scholar] [CrossRef]
- Karmezi, M.; Krigas, N.; Argyropoulou, M.D. The Invasion and long naturalization of Solanum elaeagnifolium affects the soil nematode community: Evidence from a comparative study. Agronomy 2022, 12, 2346. [Google Scholar] [CrossRef]
- Chavana, J.; Singh, S.; Vazquez, A.; Christoffersen, B.; Racelis, A.; Kariyat, R.R. Local adaptation to continuous mowing makes the noxious weed Solanum elaeagnifolium a superweed candidate by improving fitness and defense traits. Sci. Rep. 2021, 11, 6634. [Google Scholar] [CrossRef]
- Dassonville, N.; Vanderhoeven, S.; Vanparys, V.; Hayez, M.; Gruber, W.; Meerts, P. Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 2008, 157, 131–140. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 2003, 6, 503–523. [Google Scholar] [CrossRef]
- Brust, G.E. Management strategies for organic vegetable fertility. In Safety and Practice for Organic Food; Biswas, D., Micallef, S.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 193–212. [Google Scholar]
- Kulmatiski, A.; Beard, K.H.; Stark, J.M. Soil history as a primary control on plant invasion in abandoned agricultural fields. J. Appl. Ecol. 2006, 43, 868–876. [Google Scholar] [CrossRef]
- Pérez-Bejarano, A.; Mataix-Solera, J.; Zornoza, R.; Guerrero, C.; Arcenegui, V.; Mataix-Beneyto, J.; Cano-Amat, S. Influence of plant species on physical, chemical and biological soil properties in a Mediterranean forest soil. Eur. J. Forest Res. 2010, 129, 15–24. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, L.; Li, K.; Ni, R.; Han, R.; Li, C.; Zhang, C.; Shen, W.; Zhang, Z. Leaf and root litter species identity influences bacterial community composition in short-term litter decomposition. Forests 2022, 13, 1402. [Google Scholar] [CrossRef]
- Sammani, A.; Shammaa, E.; Chehna, F. Qualitative and quantitative steroidal alkaloids of Solanum species distributed widely in Syria by TLC and HPLC. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 23. [Google Scholar]
- Chiale, C.A.; Cabrera, J.L.; Juliani, H.R. Kaempferol 3-(6″-cis-cinnamoylglucoside) from Solanum elaeagnifolium. Phytochemistry 1991, 30, 1042–1043. [Google Scholar] [CrossRef]
- Balah, M.A.a. Herbicidal activity of constituents isolated from Solanum elaeagnifolium (Solanaceae). J. Crop Prot. 2015, 4, 487–496. [Google Scholar]
- Badawy, A.; Zayed, R.; Ahmed, S.; Hassanean, H. Phytochemical and pharmacological studies of Solanum elaeagnifolium growing in Egypt. J. Nat. Prod. 2013, 6, 156–167. [Google Scholar]
- Tsaballa, A.; Nikolaidis, A.; Trikka, F.; Ignea, C.; Kampranis, S.C.; Makris, A.M.; Argiriou, A. Use of the de novo transcriptome analysis of silver-leaf nightshade (Solanum elaeagnifolium) to identify gene expression changes associated with wounding and terpene biosynthesis. BMC Genom. 2015, 16, 504. [Google Scholar] [CrossRef]
- Bouslamti, M.; El Barnossi, A.; Kara, M.; Alotaibi, B.S.; Al Kamaly, O.; Assouguem, A.; Lyoussi, B.; Benjelloun, A.S. Total polyphenols content, antioxidant and antimicrobial activities of leaves of Solanum elaeagnifolium Cav. from Morocco. Molecules 2022, 27, 4322. [Google Scholar] [CrossRef] [PubMed]
- Bouslamti, M.; Metouekel, A.; Chelouati, T.; El Moussaoui, A.; Barnossi, A.E.; Chebaibi, M.; Nafidi, H.-A.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A. Solanum elaeagnifolium var. obtusifolium (Dunal) Dunal: Antioxidant, antibacterial, and antifungal activities of polyphenol-rich extracts chemically characterized by use of in vitro and in silico approaches. Molecules 2022, 27, 8688. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, N.; Swennen, R.; De Waele, D. Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant-parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. Nematology 2006, 8, 89–101. [Google Scholar] [CrossRef]
