Abstract
Achyrocline satureioides is a South American herb used in traditional medicine to treat a wide range of ailments. The healing and antimicrobial effects of this plant have already been covered by many studies, which have confirmed its beneficial effects on human health. In this study, the antimicrobial effect of A. satureioides hydroalcoholic extract against Escherichia coli ATCC10536, Staphylococcus aureus ATCC25923, Staphylococcus epidermidis ATCC12228 and Lactobacillus acidophilus INCQS00076 was determined. The cytotoxicity of the extract was tested on human HaCaT keratinocytes showing very favourable effects on the proliferation and renewal of keratinocytes. According to the results of the HPLC and GC-MS analyses, the lyophilized extract contained only a minimal amount of fragrance allergens. The extract was then used in two cosmetic formulations, and one of them showed a significant synergistic interaction with other cosmetic components. We suggest the use of A.satureioides hydroalcoholic extract as a suitable antimicrobial component of natural origin for cosmetic preparations as a substitute for commonly used preservatives that can cause skin irritation and as a material with its own biological activity.
1. Introduction
Natural extracts that exhibit antimicrobial activity are possible replacements for preservative substances added to cosmetic products. Some preservative substances are prohibited from being added to the cosmetic products due to their negative effects on human health or their potential to cause allergies. In the EU, the presence of such substances is regulated by the European Commission [1].
South America is home to a large variety of plant species that, traditionally, have many applications, e.g., in the production of dyes, drugs, antidotes, medicines, and foods [2]. Achyrocline satureioides is a native plant of South America, known by its common names “macela” or “marcela”, that grows in stony or sandy soils up to 3900 m above sea level [3,4]. Its golden yellow blossoms are the part of the plant most frequently used in native medicine [5].
Previously published studies have confirmed the antioxidant, antimicrobial, anti-inflammatory, and photoprotective effects of macela extracts [6,7,8]. Some other properties, such as sedative [9], hepatoprotective [10], antioxidant [11], immunomodulatory [12], antitumoural [13], and antiviral [14] properties, have also been attributed to this plant. Macela is traditionally used as a treatment for gastrointestinal disorders, digestive agent and antipyretic agent in infusions, macerate in cold water, decoction, syrup [9] and ingredient in the liquor industry [15]. Bidone et al. (2014) found that nano-emulsions prepared from both lyophilized and liquid extracts of A. satureioides via spontaneous emulsification provide a promising opportunity for the treatment of various skin disorders [16].
Phytochemical studies of A. satureoides have found that the antimicrobial and antioxidant activity of macela extract depends on the extract’s composition. The amount of compounds present depends on the extraction method due to the difference in dry extract yield, flavonoid profile, and the amount of free quercetin [2,17,18]. The majority of studies mostly present methods using ethanol extracts of the aerial parts of the plant [19,20,21].
The main compounds responsible for the active principles were determined to be flavonoids. Flavonoids are antioxidants that are able to absorb free radicals of reactive oxygen species (ROS) and thereby prevent damage to the skin cells [22]. Antioxidants stimulate collagen synthesis and regulate gene expression, thereby slowing skin aging, improving skin hydration, helping with pigmentation disorders, and inhibiting lipid oxidation in sebum [23]. The results of the phytochemical analysis of extracts of A. satureioides identified quercetin, 3-O-methylquercetin, and luteolin as the main flavonoids present (Figure 1) [24,25]. Further investigations confirmed that these compounds are not toxic [26]. Additionally, the anticancer properties of quercetin and luteolin were evaluated in numerous studies focusing on many types of cancer: bladder [27,28], glioma [29], colon [30,31], liver [32,33], stomach [34,35], breast [36,37], skin [38,39] and lung cancer [40,41]. Quercetin is the most abundant flavonoid in the human diet. It has been described as inhibiting lipid peroxidation by scavenging reactive oxygen species. Quercetin has higher antioxidant activity compared to other flavonoids present in the A. satureioides extracts [36,38]. Several lyophilized ethanol extract fractions were shown to be more effective than the isolated components, suggesting that a possible synergy may exist, and thus their antioxidant properties are increased [7,42].
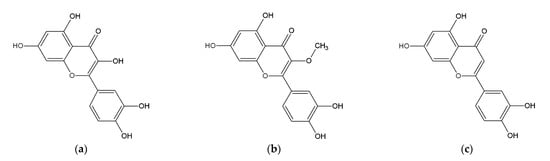
Figure 1.
Main phenolic compounds present in A. satureioides: (a) quercetin, (b) 3-O-methylquercetin, (c) luteolin.
In this study, the antimicrobial effect of A. satureioides hydroalcoholic extract against particular bacterial strains was studied. As target strains were mainly selected bacteria causing human skin disorders. Furthermore, the cytotoxicity of the extract was tested on human HaCaT keratinocytes. The presence and composition of allergens were measured using chromatography. The application of the extract in two cosmetic products as a substitute for commonly used preservatives was also tested. To our best knowledge, this application of the plant extract has never been tested before.
