Effects of Nigella sativa Oil Fractions on Reactive Oxygen Species and Chemokine Expression in Airway Smooth Muscle Cells
Abstract
1. Introduction
2. Results
2.1. Extraction Yield
2.2. Analysis of N. sativa Total Oil Lipid Components Using Gas Chromatography–Mass Spectrophotometer (GC–MS)
2.3. Antiradical Assays
2.4. MTT Assay
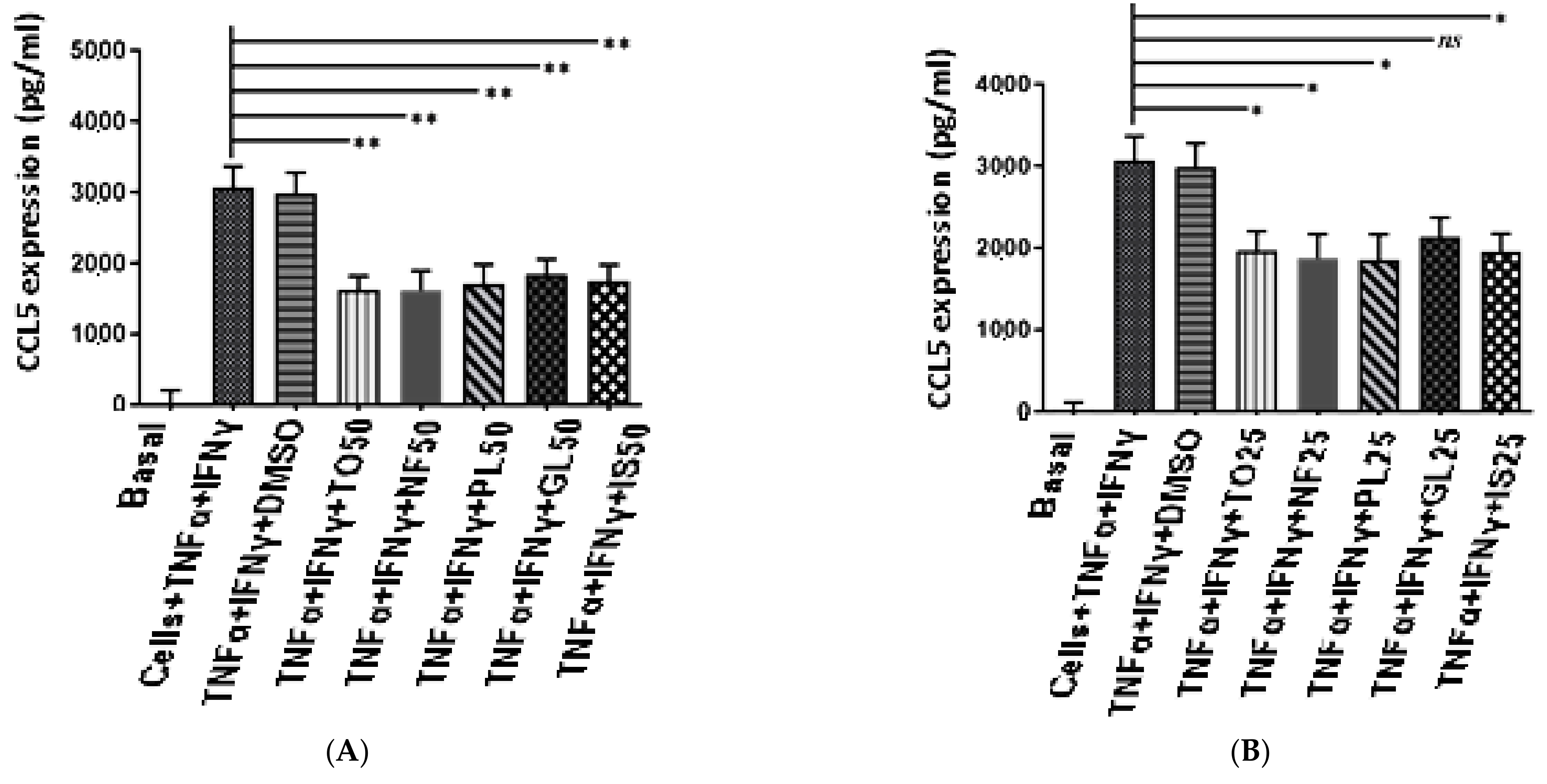
2.5. Anti-Inflammatory Activity of N. sativa Oil Fractions
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Collection of the N. sativa Plant
4.3. Human Airway Smooth Muscle (ASM) Cell Culture
4.4. Preparation of Extracts
4.5. Analysis of N. sativa Total Oil Lipid Components Using GC–MS
4.6. Superoxide Anion Radical (O2•−) Scavenging Assay
- AC:
- Absorbance of control.