- Renčo, M.; Čerevková, A.; Homolová, Z. Nematode communities indicate the negative impact of Reynoutria japonica invasion on soil fauna in ruderal habitats of tatra national park in Slovakia. Global Ecol. Conserv. 2021, 26, e01470. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Steel, H.; Buchan, D.; Bert, W.; De Neve, S. Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci. Rep. 2016, 6, 32862. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Du Preez, G.; Daneel, M.; De Goede, R.; Du Toit, M.J.; Ferris, H.; Fourie, H.; Geisen, S.; Kakouli-Duarte, T.; Korthals, G.; Sánchez-Moreno, S. Nematode-based indices in soil ecology: Application, utility, and future directions. Soil Biol. Biochem. 2022, 169, 108640. [Google Scholar] [CrossRef]
- Wasilewska, L. Soil invertebrates as bioindicators, with special reference to soil-inhabiting nematodes. Rus. J. Nematol. 1997, 5, 113–126. [Google Scholar]
- Yeates, G.; Lee, W.G. Burning in a New Zealand snow-tussock grassland: Effects on vegetation and soil fauna. N. Z. J. Ecol. 1997, 21, 73–79. [Google Scholar]
- Yannitsaros, A.; Economidou, E. Studies on the adventive flora of Greece. I. General remarks on some recently introduced taxa. Candollea 1974, 29, 111–119. [Google Scholar]
- Browicz, K. Nicotiana glauca and Solanum elaeagnifolium [Solanaceae]-two xenophytes from South America and the history of their spreading in the eastern Mediterranean. Fragm. Flor. Geobot. Suppl. 1993, 2, 299–305. [Google Scholar]
- Lagoudakis, N.; Krigas, N.; Hanlidou, E.; Kokkini, S. Plant species with alkaloids in the city of Thessaloniki (N Greece): Distribution and alkaloid content. Bot. Chron. 2002, 15, 35–44. [Google Scholar]
- Krigas, N. Flora and Human Activities in the Area of Thessaloniki: Biological Approach and Historical Consideration. Ph.D. Thesis, Aristotle University of Greece, Thessaloniki, Greece, 2004. [Google Scholar]
- Krigas, N.; Kokkini, S. A survey of the alien vascular flora of the urban and suburban area of Thessaloniki, N Greece. Willdenowia 2004, 34, 81–99. Available online: http://www.jstor.org/stable/3997464 (accessed on 30 April 2023). [CrossRef]
- Climatology of Thessaloniki. Climate Reports AUTh. Available online: https://meteo.geo.auth.gr/en/ (accessed on 30 April 2023).
- Simberloff, D.; Von Holle, B. Positive interactions of nonindigenous species: Invasional meltdown? Biol. Invas. 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Didham, R.K.; Tylianakis, J.M.; Hutchison, M.A.; Ewers, R.M.; Gemmell, N.J. Are invasive species the drivers of ecological change? Trends Ecol. Evol. 2005, 20, 470–474. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Schofield, R.; Taylor, A.W. The measurement of soil pH. Soil Sci. Soc. Am. J. 1955, 19, 164–167. [Google Scholar] [CrossRef]
- Ball, D. Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. J. Soil Sci. 1964, 15, 84–92. [Google Scholar] [CrossRef]
- Jensen, J.; Christensen, B.; Schjønning, P.; Watts, C.; Munkholm, L. Converting loss-on-ignition to organic carbon content in arable topsoil: Pitfalls and proposed procedure. Eur. J. Soil Sci. 2018, 69, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Allen, S. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK, 1974. [Google Scholar]
- Monokrousos, N.; Papatheodorou, E.M.; Orfanoudakis, M.; Jones, D.G.; Scullion, J.; Stamou, G.P. The effects of plant type, AMF inoculation and water regime on rhizosphere microbial communities. Eur. J. Soil Sci. 2020, 71, 265–278. [Google Scholar] [CrossRef]
- Spyrou, I.M.; Karpouzas, D.G.; Menkissoglu-Spiroudi, U. Do botanical pesticides alter the structure of the soil microbial community? Microb. Ecol. 2009, 58, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Xcalibur, Version 2.2; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2011. Available online: www.thermofisher.com (accessed on 30 April 2023).
