Chemical Constituents, Enantiomer Content, Antioxidant and Anticholinesterase Activities of Valeriana microphylla Kunth Essential Oil
Abstract
1. Introduction
2. Results
2.1. Physical Properties
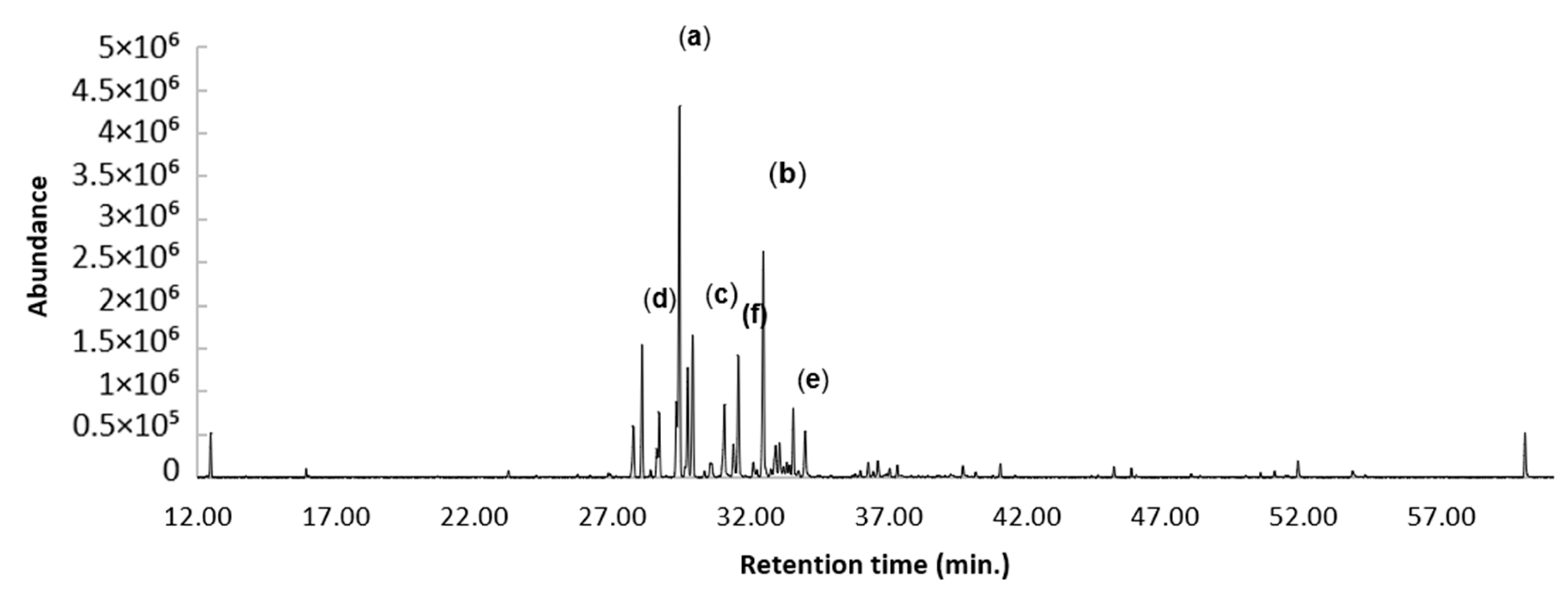
2.2. GC-EIMS and GC-FID Analyses
2.3. Enantioselective Analysis
2.4. Cholinesterase Inhibition Test
2.5. Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of the Volatile Oil
4.3. Chemical Characterization of Essential Oil
4.3.1. Qualitative and Quantitative Analysis
4.3.2. Enantioselective Analysis of Essential Oil
4.4. AChE and BuChE Inhibition Spectrophotometric Analysis
4.5. Antioxidant Spectrophotometric Analysis
4.5.1. DPPH Assay
4.5.2. ABTS Assay
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, C.D.; Donoghue, M.J. Phylogeny and biogeography of Valerianaceae (Dipsacales) with special reference to the South American valerians. Org. Div. Evol. 2005, 5, 147–159. [Google Scholar] [CrossRef]
- Kutschker, A. Revisión del género Valeriana (Valerianaceae) en Sudamérica austral. Revision of genus Valeriana (Valerianaceae) in Southern South America. Gayana Bot. 2011, 68, 2. [Google Scholar] [CrossRef]
- León-Yánez, S.; Valencia, R.; Pitmam, N.; Endara, L.; Ulloa Ulloa, C.; Navarrete, H. Libro Rojo de Plantas Endémicas del Ecuador, 2nd ed.; Publicaciones del Herbario QCA; Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2011; pp. 806–807. [Google Scholar]
- Jorgensen, P.; León-Yánez, S. Catalogue of Vascular Plants of Ecuador; Missouri Botanical Garden: San Louis, MI, USA, 1999. [Google Scholar]
- De la Torre, L.H.; Navarrete, P.; Muriel, M.; Macía, M.J.; Balslev, H. Enciclopedia de las Plantas Útiles del Ecuador, 1st ed.; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2008; pp. 613–614. [Google Scholar]
- Minga Ochoa, D.A.; Ansaloni, R.; Verdugo, A.; Ulloa Ulloa, C. Flora del Páramo del Cajas, Ecuador; Universidad del Azuay: Imprenta Don Bosco, Cuenca, Ecuador, 2016; p. 56. [Google Scholar]
- Chimbolema, S.; Suárez, D.; Peñafiel, M.; Acurio, C. Guía de Plantas de la Reserva Ecológica El Ángel, 1st ed.; Smaak Graphic Studio: Quito, Ecuador, 2010; pp. 125–126. [Google Scholar]
- Aguilar, Z.; Hidalgo, P.; Ulloa, C. Plantas Útiles de los Páramos de Zuleta, Ecuador; Proyecto de Manejo y Aprovechamiento Sustentable de Alpacas en los Páramos de Zuleta; PPA-EcoCiencia: Quito, Ecuador, 2009; pp. 93–94. [Google Scholar]
- Andrade, J.M.; Lucero-Mosquera, H.; Armijos, C. Ethnobotany of indigenous Saraguros: Medicinal plants used by community healers “Hampiyachakkuna” in the San Lucas Parish, Southern Ecuador. Biomed. Res. Int. 2017, 2017, 2314–6133. [Google Scholar] [CrossRef] [PubMed]