2. Results and Discussion
2.1. Extract Characterization
The content of total phenols in the extract was 219.7 ± 5.0 mg GAE/g of the lyophilized extract, this value complies with the results reported by Guss et al. (2017), who determined the amount of phenolic substances as 231.8 ± 9.7 mg GAE/g extract in ethanolic macerate using the same method [43]. The flavonoid content in the extract was 122.51 ± 2.64 mg CE/g of lyophilized extract. The results were thus reported as catechin equivalents. Guss et al. (2017) reported a lower value of flavonoids present, specifically 43.56 ± 1.4 mg/g of extract, but in their study, a different assay was used to determine the flavonoid content [43]. Other authors using similar extraction conditions determined the content of flavonoids via ultra-fast liquid chromatography. The final content of total flavonoids was 1 mg/mL, which corresponds to 13 mg of phenolic substances per 1 g of dried plant material [44,45]. In our case, the flavonoid content in the extract was similar, specifically 8.13 ± 0.09 mg CE/g of dried plant material.
The antioxidant activity of the extract was determined using the Trolox equivalent antioxidant capacity (TEAC) assay. The antioxidant activity was 3.91 ± 0.07 mMol of Trolox equivalent per 100 g of dry weight of inflorescences. Similar results were presented by Goltz et al. (2018), where the antioxidant activity in the case of ethanolic extract of A. satureioides ranged between 6.62 and 15.15 mMol of Trolox equivalent per 100 g of dry weight of plant material depending on extraction time. The higher antioxidant activity in comparison with our results was probably caused by the use of ultrasound extraction [46].
2.2. Determination of Allergens Content by HPLC
HPLC analysis with a reversed phase was performed according to Villa et al. (2007) [47] to determine the allergen content of the extract because of its intended use in cosmetics emulsions. The amount of fragrance allergens in A. satureioides extract, required by EU legislation [1] to be declared on the label of cosmetic products, was determined. The amount of fragrant allergenic substances detected in the extract using this method was below the EU legislative limit.
2.3. Determination of Allergens Content by GC-MS
The chemical composition of the volatile compounds of 5 mg of the lyophilized hydroalcoholic extract was determined by GS/MS (Figure 2). The extract contained linalool (Rt = 8.36 min), which comprised 143.3 ± 17.6 µg per gram of the lyophilized extract, and eugenol (Rt = 23.49 min), which comprised 12.0 ± 2.0 µg per gram of extract. The amounts of these substances present in the lyophilized hydroalcoholic extract are significantly below the EU legislative limit for fragrance allergens present in leave-on cosmetic products, e.g., emulsions (Table 1).
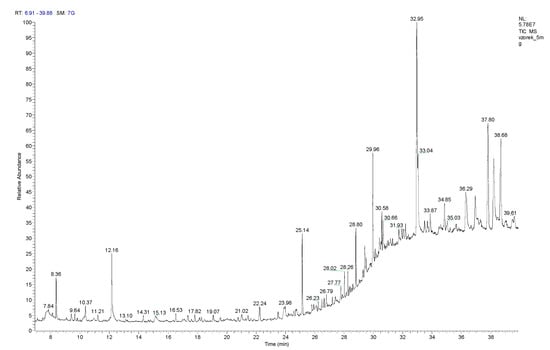
Figure 2.
GC–MS chromatogram of A. satureioides extract (5 mg).

Table 1.
The amount of fragrance substances present in lyophilized hydroethanolic extract of A. satureioides and in cosmetic emulsions.
The other fragrance substances found in the extract were caryophyllene (Rt = 9.40 min) 15.8 ± 0.9 µg; menthol (Rt = 10.24 min) 41.3 ± 0.8 µg; terpineol (Rt = 11.20 min) 10.0 ± 1.6 µg; carvone (Rt = 12.16 min) 263.8 ± 36.6 µg; anethole (Rt = 14.31 min) 22.9 ± 4.4 µg and vanillin (Rt = 29.43 min) 91.1 ± 3.2 µg per gram of lyophilized extract.
GC-MS chromatograms of Allergen Mix 1 and Allergen Mix 2 are shown in Figures S1 and S2, and their compositions are listed in Tables S1 and S2. The chemical compositions of the volatile compounds of 10 and 25 mg of the lyophilized hydroalcoholic extract are shown in Figures S3 and S4, and their compositions are listed in Tables S3 and S4.
2.4. MTT Cytotoxicity Assay
Cell viability assay using HaCaT cell line is considered to be a reliable model for assessing skin disorders and the biocompatibility and safety of various substances. HaCaT cells were exposed to the A.satureioides extract in the concentration range of 3.5–63.5 μg · mL−1 for 24 h. As shown in Figure 3, the extract did not induce cytotoxicity but increased the cell viability of keratinocytes when compared to the control in the tested concentration range of 3.5–63.5 μg·mL−1. An MTT cytotoxicity test with 24 h incubation in medium and extract mixture was used by other authors, who proposed a nanoemulsion of A. satureioides extract for wound healing [44]. Although cell viability dynamics were observed depending on the extract concentration, the incubation time was sufficient to assess whether the metabolic activity and proliferative capacity of HaCat cells is positively or negatively altered by the extract present. In our case, compared to the literature [44], the tested concentrations were even higher but still showed increased cell viability.
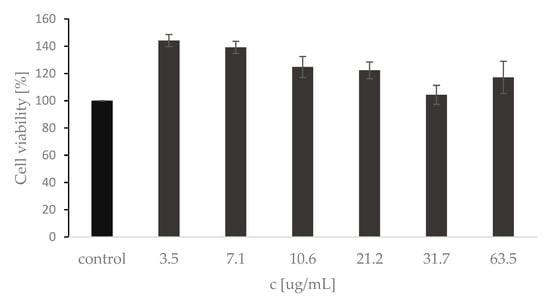
Figure 3.