- AS:
- Absorbance of samples.
4.7. Hydroxyl Radical (OH•) Scavenging Assay
- A0:
- Absorbance of control (without extract).
- A1:
- Absorbance of extracts.
4.8. Hydrogen Peroxide (H2O2) Scavenging Assay
- AC:
- Absorbance of control.
- AE:
- Absorbance of samples
4.9. Cell Viability Assay
4.10. Measurement of the Different CCL5, CXCL-10, and CXCL-8 in Human ASM Cell Supernatants
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caramori, G.; Nucera, F.; Mumby, S.; Lo Bello, F.; Adcock, I.M. Corticosteroid resistance in asthma: Cellular and molecular mechanisms. Mol. Asp. Med. 2022, 85, 100969. [Google Scholar] [CrossRef] [PubMed]
- Amrani, Y.; Panettieri, R.A.; Ramos-Ramirez, P.; Schaafsma, D.; Kaczmarek, K.; Tliba, O. Important lessons learned from studies on the pharmacology of glucocorticoids in human airway smooth muscle cells: Too much of a good thing may be a problem. Pharmacol. Ther. 2020, 213, 107589. [Google Scholar] [CrossRef] [PubMed]
- Chachi, L.; Gavrila, A.; Tliba, O.; Amrani, Y. Abnormal corticosteroid signalling in airway smooth muscle: Mechanisms and perspectives for the treatment of severe asthma. Clin. Exp. Allergy 2015, 45, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.D.; Gagliardo, R.P.; Montalbano, A.M.; Profita, M. Overview of the Mechanisms of Oxidative Stress: Impact in Inflammation of the Airway Diseases. Antioxidants 2022, 11, 2237. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.M.; Biddle, M.; Amrani, Y.; Brightling, C.E. Stressed out—The role of oxidative stress in airway smooth muscle dysfunction in asthma and COPD. Free. Radic. Biol. Med. 2022, 185, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I. Oxidative stress and gene transcription in asthma and chronic obstructive pulmonary disease: Antioxidant therapeutic targets. Curr. Drug Targets-Inflamm. Allergy 2002, 1, 291–315. [Google Scholar] [CrossRef]
- Tenscher, K.; Metzner, B.; Schöpf, E.; Norgauer, J.; Czech, W. Recombinant human eotaxin induces oxygen radical production, Ca2+-mobilization, actin reorganization, and CD11b upregulation in human eosinophils via a pertussis toxin-sensitive heterotrimeric guanine nucleotide-binding protein. Blood 1996, 88, 3195–3199. [Google Scholar] [CrossRef]
- Chihara, J.; Hayashi, N.; Kakazu, T.; Yamamoto, T.; Kurachi, D.; Nakajima, S. RANTES augments radical oxygen products from eosinophils. Int. Arch. Allergy Immunol. 1994, 104 (Suppl. S1), 52–53. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular mechanisms of oxidative stress in asthma. Mol. Asp. Med. 2022, 85, 101026. [Google Scholar] [CrossRef]
- Torequl Islam, M. Biological activities and therapeutic promises of Nigella sativa L. Int. J. Pharma Sci. Sci. Res. 2016, 2, 237–252. [Google Scholar] [CrossRef]
- Randhawa, M.A.; Alghamdi, M.S. A review of the pharmacotherapeutic effects of Nigella sativa. Pak. J. Med. Res. 2002, 41, 77–83. [Google Scholar]
- Ghaznavi, K.M. Tibbe-e-Nabvi aur Jadid Science, Al-Faisal Nasheeran wa Tajeera-e-Kutab; Urdu Bazar: Lahore, Pakistan, 1991; Volume 1, pp. 228–236. [Google Scholar]
- Ahlatci, A.; Kuzhan, A.; Taysi, S.; Demirtas, O.C.; Alkis, H.E.; Tarakcioglu, M.; Demirci, A.; Caglayan, D.; Saricicek, E.; Cinar, K. Radiation-modifying abilities of Nigella sativa and thymoquinone on radiation-induced nitrosative stress in the brain tissue. Phytomedicine 2014, 21, 740–744. [Google Scholar] [CrossRef]