- Bayer, R.; Blaskó, I.; Cola, G.; Shamma, D.; Schmidhammer, M.; Klotzer, H.; Battersby, W.; Hirst, A.B.; Mccaldin, M.; Southgate, D.; et al. Valtrates and Lignans in Valeriana microphylla. Arch. Pharm. Weinheim 1993, 59, 478–479. [Google Scholar]
- Wang, Y.; Jin, L.; Yu, S.; Shi, Q.; Gu, Y.; Kiyota, H. Chemical Constituents of Plants from the Genus Valeriana. Mini Rev. Org. Chem. 2010, 7, 161–172. [Google Scholar] [CrossRef]
- Jugran, A.K.; Rawat, S.; Bhatt, I.D.; Rawal, R.S. Essential oil composition, phenolics and antioxidant activities of Valeriana jatamansi at different phenological stages. Plant Biosyst. 2021, 155, 891–898. [Google Scholar] [CrossRef]
- Sudati, J.H.; Fachinetto, R.; Pereira, R.P.; Boligon, A.A.; Athayde, M.L.; Soares, F.A.; de Vargas Barbosa, N.B.; Rocha, J.B.T. In vitro antioxidant activity of Valeriana officinalis against different neurotoxic agents. Neurochem. Res. 2009, 34, 1372–1379. [Google Scholar] [CrossRef]
- Karakaya, S.; Koca, M.; Kılıc, C.S.; Coskun, M. Antioxidant and anticholinesterase activities of Ferulago syriaca Boiss. and F. isaurica Peșmen growing in Turkey. Med. Chem. Res. 2018, 27, 1843–1850. [Google Scholar] [CrossRef]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enz. Inh. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef]
- Sánchez-Chávez, G.; Salceda, R. Enzimas polifuncionales: El caso de la acetilcolinesterasa. Rev. Educ. Bioquímica 2008, 27, 44–51. [Google Scholar]
- McGleenon, B.M.; Dynan, K.B.; Passmore, A.P. Acetylcholinesterase inhibitors in Alzheimer’s disease. British J. Clin. Pharm. 1999, 48, 471–480. [Google Scholar] [CrossRef]
- Chen, H.W.; He, X.H.; Yuan, R.; Wei, B.J.; Chen, Z.; Dong, J.X.; Wang, J. Sesquiterpenes and a monoterpenoid with acetylcholinesterase (AChE) inhibitory activity from Valeriana officinalis var. latiofolia in vitro and in vivo. Fitoterapia 2016, 110, 142–149. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Jeszka, S.M.; Sabih, O.M. Valeriana dioscoridis aerial parts’ extracts—A new source of phytochemicals with antioxidant and enzyme inhibitory activities. Ind. Crops Prod. 2020, 148, 112273. [Google Scholar] [CrossRef]
- Caldeira, M.; Gentil, D.G. Utilização do índice de retenção linear para caracterização de compostos voláteis em café solúvel utilizando GC-MS e coluna HP-Innowax. Química Nova 2007, 30, 2031–2034. [Google Scholar]
- Armijos, C.; Matailo, A.; Bec, N.; Salinas, M.; Aguilar, G.; Solano, N.; Calva, J.; Ludeña, C.; Larroque, C.; Vidari, G. Chemical composition and selective BuChE inhibitory activity of the essential oils from aromatic plants used to prepare the traditional Ecuadorian beverage horchata lojana. J. Ethnopharmacol. 2020, 263, 113162. [Google Scholar] [CrossRef]