MTT assay of HaCaT cells: the control value represents mean ± SD for triplicate tests of the growth control with no extract. Results are expressed as the percentage of the control.
The viability of human cells was also determined using an enzymatic LDH test. The level of released LDH enzyme (units·mL−1) from damaged cells that catalyzes the interconversion of pyruvate and lactate was spectrophotometrically measured at 540 nm. The results were in accordance with the cell viability assay (MTT assay), and the extract did not induce cytotoxicity. LDH levels of extracts were compared with growth control (CC), where the LDH value of CC was determined at 461.30 ± 2.77 units·mL−1. Positive control (PC) with 10% ethanol reached LDH value at 836.67 ± 13.89 units·mL−1. All LDH values of tested samples at a concentration range of 3.5–63.5 μg·mL−1 did not achieve PC results. The highest LDH level was obtained at concentration 63,5 μg·mL−1 with an LDH value of 618.33 ± 3.33 units·mL−1.
2.5. Determination of MIC and MBC by Broth Microdilution Method
The A.satureioides hydroalcoholic extract showed antimicrobial activity against all tested bacteria strains. The antimicrobial activity of the extract has already been covered by a large number of authors [48,49]. Lemos et al. (2000) investigated the antimicrobial activity of aqueous and other extracts against E.coli and S.aureus [49]. The antimicrobial activity of these extracts was confirmed at concentrations ranging from units to hundreds of micrograms per millilitre, which is consistent with our results (Figure 4). Ampicillin is used as a reference antibiotic in the methodology to determine the values of MIC and MBC established in accordance with the Guidelines of the Institute of CLSI-Clinical and Laboratory Standards M7-A11 for bacteria [50].
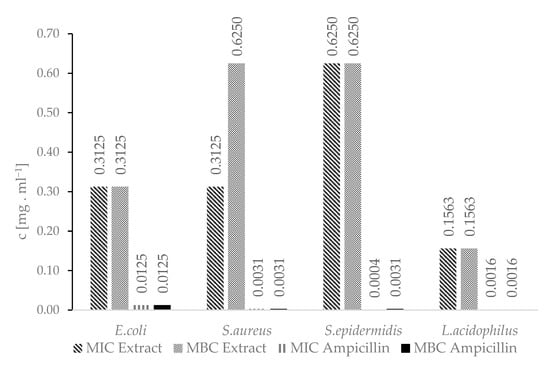
Figure 4.
MIC and MBC concentrations of the hydroalcoholic extract.
To our knowledge, no other studies have been designed to determine the antimicrobial activity of the hydroalcoholic extract of A. satureioides against lactobacilli. With an intention to add the extract to cosmetic products, we tested the antimicrobial activity of the extract against Lactobacillus acidophilus to determine if it is also suitable for cosmetic products for feminine hygiene. However, due to its strong antimicrobial activity against L. acidophilus, the hydroalcoholic extract of A. satureioides is not suitable for this purpose.
2.6. Determination of Inhibition Haloes by Agar Diffusion
The A.satureioides extract showed antimicrobial activity against all tested bacteria strains (Figure 5). Ampicillin is used as a reference antibiotic in accordance with the Institute of CLSI-Clinical and Laboratory Standards M02-A13 Guideline [51]. The results comply with the previously published literature, where the authors proved the antimicrobial activity of freeze-dried hydroalcoholic extract against Gram-negative and Gram-positive bacteria, including S.aureus [52].
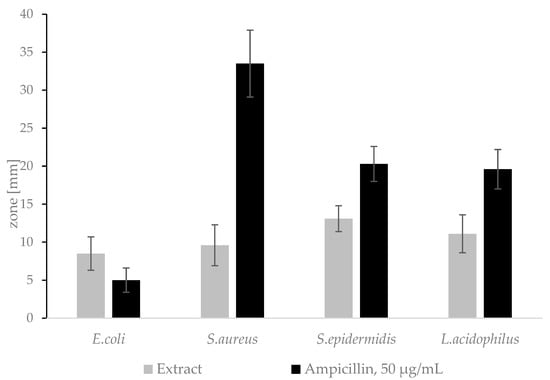
Figure 5.
Inhibition zones of the extract on agar plates.
The hydroalcoholic extract of A. satureioides is not suitable to use in cosmetic products for feminine hygiene as confirmed by both the diffusion test and the broth dilution method.
2.7. Cosmetic Emulsions
Two emulsions were prepared with the addition of the lyophilised hydroacoholic extract (1% and 2% w/w) of A.satureioides, and these emulsions had different emulsifying components. Other components were identical for both emulsions. Despite this, the antimicrobial activity of the two emulsions was significantly different. Emulsion 1 showed antimicrobial activity against all tested bacteria (Figure 6). Emulsion 2 showed antimicrobial activity against S. epidermidis and L.acidophilus, but the inhibition zones were significantly smaller. Both of the emulsions without the added extract did not show any antimicrobial effect. As mentioned above, ampicillin is used as a reference antibiotic [51]. For a better comparison of the antimicrobial effects of the emulsions with the addition of the extract and ampicillin, the antibiotic was utilized in two different concentrations. The results presented in Figure 6 refer to the antimicrobial activity of the hydroalcoholic extract of the Achyrocline satureioides inflorescences from Brazil. The hydroalcoholic extract of the Achyrocline satureioides inflorescences originating in Paraguay displayed a comparable antimicrobial activity. The zone diameter varied by 5–10% in comparison with the Brazilian sample. Such variation does not even exceed the relative standard deviations for the majority of the tested bacterial strains.