- Sharma, P.C.; Yelne, M.B.; Dennis, T.J. Database on Medicinal Plants Used in Ayurveda; Central Council for Research in Ayurveda and Siddha: New Delhi, India, 2005; pp. 420–440. [Google Scholar]
- Khare, C.P. Encyclopedia of Indian Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Mahdavi, R.; Namazi, N.; Alizadeh, M.; Farajnia, S. Nigella sativa oil with a calorie-restricted diet can improve biomarkers of systemic inflammation in obese women: A randomized double-blind, placebo-controlled clinical trial. J. Clin. Lipidol. 2016, 10, 1203–1211. [Google Scholar] [CrossRef]
- Al-Attass, S.A.; Zahran, F.M.; Turkistany, S.A. Nigella sativa and its active constituent thymoquinone in oral health. Saudi Med. J. 2016, 37, 235–244. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Zoheir, A.D.; Firoz, A. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Ahmad, S.; Beg, Z.H. Evaluation of therapeutic effect of omega-6 linoleic acid and thymoquinone enriched extracts from Nigella sativa oil in the mitigation of lipidemic oxidative stress in rats. Nutrition 2016, 32, 649–655. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Ghannadi, A.; Jafarabadi, H. Black cumin seed essential oil, as a potent analgesic and anti-inflammatory drug. Phytother. Res. 2004, 18, 195–199. [Google Scholar] [CrossRef]
- Koshak, A.; Wei, L.; Koshak, E.; Wali, S.; Alamoudi, O.; Demerdash, A.; Qutub, M.; Pushparaj, P.N.; Heinrich, M. Nigella sativa Supplementation Improves Asthma Control and Biomarkers: A Randomized, Double-Blind, Placebo-Controlled Trial. Phytother. Res. 2017, 31, 403–409. [Google Scholar] [CrossRef]
- Salem, A.M.; Bamosa, A.O.; Qutub, H.O.; Gupta, R.K.; Badar, A.; Elnour, A.; Afzal, M.N. Effect of Nigella sativa supplementation on lung function and inflammatory mediatorsin partly controlled asthma: A randomized controlled trial. Ann. Saudi Med. 2017, 37, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005, 5, 1749–1770. [Google Scholar] [CrossRef] [PubMed]
- Kalus, U.; Pruss, A.; Bystron, J.; Jurecka, M.; Smekalova, A.; Lichius, J.J.; Kiesewetter, H. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother. Res. 2003, 17, 1209–1214. [Google Scholar] [CrossRef]
- Boskabady, M.; Shahabi, M. Bronchodilatory and anticholinergic effects of Nigella sativa on isolated guinea-pig tracheal chains. Iran. J. Med. Sci. 1997, 22, 127–133. [Google Scholar]
- Boskabady, M.; Shirmohammadi, B. Effect of Nigella sativa on isolated guinea pig trachea. Arch Iran. Med. 2002, 5, 103–107. [Google Scholar]
- Boskabady, M.H.; Shirmohammadi, B.; Jandaghi, P.; Kiani, S. Possible mechanism(s) for relaxant effect of aqueous and macerated extracts from Nigella sativa on tracheal chains of guinea pig. BMC Pharmacol. 2004, 4, 3. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Mohsenpoor, N.; Takaloo, L. Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine 2010, 17, 707–713. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Kroh, L.W.; Mörsel, J.T. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrumsativum L.), and niger (Guizotia abyssinica Cass.) crude seed oils and oil fractions. J. Agric. Food Chem. 2003, 51, 6961–6969. [Google Scholar] [CrossRef]