- Fierer, N.; Schimel, J.P.; Holden, P.A. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 2003, 35, 167–176. [Google Scholar] [CrossRef]
- Salomonová, S.; Lamacova, J.; Rulík, M.; Rolcik, J.; Cap, L.; Bednar, P.; Barták, P. Determination of phospholipid fatty acids in sediments. Chemica 2003, 42, 39–49. [Google Scholar]
- Findlay, R.H. Determination of microbial community structure using phospholipid fatty acid profiles. In Molecular Microbial Ecology Manual, 2nd ed.; Kowalchuk, G.A., de Bruijn, F.J., Head, I.M., Akkermans, A.D., van Elsas, J.D., Eds.; Springer: Norwell, UK, 2004; Volume 4, pp. 983–1005. [Google Scholar]
- Kaštovská, K.; Stibal, M.; Šabacká, M.; Černá, B.; Šantrůčková, H.; Elster, J. Microbial community structure and ecology of subglacial sediments in two polythermal Svalbard glaciers characterized by epifluorescence microscopy and PLFA. Polar Biol. 2007, 30, 277–287. [Google Scholar] [CrossRef]
- Papatheodorou, E.; Kordatos, H.; Kouseras, T.; Monokrousos, N.; Menkissoglu-Spiroudi, U.; Diamantopoulos, J.; Stamou, G.; Argyropoulou, M. Differential responses of structural and functional aspects of soil microbes and nematodes to abiotic and biotic modifications of the soil environment. Appl. Soil Ecol. 2012, 61, 26–33. [Google Scholar] [CrossRef]
- Rousidou, C.; Papadopoulou, E.S.; Kortsinidou, M.; Giannakou, I.O.; Singh, B.K.; Menkissoglu-Spiroudi, U.; Karpouzas, D.G. Bio-pesticides: Harmful or harmless to ammonia oxidizing microorganisms? The case of a Paecilomyces lilacinus-based nematicide. Soil Biol. Biochem. 2013, 67, 98–105. [Google Scholar] [CrossRef]
- Ntalli, N.; Monokrousos, N.; Rumbos, C.; Kontea, D.; Zioga, D.; Argyropoulou, M.D.; Menkissoglu-Spiroudi, U.; Tsiropoulos, N.G. Greenhouse biofumigation with Melia azedarach controls Meloidogyne spp. and enhances soil biological activity. J. Pest Sci. 2018, 91, 29–40. [Google Scholar] [CrossRef]
- Ntalli, N.; Zioga, D.; Argyropoulou, M.; Papatheodorou, E.; Menkissoglu-Spiroudi, U.; Monokrousos, N. Anise, parsley and rocket as nematicidal soil amendments and their impact on non-target soil organisms. Appl. Soil Ecol. 2019, 143, 17–25. [Google Scholar] [CrossRef]
- Papatheodorou, E.; Papapostolou, A.; Monokrousos, N.; Jones, D.-W.; Scullion, J.; Stamou, G. Crust cover and prior soil moisture status affect the response of soil microbial community and function to extreme rain events in an arid area. Eur. J. Soil Biol. 2020, 101, 103243. [Google Scholar] [CrossRef]
- Jacob, J.J.; Bezooijen, V. Manual for Practical Work in Nematology; Landbouwhogeschool Wageningen: Wageningen, The Netherlands, 1984. [Google Scholar]
- Bongers, T. Identification Key: De Nematoden van Nederland; KNNV Bibliotheekuitgave: Wageningen, The Netherlands, 1994; 408p. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315. [Google Scholar] [PubMed]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Bongers, T. The Maturity Index, the evolution of nematode life history traits, adaptive radiation and cp-scaling. Plant Soil 1999, 212, 13–22. [Google Scholar] [CrossRef]
- Anderson, M.J. Permanova: A Fortran Computer Program for Permutational Multivariate Analysis of Variance; Department of Statistics, University of Auckland: Auckland, New Zealand, 2005. [Google Scholar]
- Patil, G.; Taillie, C. An overview of diversity. In Ecological Diversity in Theory and Practice; Grassle, J., Patil, G., Smith, W., Taillie, C., Eds.; International Cooperative Publishing House: Fairland, MD, USA, 1979. [Google Scholar]
- Rényi, A. On measures of entropy and information. In Proceedings of the Fourth Berkeley Symposium on Mathemtatical Statistics and Probability, Volume 1: Contributions to the Theory of Statistics, Berkley, CA, USA, 20 June–30 July 1961; pp. 547–562. [Google Scholar]
- Ricotta, C. From theoretical ecology to statistical physics and back: Self-similar landscape metrics as a synthesis of ecological diversity and geometrical complexity. Ecol. Model. 2000, 125, 245–253. [Google Scholar] [CrossRef]
- Tóthmérész, B. Comparison of different methods for diversity ordering. J. Veget. Sci. 1995, 6, 283–290. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, A.; Ryan, P. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 30 April 2023).