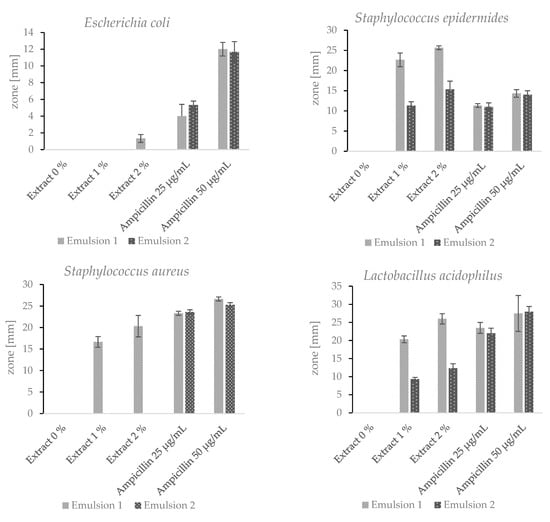
Figure 6.
Inhibition zones of Emulsions 1 and 2 with 0%, 1% and 2% (w/w) of the extract.
Emulsion 1 contained the ingredient Emulfeel® SGP, which consists of three components. Each Emulfeel® SGP component shows antimicrobial or antiseptic activity. The antimicrobial properties of Helianthus Annuus (sunflower) seed oil were investigated by Aboki et al. (2012), who found that extracted oil showed antibacterial activity against E.coli and S.aureus [53]. Xylityl sesquicaprylate is used in the cosmetic products as a surfactant, emulsifier and antiseptic compound [54]. Polyacrylic acid is an emulsion stabilizer, and many authors investigated its antimicrobial activity in copolymers. The antimicrobial activity of the copolymers increased with increasing acrylic acid concentration [55]. Despite the antimicrobial properties of the individual components of Emulfeel® SGP, no inhibition zone was observed in the case of the pure emulsion without the addition of the extract. It can therefore be concluded that the Emulfeel® SGP components have a synergistic effect with the phenolic substances contained in the extract. Thus, the antimicrobial activity of the emulsion is significantly increased. The A. satureioides hydroalcoholic extract could be used as a safe hypoallergenic natural preservative, or as a substance to reduce the amount of other preservative ingredients present, while maintaining antimicrobial activity. However, more extensive tests need to be carried out, which goes beyond the scope of this study.
3. Materials and Methods
3.1. Plant Material
To determine the antimicrobial activity of the hydroalcoholic extract of A. satureioides, the used plant material was obtained via a scientific cooperation agreement signed between the School of Pharmaceutical Sciences of Araraquara (FCFAr-UNESP), Araraquara, São Paulo, Brazil, and the Pluridisciplinary Center for Chemical, Biological and Agronomic Research (CPQBA-UNICAMP), Campinas, São Paulo, Brazil. The obtained species A. satureioides was collected in the city of Campinas-SP at coordinates 22°48’ S; 47°07’ W. It was registered and authorized for use by SISGEN under the registration number AD73F75. The antimicrobial activity of the hydroalcoholic extract of A. satureioides was confirmed with plant material commercially purchased from Diochi spol. s r.o. (Prague, CZ) and obtained from Paraguay. The extract was subsequently characterized and tested for the presence of fragrance allergens and cell cytotoxic activity. Only plant material from Paraguay was available via the free market in CZ, likely due to strict rules about exporting plant material outside of Brazil [56].
3.2. Plant Extract Preparation
The preparation was carried out with 50 g of air-dried inflorescences of A. satureioides, which were mechanically ground and macerated in 1000 mL of hexane. In this step, the lipid fraction was removed to increase the water solubility of the final extract and efficiently extract active compounds. The mixture was tempered at 40 °C for 24 h, and the macerate was filtered. The plant material was used for the three-step maceration. In the first step, the plant material was tempered at 40 °C for 24 h in 500 mL of 70% EtOH; in the second and the third steps, the plant material was tempered at 40 °C. In each step, the macerate was filtered, and the ethanol was completely removed via rotary evaporation under reduced pressure.
The freeze-dried extract (FDE) was prepared using Edwards Modulyo (BOC Edwards, Tonawanda, NY, USA) freeze-drying equipment. The extract was freeze-dried under a controlled temperature (−60 °C) and pressure (−10−2 bar). The final dry extract powder consisted of all three ethanolic extracts and was stored in a freezer (−20 °C) until further use.
The lyophilized extract from commercially purchased plant material was prepared in the same way using FreeZone Triad (Labconco, Kansas City, MI, USA) freeze-drying equipment.