- Paolini, J.; Muselli, A.; Bernardini, A.F.; Bighelli, A.; Casanova, J.; Costa, J. Thymol derivatives from essential oil of Doronicum corsicum L. Flav. Frag. J. 2007, 22, 479–487. [Google Scholar] [CrossRef]
- Özek, G.; Özek, T.; İşcan, G.; Başer, K.H.C.; Hamzaoglu, E.R.G.I.N.; Duran, A. Comparison of hydrodistillation and microdistillation methods for the analysis of fruit volatiles of Prangos pabularia Lindl., and evaluation of its antimicrobial activity. South Afr. J. Bot. 2007, 73, 563–569. [Google Scholar] [CrossRef]
- Shimizu, Y.; Imayoshi, Y.; Kato, M.; Maeda, K.; Iwabuchi, H.; Shimomura, K. Volatiles from leaves of field-grown plants and shoot cultures of Gynura bicolor DC. Flav. Frag. J. 2009, 24, 251–258. [Google Scholar] [CrossRef]
- Calvopiña, K.; Malagón, O.; Capetti, F.; Sgorbini, B.; Verdugo, V.; Gilardoni, G. A New Sesquiterpene Essential Oil from the Native Andean Species Jungia rugosa Less (Asteraceae): Chemical Analysis, Enantiomeric Evaluation, and Cholinergic Activity. Plants 2021, 10, 2102. [Google Scholar] [CrossRef]
- Demirci, F.; Guven, K.; Demirci, B.; Dadandi, M.Y.; Baser, K.H.C. Antibacterial activity of two Phlomis essential oils against food pathogens. Food Control 2008, 19, 1159–1164. [Google Scholar] [CrossRef]
- Salinas, M.; Bec, N.; Calva, J.; Ramírez, J.; Andrade, J.M.; Larroque, C.; Vidari, G.; Armijos, C. Chemical composition and anticholinesterase activity of the essential oil from the Ecuadorian plant Salvia pichinchensis Benth. Rec. Nat. Prod. 2020, 14, 276–285. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Demirci, B.; Kirimer, N.E.; Satil, F.; Tümen, G. The essential oils of Thymus migricus and T. fedtschenkoi var. handelii from Turkey. Flavour Frag. J. 2002, 17, 41–45. [Google Scholar] [CrossRef]
- Formisano, C.; Senatore, F.; Bancheva, S.; Bruno, M.; Maggio, A.; Rosselli, S. Volatile components of Centaurea bracteata and C. pannonica subsp. pannonica growing wild in Croatia. Nat. Prod. Comm. 2010, 5, 1649–1654. [Google Scholar] [CrossRef]
- Wijaya, C.H.; Ulrich, D.; Lestari, R.; Schippel, K.; Ebert, G. Identification of potent odorants in different cultivars of snake fruit [Salacca zalacca (Gaert.) Voss] using gas chromatography−olfactometry. J. Agric. Food Chem. 2005, 53, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Ennigrou, A.; Casabianca, H.; Laarif, A.; Hanchi, B.; Hosni, K. Maturation-related changes in phytochemicals and biological activities of the Brazilian pepper tree (Schinus terebinthifolius Raddi) fruits. South Afr. J. Bot. 2017, 108, 407–415. [Google Scholar] [CrossRef]