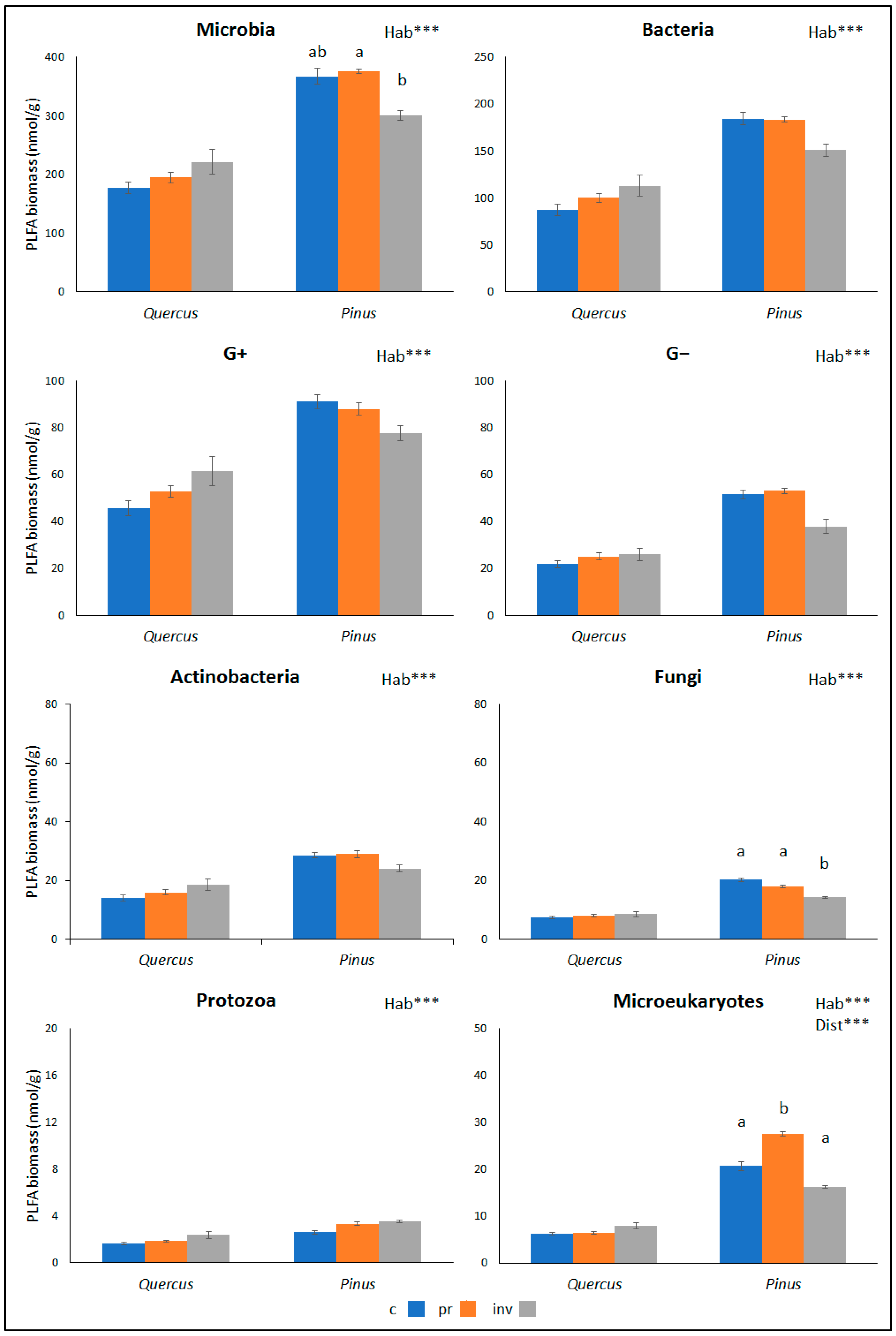

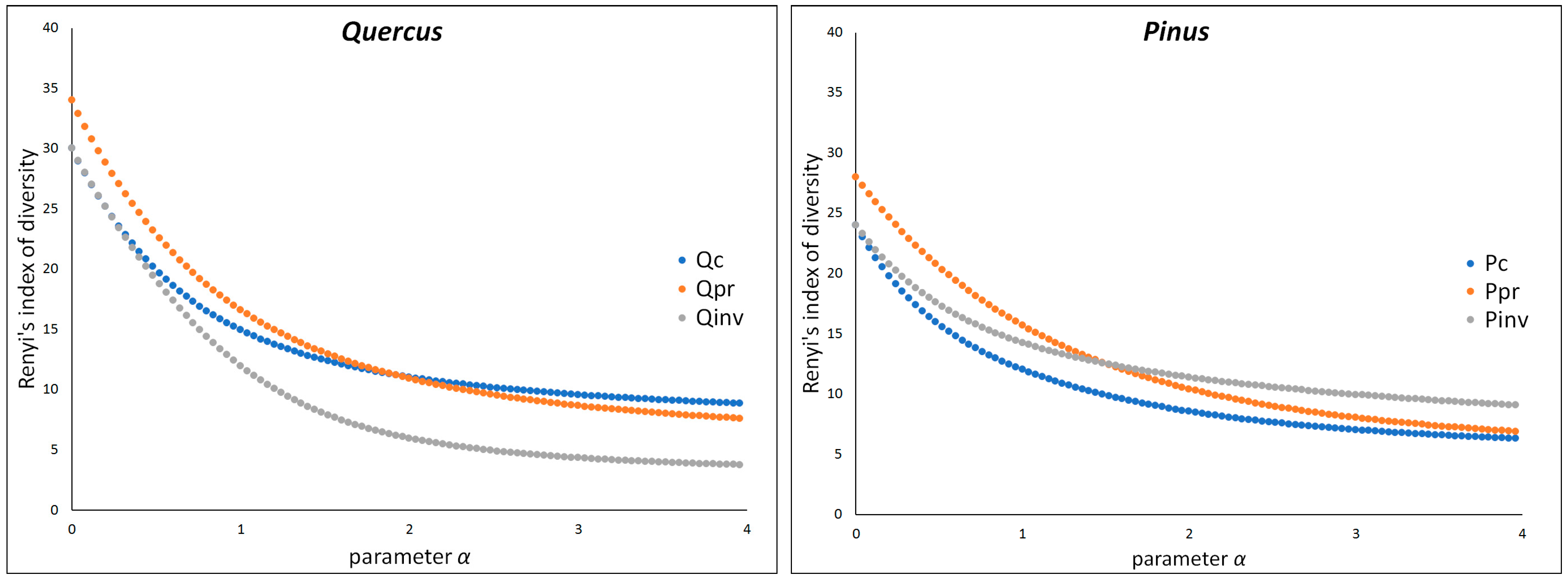
| Soil Properties | Quercus coccifera | Pinus brutia | Hab | Dist | ||||
|---|---|---|---|---|---|---|---|---|
| Qc | Qpr | Qinv | Pc | Ppr | Pinv | |||
| Clay (%) | 5.75 (1.14) a | 9.11 (0.59) b | 9.93 (1.45) ab | 8.52 (1.27) ab | 7.11 (0.45) a | 11.61 (1.00) b | ns | * |
| Silt (%) | 33.37 (1.35) a | 22.36 (0.90) b | 29.68 (2.69) ab | 30.27 (2.46) | 35.18 (2.14) | 34.75 (0.77) | * | ** |
| Sand (%) | 60.89 (1.88) a | 68.53 (1.48) b | 60.39 (2.48) a | 61.21 (2.59) a | 57.71 (2.14) ab | 53.64 (0.91) b | ** | * |
| Water content (%) | 2.91 (0.82) | 2.22 (0.65) | 2.22 (0.20) | 2.99 (0.44) | 3.81 (0.51) | 3.38 (0.28) | * | ns |
| pH | 6.39 (0.05) | 6.44 (0.06) | 6.25 (0.12) | 6.28 (0.10) | 6.33 (0.13) | 6.49 (0.09) | ns | ns |
| Organic C (%) | 1.65 (0.29) | 1.51 (0.27) | 1.79 (0.27) | 4.92 (0.98) ab | 4.68 (0.69) a | 2.64 (0.17) b | *** | ns |
| Organic N (%) | 0.15 (0.02) ab | 0.11 (0.02) a | 0.24 (0.04) b | 0.37 (0.07) | 0.41 (0.08) | 0.26 (0.03) | *** | ns |
| Corg/Norg | 11.55 (1.34) ab | 14.21 (1.44) a | 7.68 (1.05) b | 13.44 (1.03) | 11.84 (0.89) | 10.62 (1.09) | ns | ** |
| Quercus coccifera | Pinus brutia | Hab | Dist | |||||
|---|---|---|---|---|---|---|---|---|
| Qc | Qpr | Qinv | Pc | Ppr | Pinv | |||
| Maturity Index | 2.07 (0.06) | 2.15 (0.08) | 1.97 (0.05) | 2.05 (0.02) | 2.08 (0.06) | 2.02 (0.01) | ns | ns |
| Plant Parasitic Index | 2.19 (0.06) | 2.15 (0.03) | 2.11 (0.08) | 2.43 (0.20) | 2.04 (0.04) | 2.14 (0.09) | ns | ns |