3.3. Antioxidant Activity
The antioxidant activity of the hydroalcoholic extract was measured using the Trolox equivalent antioxidant capacity (TEAC) assay [57]. The ABTS·+ solution was prepared by diluting 7 mM ABTS (2,2′–azino–bis(3–ethylbenzothiazoline–6–sulfuric acid) diammonium salt) (Sigma–Aldrich, Burlington, MA, USA) and 2.45 mM potassium persulphate (Sigma–Aldrich, USA) in deionized water. The solution was stored in a dark place at room temperature for 12–16 h before use. The blank solution was prepared by diluting the ABTS·+ solution with UV–VIS ethanol to a final absorption value: A734 nm = 0.70 ± 0.02. The antioxidant activity of the hydroalcoholic extract was measured by adding 10 μL to 1 mL of diluted ABTS·+, and the decrease in absorption was measured. The Trolox calibration standards were prepared in the range of concentration 0.2–1.6 μmol·mL−1. The absorbance of the samples was measured in triplicate.
3.4. Analysis of Total Phenols
The amount of total phenols present in the hydroalcoholic extract was measured by the Folin–Ciocalteu assay [58]. The solution of 1 mL Folin–Ciocalteu reagent (Penta Chemicals, CZ, 10 times diluted), 1 mL of deionized water and 50 μL of the hydroalcoholic extract was prepared, mixed and incubated for 5 min at room temperature. The blank solution was prepared with 50 μL of deionized water. Saturated Na2CO3 solution (1 mL) was added to the measured solutions, which were incubated for 15 min at room temperature. The absorbance of solutions was measured at a wavelength of 750 nm. The calibration standards of gallic acid (Sigma–Aldrich, USA) were prepared in the range of concentrations: 0.1–0.7 mg · mL−1. The absorbance of the samples was measured in triplicate, and the results are expressed as mg of gallic acid equivalent (GAE)/g of each sample.
3.5. Analysis of Total Flavonoids
The amount of total flavonoids present in the extract was measured using a flavonoid colorimetric assay [59]. A solution of 0.5 mL hydroalcoholic extract, 1.5 mL deionized water, and 0.2 mL 5% NaNO2 (Lach-Ner, CZ) was prepared, mixed and incubated for 5 min at room temperature. In the case of a blank solution, the 0.5 mL of extract was replaced with the same amount of deionized water. Then, 0.2 mL of 10% AlCl3 (Lach-Ner, CZ) solution was added, mixed and incubated at room temperature for 5 min. Then, 1.5 mL of 5% NaOH (Lach-Ner, CZ) solution and 1.0 mL of deionized water were added, mixed and incubated for 15 min at room temperature. The absorbance was measured at a wavelength of 510 nm. The calibration standards of catechin (Sigma–Aldrich, USA) were prepared in a range of concentrations: 0.05–0.3 mg · mL−1. The absorbance of the samples was measured in triplicate, and the results were expressed as milligram of catechin equivalent (CE) per gram of dry weight (mg CE/g dw) of sample.
3.6. Determination of Allergen Content by HPLC-DAD
Reversed-phase HPLC analysis was performed according to Villa et al. (2007) [47] to determine the fragrance allergen content of the extract. The analysis was performed in triplicate using HPLC/PDA Dionex UltiMate 3000 (Thermo Fischer, Waltham, MA, USA), DAD detector Vanquish series (Thermo Fischer, USA), column YMC-Triart C18 ExRS S-3µm, 8nm Analytical HPLC Column, 150 × 4.6 mm. Allergen standards were obtained from Accustandard Inc. (New Haven, CT, USA).
3.7. Determination of Allergen Content by GC-MS
The major volatile compounds of the lyophilized hydroalcoholic extract of Achyrocline satureioides were analysed using a Trace™ 1310 gas chromatograph with the TSQ 9000 mass detector (Thermo Fisher Scientific Inc., Waltham, MA, USA) and the Supelcowax capillary column (30 m × 0.25 mm; I.D. 0.25 μm, Supelco, Bellefonte, PA, USA). Helium was used as carrying gas at a flow rate of 1 mL·min−1.
The lyophilized extract material was extracted in amounts of 5, 10 and 25 mg using ethyl acetate for 10 min, and then filtered through a nylon filter (0.22 μm). One microliter of the sample was injected onto the column (splitless mode, 1 min). The temperature of the injector was 250 °C, and the temperature of the transfer line was 220 °C. The conditions of the analysis were as follows: incubation at 70 °C for 1 min, followed by temperature increase up to 135 °C (10 °C/min), and the temperature was maintained at 135 °C for 2 min. The temperature was increased up to 170 °C (3 °C/min) and maintained for 1 min. This was followed by an increase in temperature to 250 °C (10 °C/min.), where the temperature was maintained for 20 min. The TSQ 9000 MS detector was used in electron ionization mode and set at 70 eV, ion source temperature: 250 °C, scanning range: 35–350 m/z and scanning speed: 0.2 s.
3.8. Cell Culture and Treatment
The assay was performed with human epidermal keratinocytes (HaCaT), and the cells were obtained from CLS Cell Lines Service GmbH (Eppelheim, Germany). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Lonza Biotec, CZ, USA) without sodium pyruvate, supplemented with high glucose, 0.4 mM L-glutamine, 1% penicillin/streptomycin/amphotericin B solution (Antimycotin-Antibiotic 100×, Sigma-Aldrich, Burlington, MA, USA), and 10% of heat-inactivated fetal bovine serum (FBS). HaCaT cells were incubated in a humidified 5% (v/v) CO2 atmosphere at 37 °C for 24 h. The lyophilized extract was dissolved in 70% EtOH. Samples were diluted with DMEM up to a concentration range of 3.5–63.5 μg·mL−1. The control was DMEM medium, and 10% ethanol was used as a negative control. After 24 h of incubation in a humidified 5% (v/v) CO2 atmosphere at 37 °C, cell viability was determined [60].