- Saïdana, D.; Mahjoub, S.; Boussaada, O.; Chriaa, J.; Mahjoub, M.A.; Chéraif, I.; DaamiRemadi, M.; Gannoun, S.; Helal, A.N. Antibacterial and antifungal activities of the essential oils of two saltcedar species from Tunisia. J. Am. Oil Chem. Soc. 2008, 85, 817–826. [Google Scholar] [CrossRef]
- Noorizadeh, H.; Farmany, A. Exploration of linear and nonlinear modeling techniques to predict of retention index of essential oils. J. Chin. Chem. S 2010, 57, 1268–1277. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas, Chromatography/Mass Spectrometry, 4th ed.; AllurePublishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-1932633219. [Google Scholar]
- Gilardoni, G.; Montalván, M.; Ortiz, M.; Vinueza, D.; Montesinos, J.V. The flower essential oil of Dalea mutisii Kunth (Fabaceae) from Ecuador: Chemical, enantioselective, and olfactometric analyses. Plants 2020, 9, 1403. [Google Scholar] [CrossRef]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C.; Gilardoni, G. A novel chemical profile of a selective in vitro cholinergic essential oil from Clinopodium taxifolium (Kunth) Govaerts (Lamiaceae), a native Andean species of Ecuador. Molecules 2021, 26, 45. [Google Scholar] [CrossRef]
- Valarezo, E.; Ojeda-Riascos, S.; Cartuche, L.; Andrade-González, N.; González-Sánchez, I.; Meneses, M.A. Extraction and study of the essential oil of copal (Dacryodes peruviana), an amazonian fruit with the highest yield worldwide. Plants 2020, 9, 1658. [Google Scholar] [CrossRef]
- Ochoa, K.; Paredes, L.; Bejarano, D.; Silva, R. Extraction, characterization and evaluation of antibacterial activity of essential oil of Senecio graveolens Wedd (Wiskataya). Sci. Agropecu. 2012, 3, 291–302. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Nasshorudin, D.; Mamat, A.S. Novel Closed System Extraction of Essential Oil: Impact on Yield and Physical Characterization. Int. Conf. Biotech Env. Manag. 2014, 75, 42–46. [Google Scholar]
- Barra, A. Factors Affecting Chemical Variability of Essential Oils: A Review of Recent Developments. Nat. Prod. Com. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Das, J.; Mao, A.A.; Handique, P.J. Terpenoid Compositions and Antioxidant Activities of Two Indian Valerian Oils from the Khasi Hills of North-east India. Nat. Prod. Com. 2011, 6, 129–132. [Google Scholar] [CrossRef]
- Safaralie, A.; Fatemi, S.; Sefidkon, F. Essential oil composition of Valeriana officinalis L. roots cultivated in Iran. Comparative analysis between supercritical CO2 extraction and hydrodistillation. J. Chromatogr. A 2008, 1180, 159–164. [Google Scholar] [CrossRef]
- Bardakcı, H.; Demirci, B.; Yesilada, E.; Kırmızıbekmez, H. Chemical Composition of the Essential Oil of the Subterranean Parts of Valeriana alliariifolia. Rec. Nat. Prod. 2014, 6, 89–92. [Google Scholar]