- Thilakarathna, R.C.N.; Madhusankha, G.D.M.P.; Navaratne, S.B. Morphological characteristics of black cumin (Nigella sativa) seeds. Chem. Res. J. 2018, 3, 40–45. [Google Scholar]
- Bonesi, R.; Saab, M.; Tenuta, A.M.; Leporini, M.C.; Saab, M.; Loizzo, M.J.; Tundis, M.R. Screening of traditional Lebanese medicinal plants as antioxidants and inhibitors of key enzymes linked to type 2 diabetes. Plant Biosyst. 2020, 154, 656–662. [Google Scholar] [CrossRef]
- Babar, Z.M.; Azizi, W.M.; Ichwan, S.J.A.; Ahmed, Q.U.; Azad, A.K.; Mawa, I. A simple method for extracting both active oily and water soluble extract (WSE) from Nigella sativa (L.) seeds using a single solvent system. Nat. Prod. Res. 2019, 33, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Dalli, M.; Bekkouch, O.; Azizi, S.-e.; Azghar, A.; Gseyra, N.; Kim, B. Nigella sativa L. Phytochemistry and Pharmacological Activities: A Review (2019–2021). Biomolecules 2022, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Afifa, Z.B.; Meriem, J.; Mansour, Z.; Asma, A.Z.; Hichem, B.J. Physico-chemical properties, composition and antioxidant activityof seed oil from the Tunisian virginia creeper (Parthenocissus quinquefolia (L.) planch). J. Tunis. Chem. Soc. 2016, 18, 89–95. [Google Scholar]
- Kumar, P.P.; Kumaravel, S.; Lalitha, C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitexnegundo. Afr. J. Biochem. Res. 2010, 4, 191–195. [Google Scholar]
- Sugiura, H.; Ichinose, M. Oxidative and nitrative stress in bronchial asthma. Antioxid. Redox Signal. 2008, 10, 785–797. [Google Scholar] [CrossRef]
- Riedl, M.A.; Nel, A.E. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 49–56. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. Consumption of Anacardium occidentale L. (Cashew Nuts) Inhibits Oxidative Stress through Modulation of the Nrf2/HO−1 and NF-kB Pathways. Molecules 2020, 25, 4426. [Google Scholar] [CrossRef]
- Saadat, S.; Aslani, M.R.; Ghorani, V.; Keyhanmanesh, R.; Boskabady, M.H. The effects of Nigella sativa on respiratory, allergic and immunologic disorders, evidence from experimental and clinical studies, a comprehensive and updated review. Phytother. Res. 2021, 35, 2968–2996. [Google Scholar] [CrossRef]
- Oliver, B.G.; Black, J.L. Airway smooth muscle and asthma. Allergol. Int. 2006, 55, 215–223. [Google Scholar] [CrossRef]
- Chung, K.F. The role of airway smooth muscle in the pathogenesis of airway wall remodeling in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005, 2, 347–354, discussion 371. [Google Scholar] [CrossRef]
- Tliba, O.; Amrani, Y. Airway smooth muscle cell as an inflammatory cell: Lessons learned from interferon signaling pathways. Proc. Am. Thorac. Soc. 2008, 5, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Halayko, A.J.; Amrani, Y. Mechanisms of inflammation-mediated airway smooth muscle plasticity and airways remodeling in asthma. Respir. Physiol. Neurobiol. 2003, 137, 209–222. [Google Scholar] [CrossRef]
- John, M.; Hirst, S.J.; Jose, P.J.; Robichaud, A.; Berkman, N.; Witt, C.; Twort, C.H.; Barnes, P.J.; Chung, K.F. Human airway smooth muscle cells express and release RANTES in response to T helper 1 cytokines: Regulation by T helper 2 cytokines and corticosteroids. J. Immunol. 1997, 158, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Keyhanmanesh, R.; Saadatloo, M.A. Relaxant effects of different fractions from Nigella sativa L. on guinea pig tracheal chains and its possible mechanism(s). Indian J. Exp. Biol. 2008, 46, 805–810. [Google Scholar] [PubMed]