3.9. MTT Assay
Cell viability was assessed using a colorimetric MTT reduction assay to determine cell metabolic activity. Following the treatment, 20 μL of MTT dissolved in PBS (2.5 mg·mL−1) was added to each sample and incubated for 3 h in humidified 5% (v/v) CO2 atmosphere at 37 °C, and then 100 μL of 10% SDS in PBS was added to each well. Plates were stored in the dark and evaluated the next day using ELISA Reader at 562 nm [61].
3.10. Microorganisms
The following reference strains were used: Escherichia coli ATCC10536, Staphylococcus aureus ATCC25923, Staphylococcus epidermidis ATCC12228, and Lactobacillus acidophilus INCQS00076. The bacteria were transferred to a fresh brain infusion medium (BHI) and allowed to grow aerobically for 24 h at 37 °C. The inoculums for the assays were made by diluting bacterial suspensions up to McFarland standard turbidity of 0.5 (108 CFU·mL−1) [50], except for L. acidophilus, which was diluted to 5.0 McFarland standard (109 CFU·mL−1) [62].
3.11. Broth Microdilution Method
Minimal inhibitory concentrations (MIC) were established in accordance with the Guidelines of the Institute of Clinical and Laboratory Standards M7-A11 for bacteria [50]. The test was performed on 96-well sterile microplates. The extract in DMSO solution (DMSO:medium, 1:10 v/v) was transferred to wells of microplates in order to obtain double serial dilutions in a concentration range of 2.5 to 0.0012 mg·mL−1. Each microplate contained an antibiotic control, ampicillin (Sigma Chemical Co., Burlington, MA, USA), with concentrations ranging from 12.5 to 0.006 μg·mL−1. An inoculum containing 5 × 106 CFU·mL−1 of bacteria in BHI broth (the final concentration in the well was 2.5 × 106 CFU·mL−1) was added to each well. A number of wells on each plate belonged to control of sterility of media, the viability of inoculum, the sterility of the extract solution, and controls to exclude the inhibitory effect of DMSO. The plates were prepared in triplicates and aerobically incubated for 24 h at 37 °C. After incubation, the 0.5% aqueous resazurin solution was added to each well in order to observe the viability of bacteria.
The lowest extract concentration that prevented growth was determined to be MIC. By sub-cultivating microplate wells on Müller Hinton agar plates (MHA), which were aerobically incubated for 24 h at 37 °C, the minimal bactericidal concentration (MBC) was determined.
3.12. Method of Agar Diffusion
According to the Institute of Clinical and Laboratory Standards’ M02-A13 Guideline, the test for bacteria was performed on Müller Hinton agar (MHA) [51]. Each plate was seeded separately with 1% (v/v) bacterial cell suspension (106 CFU·mL−1). A 1% (v/v) L. acidophilus bacterial cell culture containing 107 CFU · mL−1 was inoculated on MRS agar [51]. Next, 100 μL of the extract at a concentration of 5.0 mg·mL−1 in dimethyl sulfoxide in saline solution (saline:DMSO, 5:1 v/v) was added to the agar plate. Each plate contained ampicillin (50 μg·mL−1) and DMSO solvent control.
3.13. Preparation of Cosmetic Emulsions
Two emulsions with different ingredients and two temperatures were prepared, and their compositions are shown in Table 2 and Table 3. The lyophilized hydroalcoholic extract of Achyrocline satureioides was added to prepared emulsions at room temperature at two concentrations: 1% w/w and 2% w/w.

Table 2.
Composition of Emulsion 1.

Table 3.
Composition of Emulsion 2.
3.14. Antimicrobial Activity of Emulsions
According to the Institute of Clinical and Laboratory Standards’ M02-A13 Guideline, the test for bacteria was performed on Müller Hinton agar (MHA) [51]. Plates were seeded separately with each 1% (v/v) bacterial cell suspension (106 CFU·mL−1). A 1% (v/v) L. acidophilus bacterial cell culture containing 107 CFU·mL−1 was inoculated on MRS agar [51]. Each agar plate contained 100 µL of an emulsion with 0%, 1% and 2% additions of the extract, ampicillin (25 and 50 μg·mL−1). Both emulsions were tested in triplicate for all bacteria.
3.15. Statistical Analysis
All experiments were performed in triplicates. Results are presented as mean ± standard deviation (SD). For hypothesis testing, one-way ANOVA was used and set at a significance level of 0.05. Data were evaluated using Tukey’s HSD test.