- Matailo, A.; Bec, N.; Calva, J.; Ramírez, J.; Andrade, J.M.; Larroque, C.; Vidari, G.; Armijos, C. Selective BuChE inhibitory activity, chemical composition, and enantiomer content of the volatile oil from the Ecuadorian plant Clinopodium brownei. Rev. Bras. Farmacogn. 2019, 29, 749–750. [Google Scholar] [CrossRef]
- Yoshihara, K.; Ohta, Y.; Sakai, T.; Hirose, Y. Germacrene D, a key intermediate of cadinene group compounds and bourbonenes. Tetrahedron Lett. 1969, 27, 2263–2264. [Google Scholar] [CrossRef]
- Stranden, M.; Borg-Karlson, A.-K.; Mustaparta, H. Receptor Neuron Discrimination of the Germacrene D Enantiomers in the Moth Helicoverpa armigera. Chem. Senses 2002, 27, 143–152. [Google Scholar] [CrossRef]
- Silva, R.A.; Lopes, M.P.; Azevedo, B.M.; Costa, M.D.; Alviano, S.C.; Alviano, S.D. Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef]
- Thusoo, S.; Gupta, S.; Sudan, R.; Kour, J.; Bhagat, S.; Hussain, R.; Bhagat, M. Antioxidant activity of essential oil and extracts of Valeriana jatamansi Roots. BioMed. Res. Int. 2014, 2014, 614187. [Google Scholar] [CrossRef] [PubMed]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Liu, H.; Zhou, L.; Liu, Z.; Wang, J.; Han, J.; Yu, Z.; Yang, F. Chemical analysis and biological activity of the essential oils of two valerianaceous species from China: Nardostachys chinensis and Valeriana officinalis. Molecules 2010, 15, 6411–6422. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Bhatt, I.D.; Rawal, R.S.; Nandi, S.K. Effect of developmental stages on total phenolic composition and anti-oxidant activities in Hedychium spicatum Buch. Ham. ex D.Don (Vanhaldi). J. Hort. Sci. Biotech. 2014, 89, 557–563. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Dar, M.Y.; Wani, B.A.; Shah, W.A.; Bhat, B.A.; Ganai, B.A.; Bhat, K.A.; Anand, R.; Qurishi, M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine 2012, 19, 1185–1190. [Google Scholar] [CrossRef]
- Pérez, M.L.; Cabeza, M.; Nuñez, N. In vitro effect of rosemary oil (Rosmarinus officinalis) as inhibitor of acetylcholinesterase activity as an alternative therapy in Alzheimer’s Syndrome. Health Probl. 2020, 26, 93–102. [Google Scholar]
- Marcucci, C.; Rademacher, M.; Kamecki, F.; Pastore, V.; Bach, H.G.; Ricco, R.A.; Wagner, M.L.; Knez, D.; Gobec, S.; Colettis, N.; et al. Biological Evaluation of Valeriana Extracts from Argentina with Potent Cholinesterase Inhibition for the Treatment of Neurodegenerative Disorders and Their Comorbidities—The Case of Valeriana carnosa Sm. (Caprifoliaceae) Studied in Mice. Pharmaceuticals 2023, 16, 129. [Google Scholar] [CrossRef]