- Boskabady, M.; Kiani, S.; Jandaghi, P. Stimulatory effect of Nigella sativa on Β2-adrenoceptors of guinea pig tracheal chains. Med. J. Islam. Repub. Iran (MJIRI) 2004, 18, 153–158. [Google Scholar]
- Maier, E.; Duschl, A.; Horejs-Hoeck, J. STAT6-dependent and –independent mechanisms in Th2 polarization. Eur. J. Immunol. 2012, 42, 2827–2833. [Google Scholar] [CrossRef]
- Barlianto, W.; Rachmawati, M.; Irawan, M.; Wulandari, D. Effects of Nigella sativa oil on Th1/Th2, cytokine balance, and improvement of asthma control. Paediatr. Indones. 2017, 57, 223–228. [Google Scholar] [CrossRef]
- Chachi, L.; Abbasian, M.; Gavrila, A.; Alzahrani, A.; Tliba, O.; Bradding, P.; Wardlaw, A.J.; Brightling, C.; Amrani, Y. Protein phosphatase 5 mediates corticosteroid insensitivity in airway smooth muscle in patients with severe asthma. Allergy 2017, 72, 126–136. [Google Scholar] [CrossRef]
- Chachi, L.; Shikotra, A.; Duffy, S.M.; Tliba, O.; Brightling, C.; Bradding, P.; Amrani, Y. Functional KCa3.1 channels regulate steroid insensitivity in bronchial smooth muscle cells. J. Immunol. 2013, 191, 2624–2636. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mörsel, J. Direct isocratic normal-phase HPLC assay of fat-soluble vitamins and β-carotene in oilseeds. Eur. Food Res. Technol. 2002, 214, 521–527. [Google Scholar] [CrossRef]
- Nieto, G.; Martínez, L.; Castillo, J.; Ros, G. Effect of hydroxytyrosol, walnut and olive oil on nutritional profile of Low-Fat Chicken Frankfurters. Eur. J. Lipid Sci. Technol. 2017, 119, 1600518. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Fe, Zn and Se Bioavailability in Chicken Meat Emulsions Enriched with Minerals, Hydroxytyrosol and Extra Virgin Olive Oil as Measured by Caco-2 Cell Model. Nutrients 2018, 10, 969. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.N. Incorporation of by-products of rosemary and thyme in the diet of ewes: Effect on the fatty acid profile of lamb. Eur. Food Res. Technol. 2013, 236, 379–389. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Effect of natural extracts obtained from food industry by-products on nutritional quality and shelf life of chicken nuggets enriched with organic Zn and Se provided in broiler diet. Poult. Sci. 2020, 99, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Bañón, S.; Garrido, M. Administration of distillate thyme leaves into the diet of Segureña ewes: Effect on lamb meat quality. Animal 2012, 6, 2048–2056. [Google Scholar] [CrossRef]
- Macho-González, A.; Garcimartín, A.; López-Oliva, M.E.; Bastida, S.; Benedí, J.; Ros, G.; Nieto, G.; Sánchez-Muniz, F.J. Can meat and meat-products induce oxidative stress? Antioxidants 2020, 9, 638. [Google Scholar] [CrossRef]
- Nieto, G.; Bañón, S.; Garrido, M.D. Incorporation of thyme leaves in the diet of pregnant and lactating ewes: Effect on the fattyacid profile of lamb. Small Rumin. Res. 2012, 105, 140–147. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Nieto, G.; Pateiro, M.; Lorenzo, J.M. Phenolic compounds obtained from olea europaea by-products and their use to improve the quality and shelf life of meat and meat products—A review. Antioxidants 2020, 9, 1061. [Google Scholar] [CrossRef]