| Structure Index | 14.81 (8.72) | 30.90 (8.38) | 16.11 (6.65) | 15.47 (2.96) | 19.44 (8.37) | 9.86 (1.50) | ns | ns |
| Enrichment Index | 34.10 (2.81) a | 38.60 (5.30) ab | 47.50 (4.0) b | 38.05 (1.53) a | 40.36 (1.58) a | 31.27 (1.6) b | ns | * |
| Channel Index | 84.00 (9.24) | 74.22 (9.25) | 46.42 (15.77) | 74.84 (9.35) | 76.23 (8.67) | 75.34 (6.21) | ns | ns |
| Habitat | Disturbance | Pair–Wise Tests | |
|---|---|---|---|
| All genera | *** | *** | Qpr ≠ Qinv * Pc ≠ Pinv * Ppr ≠ Pinv ** |
| Root/fungal feeding genera | *** | ** | Qc ≠ Qinv * Qpr ≠ Qinv * |
| Plant parasitic genera | ** | * | |
| Bacterivorous genera | ** | ** | Ppr ≠ Pinv * |
| Fungivorous genera | ** | ** | Pc ≠ Pinv * Ppr ≠ Pinv ** |
| Predatory genera | ns | ns | |
| Omnivorous genera | ns | ns |
| Genus | c-p | Qc | Qpr | Qinv | Pc | Ppr | Pinv | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab. | Part. | Ab. | Part. | Ab. | Part. | Ab. | Part. | Ab. | Part. | Ab. | Part. | ||
| Root/fungal feeders | |||||||||||||
| Boleodorus | 2 | 74.58 | 5.03 | 1.82 | 0.19 | 27.20 | 1.86 | 44.17 | 2.41 | 20.12 | 2.63 | ||
| Filenchus | 2 | 232.42 | 15.68 | 197.45 | 20.40 | 36.40 | 2.49 | 76.75 | 1.85 | 141.62 | 7.73 | 17.03 | 2.22 |
| Malenchus | 2 | 31.05 | 2.10 | 37.74 | 3.90 | 147.17 | 10.06 | 11.34 | 0.62 | 16.76 | 2.19 | ||
| Tylenchus | 2 | 14.06 | 0.95 | 9.75 | 1.01 | 18.62 | 1.27 | 13.11 | 0.72 | 3.28 | 0.43 | ||
| Parasitic plant feeders | |||||||||||||
| Bitylenchus | 3 | 8.68 | 0.59 | 1.82 | 0.19 | 8.33 | 0.57 | ||||||