- Hung, N.H.; Quan, P.M.; Satyal, P.; Dai, D.N.; Hoa, V.; van Huy, N.G.; Giang, L.D.; Ha, N.T.; Huong, L.T.; Hien, V.T.; et al. Acetylcholinesterase Inhibitory Activities of Essential Oils from Vietnamese Traditional Medicinal Plants. Molecules 2022, 27, 7092. [Google Scholar] [CrossRef]
- Calva, J.; Bec, N.; Gilardoni, G.; Larroque, C.; Cartuche, L.; Bicchi, C.; Montesinos, J.V. Acorenone B: AChE and BChE Inhibitor as a Major Compound of the Essential Oil Distilled from the Ecuadorian Species Niphogeton dissecta (Benth.) J.F. Macbr. Pharmaceuticals 2017, 10, 84. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Villalta, G.; Salinas, M.; Calva, J.; Bec, N.; Larroque, C.; Vidari, G.; Armijos, C. Selective BuChE Inhibitory Activity, Chemical Composition, and Enantiomeric Content of the Essential Oil from Salvia leucantha Cav. Collected in Ecuador. Plants 2021, 10, 1169. [Google Scholar] [CrossRef]
- Gilardoni, G.; Matute, Y.; Ramírez, J. Chemical and Enantioselective Analysis of the Leaf Essential Oil from Piper coruscans Kunth (Piperaceae), a Costal and Amazonian Native Species of Ecuador. Plants 2020, 9, 791. [Google Scholar] [CrossRef]
- Calva, J.; Cartuche, L.; González, S.; Montesinos, J.V.; Morocho, V. Chemical composition, enantiomeric analysis and anticholinesterase activity of Lepechinia betonicifolia essential oil from Ecuador. Pharm. Biol. 2022, 60, 206–211. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]

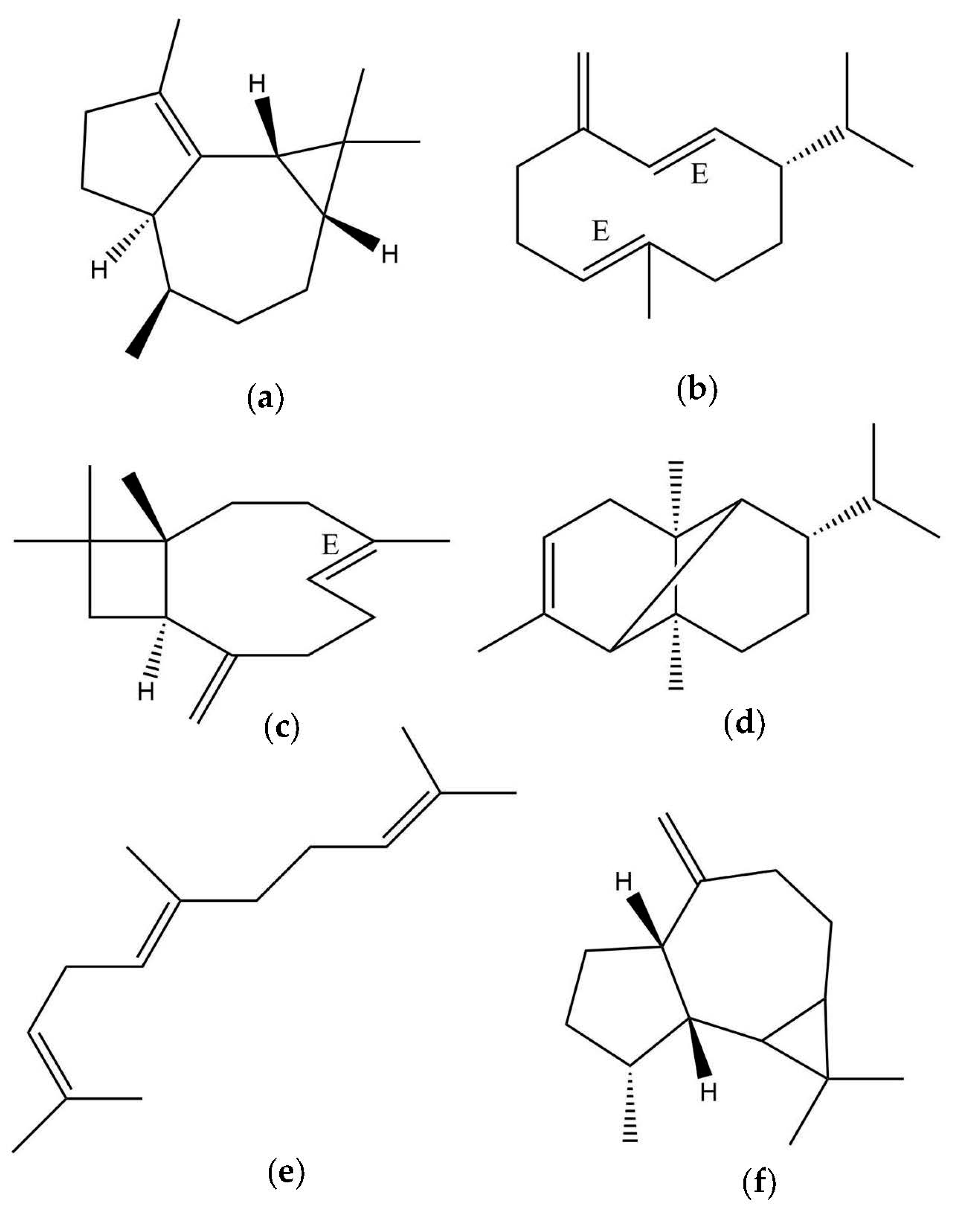
| DB-5ms | HP-INNOwax | |||||||
|---|---|---|---|---|---|---|---|---|
| N° | Compounds | LRI a | LRI b | % ± SD | LRI a | LRI c | Ref. | % ± SD |
| 1 | Isovaleric acid | 831 | 827 | 0.64 ± 0.65 | 1676 | 1680 | [20] | 2.58 ± 0.07 |
| 2 | α-Pinene | 938 | 932 | 0.27 ± 0.25 | ||||
| 3 | 1,8-Cineole | 1031 | 1026 | 1.62 ± 0.15 | 1205 | 1206 | [21] | 1.43 ± 0.81 |
| 4 | n-Nonanal | 1105 | 1100 | 0.59 ± 0.05 | 1391 | 1395 | [22] | 0.39 ± 0.01 |
| 5 | (2E)-Decenal | 1262 | 1260 | 0.93 ± 0.42 | ||||
| 6 | (E)-Anethole | 1285 | 1282 | 1.16 ± 0.04 | 1827 | 1845 | [23] | 0.28 ± 0.01 |
| 7 | α-Cubebene | 1346 | 1348 | 0.48 ± 0.14 | 1451 | 1460 | [22] | 0.32 ± 0.07 |
| 8 | trans-Piperitol acetate | 1346 | 1343 | 0.33 ± 0.13 | ||||
| 9 | Cyclosativene | 1367 | 1369 | 2.29 ± 0.20 | 1470 | 1490 | [24] | 2.03 ± 0.53 |
| 10 | α-Copaene | 1375 | 1374 | 6.76 ± 1.80 | 1462 | 1464 | [25] | 6.91 ± 1.59 |
| 11 | β-Cubebene | 1386 | 1387 | 0.95 ± 0.73 | 1531 | 1549 | [26] | 1.19 ± 0.97 |
| 12 | β-Elemene | 1388 | 1389 | 2.83 ± 1.46 | ||||
| 13 | Sesquithujene | 1402 | 1405 | 2.19 ± 0.66 | ||||
| 14 | α-Gurjunene | 1406 | 1409 | 11.98 ± 3.68 | 1513 | 1520 | [27] | 12.74 ± 5.90 |
| 15 | α-cis-Bergamotene | 1412 | 1411 | 3.90 ± 1.16 | 1558 | 1557 | [22] | 3.26 ± 2.14 |
| 16 | (E)-Caryophyllene | 1418 | 1417 | 7.05 ± 0.43 | 1586 | 1593 | [22] | 7.78 ± 0.97 |