4. Conclusions
The antimicrobial and antioxidant activity of the hydroalcoholic extract confirmed the results of the previously published studies. Antimicrobial activity against L. acidophilus was determined. Unfortunately, due to the strong antimicrobial activity of the extract against L. acidophilus, the use of this extract in feminine hygiene products is not recommended. It was found that, in a suitable combination with other components of the emulsion formula, the antimicrobial activity against the tested bacteria increased. Thus, it could be possible to limit the amount of preservatives necessary to maintain the microbiological stability of the cosmetic product. Moreover, the high antimicrobial activity of A. satureioides extract can be considered an additional biological activity. In addition, the hydroalcoholic extract of A. satureioides did not show a toxic effect on keratinocytes; on the contrary, very favourable effects on cell proliferation and renewal were observed. HPLC analysis also confirmed that the extract does not contain any fragrance allergens, which significantly reduces the likelihood of a possible allergic reaction to this component of the cosmetic product. A very low amount of fragrance allergens was detected via GC-MS analysis, but these concentrations are insignificant from a legislative point of view. In conclusion, the hydroalcoholic extract of A. satureioides could serve as a safe hypoallergenic natural preservative for use in cosmetic products. This application of the plant extract has never been tested before.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12102027/s1, Figure S1: GC-MS chromatogram Allergen Mix 1; Figure S2: GC-MS chromatogram Allergen Mix 2; Figure S3: GC-MS chromatogram of A.satureioides lyophilized extract (10 mg); Figure S4. GC-MS chromatogram of A.satureioides lyophilized extract (25 mg); Table S1: GC-MS Allergen Mix 1 composition; Table S2: GC-MS Allergen Mix 2 composition; Table S3: Fragrance allergen composition of A.satureioides lyophilized extract (10 mg); Table S4: Fragrance allergen composition of A.satureioides lyophilized extract (25 mg).
Author Contributions
Conceptualization, D.L., M.A.C., R.C.L.R.P. and I.M.; methodology, M.A.C., R.C.L.R.P., R.S., D.L., J.H. and R.M.; validation, D.L., J.H., Z.S., R.S. and M.A.M.C.; formal analysis, D.L., J.H. and R.M.; investigation, D.L., M.A.M.C., R.S., J.H. and Z.S.; resources, R.C.L.R.P. and I.M.; data curation, D.L. and R.M.; writing—original draft preparation, D.L.; writing—review and editing, R.C.L.R.P. and I.M.; visualization, D.L.; supervision, M.A.C., R.C.L.R.P. and I.M.; project administration, I.M.; funding acquisition, I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Internal Grant Agency of the Brno University of Technology, grant number FCH-S-23-8330.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Publications Office of the European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products; Publications Office of the European Union: Luxembourg, 2009; Volume 59. [Google Scholar]
- Retta, D.; Dellacassa, E.; Villamil, J.; Suárez, S.A.; Bandoni, A.L. Marcela, a promising medicinal and aromatic plant from Latin America: A review. Ind. Crop. Prod. 2012, 38, 27–38. [Google Scholar] [CrossRef]
- Lorenzo, D.; Atti-Serafini, L.; Santos, A.C.; Frizzo, C.D.; Paroul, N.; Paz, D.; Dellacassa, E.; Moyna, P. Achyrocline satureioides Essential Oils from Southern Brazil and Uruguay. Planta Med. 2000, 66, 476–477. [Google Scholar] [CrossRef] [PubMed]
- De Souza, K.; Bassani, V.; Schapoval, E. Influence of excipients and technological process on anti-inflammatory activity of quercetin and Achyrocline satureioides (Lam.) D.C. extracts by oral route. Phytomedicine 2007, 14, 102–108. [Google Scholar] [CrossRef]
- Davies, P.; Villamil, J. Estudios en Domestication y Cultivo de Especies Medicinales y Aromáticas Nativas, 11th ed.; Instituto Nacional de Investigacion Agropecuaria: Rincon del Colorado, Uruguay, 2004. [Google Scholar]
- Morquio, A.; Rivera-Megret, F.; Dajas, F. Photoprotection by topical application of Achyrocline satureioides (‘Marcela’). Phytother. Res. 2005, 19, 486–490. [Google Scholar] [CrossRef]
- Polydoro, M.; de Souza, K.; Andrades, M.; Da Silva, E.; Bonatto, F.; Heydrich, J.; Pizzol, F.D.; Schapoval, E.; Bassani, V.; Moreira, J. Antioxidant, a pro-oxidant and cytotoxic effects of Achyrocline satureioides extracts. Life Sci. 2004, 74, 2815–2826. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, M.; Blasina, F.; Echeverry, C.; Morquio, A.; Ferreira, M.; Abin-Carriquiry, J.; Lafon, L.; Dajas, F. Cytoprotection by Achyrocline satureioides (Lam) D.C. and some of its main flavonoids against oxidative stress. J. Ethnopharmacol. 2004, 91, 13–20. [Google Scholar] [CrossRef]