| Helicotylenchus | 3 | 10.84 | 0.73 | 5.08 | 0.53 | 6.13 | 0.42 | 3.37 | 0.08 | ||||
| Hemicycliophora | 3 | 27.90 | 2.88 | 117.57 | 2.83 | ||||||||
| Heterodera | 3 | 4.15 | 0.28 | ||||||||||
| Meloidogyne | 3 | 4.69 | 0.61 | ||||||||||
| Merlinius | 3 | 25.72 | 1.74 | 19.61 | 1.34 | 5.52 | 0.30 | ||||||
| Paratylenchus | 2 | 85.59 | 5.78 | 86.93 | 8.98 | 543.55 | 37.15 | 14.38 | 0.35 | 47.39 | 2.59 | 87.12 | 11.37 |
| Pratylenchus | 3 | 7.28 | 0.50 | 11.04 | 0.60 | 1.27 | 0.17 | ||||||
| Pungentus | 4 | 8.57 | 0.58 | 1.82 | 0.19 | 17.27 | 0.42 | ||||||
| Scutylenchus | 3 | 44.97 | 3.04 | 11.57 | 1.20 | 7.60 | 0.52 | 9.11 | 1.19 | ||||
| Trichodorus | 4 | 4.34 | 0.29 | 2.90 | 0.30 | ||||||||
| Bacterivores | |||||||||||||
| Acrobeles | 2 | 168.61 | 11.38 | 41.94 | 4.33 | 45.32 | 3.10 | 954.61 | 22.96 | 101.53 | 5.54 | 60.61 | 7.91 |
| Acrobeloides | 2 | 120.06 | 8.10 | 32.57 | 3.37 | 119.78 | 8.19 | 300.32 | 7.22 | 162.02 | 8.84 | 71.87 | 9.38 |
| Cervidellus | 2 | 57.63 | 3.89 | 38.81 | 4.01 | 68.37 | 4.67 | 289.24 | 6.96 | 82.82 | 4.52 | 31.24 | 4.08 |
| Chiloplacus | 2 | 3.42 | 0.23 | 21.68 | 2.24 | 18.87 | 1.29 | 53.93 | 1.30 | 43.41 | 2.37 | 133.64 | 17.44 |
| Chronogaster | 3 | 2.90 | 0.30 | 1.27 | 0.17 | ||||||||
| Drilocephalobus | 2 | 12.46 | 0.85 | ||||||||||
| Eucephalobus | 2 | 84.43 | 5.70 | 65.44 | 6.76 | 37.93 | 2.59 | 165.90 | 3.99 | 44.09 | 2.41 | 35.15 | 4.59 |
| Eumonhystera | 2 | 3.42 | 0.23 | 3.27 | 0.34 | 63.09 | 1.52 | 144.24 | 7.87 | 2.54 | 0.33 | ||