| 17 | β-Copaene | 1425 | 1430 | 0.43 ± 0.19 | 1580 | 1585 | [22] | 2.47 ± 0.80 |
| 18 | α-trans-Bergamotene | 1432 | 1432 | 0.69 ± 0.31 | 1563 | 1580 | [22] | 1.92 ± 0.51 |
| 19 | Seychellene | 1446 | 1444 | 3.03 ± 0.61 | 1622 | [25] | 3.69 ± 2.65 | |
| 20 | Sesquisabinene A | 1641 | 1629 | [22] | 1.74 ± 0.04 | |||
| 21 | α-Humulene | 1453 | 1444 | 2.46 ± 0.47 | 1657 | 1661 | [22] | 1.78 ± 0.28 |
| 22 | allo-Aromadendrene | 1458 | 1458 | 4.25 ± 1.59 | 1632 | 1631 | [22] | 2.55 ± 0.31 |
| 23 | (Z)-Cadina-1(6),4-diene | 1468 | 1461 | 1.39 ± 0.44 | ||||
| 24 | (Z)-β-Farnesene | 1647 | 1665 | [22] | 0.81 ± 0.20 | |||
| 25 | (E)-β-Farnesene | 1668 | 1665 | [22] | 0.54 ± 0.10 | |||
| 26 | Ledene | 1676 | 1686 | [22] | 0.76 ± 0.01 | |||
| 27 | Germacrene D | 1480 | 1480 | 11.47 ± 1.59 | 1698 | 1700 | [22] | 14.93 ± 3.68 |
| 28 | γ-Muurolene | 1680 | 1681 | [22] | 0.90 ± 0.27 | |||
| 29 | δ-Selinene | 1488 | 1492 | 2.43 ± 0.19 | 1681 | 1707 | [28] | 1.78 ± 0.02 |
| 30 | Bicyclogermacrene | 1494 | 1500 | 2.41 ± 1.15 | 1722 | 1724 | [22] | 1.40 ± 0.03 |
| 31 | Isodaucene | 1497 | 1500 | 0.67 ± 0.24 | ||||
| 32 | Pentadecane | 1500 | 1500 | 0.83 ± 0.83 | 1501 | 1500 | [29] | 0.57 ± 0.04 |
| 33 | (E,E)-α-Farnesene | 1504 | 1514 | 4.78 ± 1.31 | 1748 | 1746 | [22] | 4.13 ± 0.51 |
| 34 | δ-Amorphene | 1508 | 1511 | 0.26 ± 0.06 | 1716 | 1704 | [25] | 1.05 ± 0.01 |
| 35 | γ-Cadinene | 1517 | 1513 | 2.4 ± 0.40 | 1750 | 1748 | [22] | 3.60 ± 1.29 |
| 36 | ar-Curcumene | 1771 | 1771 | [27] | 0.71 ± 0.05 | |||
| 37 | Valeric acid | 1798 | 1780 | [30] | 0.45 ± 0.41 | |||
| 38 | (E)-Nerolidol | 1561 | 1561 | 0.56 ± 0.25 | 2046 | 2047 | [21] | 0.39 ± 0.20 |
| 39 | (Z)-calamenene | 1814 | 1808 | [22] | 0.34 ± 0.01 | |||
| 40 | Palustrol | 1565 | 1567 | 0.46 ± 0.26 | 1905 | 1915 | [27] | 0.31 ± 0.02 |
| 41 | Spathulenol | 1574 | 1577 | 0.47 ± 031 | 2118 | 2103 | [22] | 0.32 ± 0.11 |
| 42 | Caryophyllene oxide | 1577 | 1582 | 0.43 ± 0.11 | 1954 | 1940 | [22] | 0.30± 0.01 |
| 43 | Globulol | 1582 | 1590 | 0.91 ± 0.17 | 2061 | 2051 | [22] | 0.78 ± 0 |
| 44 | Viridiflorol | 1590 | 1592 | 0.85 ± 0.41 | ||||
| 45 | Ledol | 1600 | 1602 | 0.50 ± 0.02 | 2008 | 2017 | [27] | 0.88 ± 0.02 |
| 46 | Hexadecanal | 2132 | 2119 | [22] | 0.86 ± 0.02 | |||
| 47 | α-Cadinol | 1653 | 1652 | 0.45 ± 0.20 | 2222 | 2218 | [22] | 0.09± 0.01 |
| 48 | Valerianol | 1663 | 1656 | 0.45 ± 0.14 | 2243 | 2230 | [31] | 0.43 ± 0.05 |
| 49 | n-Tetradecanol | 1677 | 1671 | 0.70 ± 0.33 | ||||
| 50 | n-Heptadecane | 1701 | 1700 | 0.64 ± 0.08 | 1700 | 1700 | [32] | 2.09± 0.03 |
| 51 | Octadecane | 1800 | 1800 | 0.14 ± 0.08 | 1800 | 1800 | [26] | 0.33 ± 0.03 |
| 52 | (2E,6E)-Farnesyl acetate | 1845 | 1845 | 0.65 ± 0.21 | ||||