- Dalli, M.; Daoudi, N.E.; Azizi, S.E.; Benouda, H.; Bnouham, M.; Gseyra, N. Chemical Composition Analysis Using HPLC-UV/GC-MS and Inhibitory Activity of Different Nigella sativa Fractions on Pancreatic α-Amylase and Intestinal Glucose Absorption. Biomed. Res. Int. 2021, 2021, 9979419. [Google Scholar] [CrossRef]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.; Denev, S.; Dutton, M.; Nkosi, B. Cytotoxic effect of some mycotoxins and their combinations on human peripheral blood mononuclear cells as measured by the MTT assay. Open Toxinology J. 2009, 2, 1–8. [Google Scholar] [CrossRef]
- Tliba, O.; Panettieri, R.A.; Tliba, S.; Walseth, T.F.; Amrani, Y. Tumor necrosis factor-alpha differentially regulates the expression of proinflammatory genes in human airway smooth muscle cells by activation of interferon-beta-dependent CD38 pathway. Mol. Pharmacol. 2004, 66, 322–329. [Google Scholar] [CrossRef]




| Fractions | Yield (g/mL) | Yield (%) |
|---|---|---|
| Total oil (TO) | 11.79 | 13.61 of seeds weight |
| Neutral lipids (NLs) | 10.08 | 93.97 of TO |
| Glycolipids (GLs) | 0.35 | 2.98 of TO |
| Phospholipids (FLs) | 0.13 | 1.11 of TO |
| Retention Time (min) | Components | Content (%) |
|---|---|---|
| 14.44 | o-Cymen | 3.5 |
| 15.15 | Gamma-Terpinene | 1.04 |
| 15.82 | p-Cymene | 0.19 |
| 16.54 | m-Cymene | 0.19 |
| 19.32 | Alpha-Longipinene | 0.16 |
| 21.67 | (+)-Longifolene | 1.59 |
| 22.95 | Squalene | 0.53 |
| 16.25 | Cumene | 0.12 |
| 16.92 | 1-Ethyl-3,5-Dimethyl-Benzene | 0.51 |
| 18.59 | Prehnitol | 0.38 |
| 22.62 | (E)5-Octadecene | 0.75 |
| 23.64 | Heptadecane | 1.04 |
| 23.94 | Not identified | 26.21 |
| 30.74 | Sandaracopimaradiene | 3.28 |
| 29.89 | Palmitic acid | 8.81 |
| 32.51 | Stearic acid | 4.09 |
| 32.90 | Oleic acid | 22.58 |
| 33.72 | Linoleic acid | 25.03 |
| Fatty Acid Composition | Total Oil Content (%) | |||
|---|---|---|---|---|
| Algerian | Egyptian | Indian | Ethiopian | |
| Oleic acid | 22.58 | 24.7 | 19.09 | 17.63 |
| Linoleic acid | 25.03 | 51.8 | 50.24 | 61.25 |
| Palmitic acid | 8.81 | 18.4 | 10.83 | 11.36 |
| Stearic acid | 4.09 | 2.07 | 2.47 | 2.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosbah, A.; Khither, H.; Mosbah, C.; Slimani, A.; Mahrouk, A.; Akkal, S.; Nieto, G. Effects of Nigella sativa Oil Fractions on Reactive Oxygen Species and Chemokine Expression in Airway Smooth Muscle Cells. Plants 2023, 12, 2171. https://doi.org/10.3390/plants12112171
Mosbah A, Khither H, Mosbah C, Slimani A, Mahrouk A, Akkal S, Nieto G. Effects of Nigella sativa Oil Fractions on Reactive Oxygen Species and Chemokine Expression in Airway Smooth Muscle Cells. Plants. 2023; 12(11):2171. https://doi.org/10.3390/plants12112171
Chicago/Turabian StyleMosbah, Asma, Hanane Khither, Camélia Mosbah, Abdelkader Slimani, Abdelkader Mahrouk, Salah Akkal, and Gema Nieto. 2023. "Effects of Nigella sativa Oil Fractions on Reactive Oxygen Species and Chemokine Expression in Airway Smooth Muscle Cells" Plants 12, no. 11: 2171. https://doi.org/10.3390/plants12112171
APA StyleMosbah, A., Khither, H., Mosbah, C., Slimani, A., Mahrouk, A., Akkal, S., & Nieto, G. (2023). Effects of Nigella sativa Oil Fractions on Reactive Oxygen Species and Chemokine Expression in Airway Smooth Muscle Cells. Plants, 12(11), 2171. https://doi.org/10.3390/plants12112171