| Geomonhystera | 2 | 24.16 | 1.32 | ||||||||||
| Mesorhabditis | 1 | 2.17 | 0.15 | 1.82 | 0.19 | 18.40 | 1.26 | 235.13 | 5.65 | 14.20 | 0.78 | 1.27 | 0.17 |
| Monhystera | 2 | 2.44 | 0.25 | 5.10 | 0.28 | ||||||||
| Panagrolaimus | 1 | 9.01 | 0.61 | 22.09 | 2.28 | 56.43 | 3.86 | 17.10 | 0.41 | 39.75 | 2.17 | 16.92 | 2.21 |
| Plectus | 2 | 10.77 | 0.73 | 26.29 | 2.72 | 20.77 | 1.42 | 218.41 | 5.25 | 49.11 | 2.68 | 7.13 | 0.93 |
| Prismatolaimus | 3 | 20.51 | 1.38 | 10.58 | 1.09 | 8.64 | 0.21 | ||||||
| Rhabditis | 1 | 14.44 | 0.99 | 3.37 | 0.08 | 8.43 | 0.46 | ||||||
| Rhabdolaimus | 3 | 3.42 | 0.23 | 7.27 | 0.75 | 26.04 | 1.42 | ||||||
| Teratocephalus | 3 | 19.06 | 1.97 | 73.88 | 1.78 | 39.93 | 2.18 | ||||||
| Wilsonema | 2 | 49.83 | 3.36 | 28.40 | 2.93 | 24.73 | 1.69 | 113.39 | 2.73 | 50.19 | 2.74 | 42.41 | 5.53 |
| Fungivores | |||||||||||||
| Aphelenchoides | 2 | 181.94 | 12.28 | 69.25 | 7.16 | 36.39 | 2.49 | 367.46 | 8.84 | 184.41 | 10.07 | 67.11 | 8.76 |
| Aphelenchus | 2 | 26.16 | 1.77 | 15.96 | 1.65 | 47.51 | 3.25 | 159.79 | 3.84 | 72.51 | 3.96 | 62.92 | 8.21 |
| Diphtherophora | 3 | 5.45 | 0.56 | ||||||||||
| Ditylenchus | 2 | 240.94 | 16.26 | 141.23 | 14.59 | 84.80 | 5.80 | 765.71 | 18.41 | 424.24 | 23.16 | 57.19 | 7.46 |
| Funaria | 4 | 2.08 | 0.14 | ||||||||||
| Tylencholaimellus | 4 | 12.46 | 0.85 | 131.58 | 3.16 | 23.29 | 1.27 | ||||||
| Tylencholaimus | 4 | 10.26 | 0.69 | 6.16 | 0.64 | 2.08 | 0.14 | ||||||
| Predators | |||||||||||||
| Aporcelaimellus | 5 | 16.15 | 1.09 | 9.43 | 0.97 | 4.15 | 0.28 | ||||||