| 53 | 2-Pentadecanone, 6,10,14-trimethyl- | 1850 | 1847 | 0.42 ± 0.26 | ||||
| 54 | n-Nonadecane | 1901 | 1900 | 0.25 ± 0.02 | ||||
| 55 | (E,Z)-Geranyl linalool | 1984 | 1987 | 0.65 ± 0.19 | ||||
| 56 | 1-Eicosene | 1996 | 1987 | 1.11 ± 0.86 | ||||
| 57 | Octadecanal | 2345 | 2345 | [33] | 0.60 ± 0 | |||
| 58 | (E,E)-Geranyl linalool | 2025 | 2026 | 1.62 ± 0.04 | ||||
| 59 | n-Octadecanol | 2087 | 2077 | 2.01 ± 0.16 | ||||
| 60 | n-Heneicosane | 2101 | 2100 | 0.34 ± 0.11 | ||||
| 61 | n-Tricosane | 2302 | 2300 | 2.95 ± 1.17 | 2302 | 2300 | [22] | 3.99± 1.32 |
| 62 | n-Pentacosane | 2500 | 2500 | [22] | 1.91 ± 0.79 | |||
| Oxygenated monoterpenes | 2.77 | 1.71 | ||||||
| Monoterpene hydrocarbons | 0.27 | 0 | ||||||
| Oxygenated sesquiterpenes | 5.07 | 3.49 | ||||||
| Sesquiterpene hydrocarbons | 75.15 | 79.31 | ||||||
| Aliphatic hydrocarbons | 6.27 | 8.89 | ||||||
| Fatty acids | 0.64 | 3.03 | ||||||
| Alcohol | 2.71 | 0 | ||||||
| Others | 5.20 | 1.85 | ||||||
| Total identified oil constituents (%) | 98.08 | 98.29 | ||||||
| Enantiomer | LRI a | LRI b | Ref. | Enantiomeric Distribution (%) | ee (%) |
|---|---|---|---|---|---|
| (+)-α-Pinene | 931 | 932 | [35] | 100 | 100 |
| (R)-(+)-Germacrene | 1436 | 1474 | [36] | 100 |
| Sample | AChE, IC50 ± SD (µg/mL) | BuChE, IC50 ± SD (µg/mL) |
|---|---|---|
| V. microphylla | >250 | >250 |
| Donepezil | 0.04 ± 0.01 | 3.60 ± 0.20 |
| Sample | ABTS | DPPH |
|---|---|---|
| SC50 (µg/mL) | ||
| V. microphylla | 41.82 ± 1.62 | 89.60 ± 1.31 |
| Trolox | 23.27 ± 1.05 | 29.99 ± 1.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, G.; Calva, J.; Cartuche, L.; Salinas, M.; Armijos, C. Chemical Constituents, Enantiomer Content, Antioxidant and Anticholinesterase Activities of Valeriana microphylla Kunth Essential Oil. Plants 2023, 12, 2155. https://doi.org/10.3390/plants12112155
Aguilar G, Calva J, Cartuche L, Salinas M, Armijos C. Chemical Constituents, Enantiomer Content, Antioxidant and Anticholinesterase Activities of Valeriana microphylla Kunth Essential Oil. Plants. 2023; 12(11):2155. https://doi.org/10.3390/plants12112155
Chicago/Turabian StyleAguilar, Gabriela, James Calva, Luis Cartuche, Melissa Salinas, and Chabaco Armijos. 2023. "Chemical Constituents, Enantiomer Content, Antioxidant and Anticholinesterase Activities of Valeriana microphylla Kunth Essential Oil" Plants 12, no. 11: 2155. https://doi.org/10.3390/plants12112155
APA StyleAguilar, G., Calva, J., Cartuche, L., Salinas, M., & Armijos, C. (2023). Chemical Constituents, Enantiomer Content, Antioxidant and Anticholinesterase Activities of Valeriana microphylla Kunth Essential Oil. Plants, 12(11), 2155. https://doi.org/10.3390/plants12112155