| Aporcelaimus | 5 | 2.91 | 0.16 | ||||||||||
| Discolaimus | 5 | 3.37 | 0.08 | ||||||||||
| Eudorylaimus | 4 | 3.42 | 0.23 | 4.32 | 0.10 | 15.29 | 0.83 | 13.22 | 1.72 | ||||
| Prionchulus | 4 | 9.09 | 0.94 | ||||||||||
| Thonus | 4 | 3.42 | 0.23 | 1.82 | 0.19 | ||||||||
| Omnivores | |||||||||||||
| Microdorylaimus | 4 | 10.29 | 0.70 | 2.54 | 0.33 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karmezi, M.; Krigas, N.; Papatheodorou, E.M.; Argyropoulou, M.D. The Invasion of Alien Populations of Solanum elaeagnifolium in Two Mediterranean Habitats Modifies the Soil Communities in Different Ways. Plants 2023, 12, 2193. https://doi.org/10.3390/plants12112193
Karmezi M, Krigas N, Papatheodorou EM, Argyropoulou MD. The Invasion of Alien Populations of Solanum elaeagnifolium in Two Mediterranean Habitats Modifies the Soil Communities in Different Ways. Plants. 2023; 12(11):2193. https://doi.org/10.3390/plants12112193
Chicago/Turabian StyleKarmezi, Maria, Nikos Krigas, Efimia M. Papatheodorou, and Maria D. Argyropoulou. 2023. "The Invasion of Alien Populations of Solanum elaeagnifolium in Two Mediterranean Habitats Modifies the Soil Communities in Different Ways" Plants 12, no. 11: 2193. https://doi.org/10.3390/plants12112193
APA StyleKarmezi, M., Krigas, N., Papatheodorou, E. M., & Argyropoulou, M. D. (2023). The Invasion of Alien Populations of Solanum elaeagnifolium in Two Mediterranean Habitats Modifies the Soil Communities in Different Ways. Plants, 12(11), 2193. https://doi.org/10.3390/plants12112193