- Simões, C.M.O.; Schenkel, E.P.; Bauer, L.; Langeloh, A. Pharmacological investigations on Achyrocline satureioides (Lam.) DC., compositae. J. Ethnopharmacol. 1988, 22, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Kadarian, C.; Broussalis, A.; Miño, J.; Lopez, P.; Gorzalczany, S.; Ferraro, G.; Acevedo, C. Hepatoprotective activity of Achyrocline satureioides (Lam) D. C. Pharmacol. Res. 2002, 45, 57–61. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Coussio, J.; Ciccia, G. Antioxidant and free radical scavenging effects in extracts of the medicinal herb Achyrocline satureioides (Lam.) DC. (“marcela”). Braz. J. Med. Biol. Res. 1998, 31, 1163–1170. [Google Scholar] [CrossRef]
- Cosentino, M.; Bombelli, R.; Carcano, E.; Luini, A.; Marino, F.; Crema, F.; Dajas, F.; Lecchini, S. Immunomodulatory properties of Achyrocline satureioides (Lam.) D.C. infusion: A study on human leukocytes. J. Ethnopharmacol. 2008, 116, 501–507. [Google Scholar] [CrossRef]
- Souza, P.; Bianchi, S.; Figueiró, F.; Heimfarth, L.; Moresco, K.; Gonçalves, R.; Hoppe, J.; Klein, C.; Salbego, C.; Gelain, D.; et al. Anticancer activity of flavonoids isolated from Achyrocline satureioides in gliomas cell lines. Toxicol. Vitr. 2018, 51, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Bettega, J.M.R.; Teixeira, H.; Bassani, V.L.; Barardi, C.R.M.; Simões, C.M.O. Evaluation of the antiherpetic activity of standardized extracts of Achyrocline satureioides. Phytother. Res. 2004, 18, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Guariniello, J.; Iannicelli, J.; Peralta, P.A.; Escandón, A.S. In vivo and in vitro propagation of “macela”: A medicinal-aromatic native plant with ornamental potential. Ornam. Hortic. 2018, 24, 361–370. [Google Scholar] [CrossRef]
- Bidone, J.; Zorzi, G.K.; Carvalho, E.L.; Simões, C.M.; Koester, L.S.; Bassani, V.L.; Teixeira, H.F. Incorporation of Achyrocline satureioides (Lam.) DC extracts into topical nanoemulsions obtained by means of spontaneous emulsification procedure. Ind. Crop. Prod. 2014, 62, 421–429. [Google Scholar] [CrossRef]
- Diaz, C.; Heinzen, H. Variations in the flavonoid profile and free quercetin content in different extracts of Achyrocline satureoides. Acta Farm. Bonaer. 2006, 25, 574–577. [Google Scholar]
- Takeuchi, T.M.; Rubano, M.L.; Meireles, M.A.A. Characterization and Functional Properties of Macela (Achyrocline Satureioides) Extracts Obtained by Supercritical Fluid Extraction Using Mixtures of CO2 Plus Ethanol. Food Bioprocess Technol. 2010, 3, 804–812. [Google Scholar] [CrossRef]
- Casero, C.; Machín, F.; Méndez-Álvarez, S.; Demo, M.; Ravelo, G.; Pérez-Hernández, N.; Joseph-Nathan, P.; Estévez-Braun, A. Structure and Antimicrobial Activity of Phloroglucinol Derivatives from Achyrocline satureioides. J. Nat. Prod. 2014, 78, 93–102. [Google Scholar] [CrossRef]
- Kaloga, M.; Hänsel, R.; Cybulski, E.-M. Isolierung eines Kawapyrons aus Achyrocline satureioides. Planta Med. 1983, 48, 103–104. [Google Scholar] [CrossRef]
- Ferraro, G.E.; Norbedo, C.; Coussio, J.D. Polyphenols from Achyrocline satureioides. Phytochemistry 1981, 20, 2053–2054. [Google Scholar] [CrossRef]
- Nimse, S.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Baumann, L. How to Use Oral and Topical Cosmeceuticals to Prevent and Treat Skin Aging. Facial Plast. Surg. Clin. N. Am. 2018, 26, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, A.C.; Folmer, V.; da Rosa, H.S.; Costa, M.T.; Boligon, A.A.; Paula, F.R.; Roos, D.H.; Puntel, G.O. In vitro and in silico antioxidant and toxicological activities of Achyrocline satureioides. J. Ethnopharmacol. 2016, 194, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.; Anesini, C.; Ouvina, A.; Retta, D.; Filip, R.; Gattuso, M.; Gattuso, S.; Hnatyszyn, O.; Bandoni, A. Total phenolic content and antioxidant activity of extracts of Achyrocline satureioides flowers from different zones in Argentina. Lat. Am. J. Pharm. 2008, 27, 626–628. [Google Scholar]
- Duke, J. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780849312847. [Google Scholar]
- Kim, Y.; Kim, W.-J.; Cha, E.-J. Quercetin-induced Growth Inhibition in Human Bladder Cancer Cells Is Associated with an Increase in Ca2+-activated K+ Channels. Korean J. Physiol. Pharmacol. 2011, 15, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Kilani-Jaziri, S.; Frachet, V.; Bhouri, W.; Ghedira, K.; Chekir-Ghedira, L.; Ronot, X. Flavones inhibit the proliferation of human tumor cancer cell lines by inducing apoptosis. Drug Chem. Toxicol. 2011, 35, 1–10. [Google Scholar] [CrossRef]
- Michaud-Levesque, J.; Bousquet-Gagnon, N.; Béliveau, R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp. Cell Res. 2012, 318, 925–935. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, S.-K.; Kim, B.-S.; Lee, S.-H.; Park, Y.-S.; Park, B.-K.; Kim, S.-J.; Kim, J.; Choi, C.; Kim, J.-S.; et al. Apoptotic Effect of Quercetin on HT-29 Colon Cancer Cells via the AMPK Signaling Pathway. J. Agric. Food Chem. 2010, 58, 8643–8650. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.Y.; Jeong, Y.; Tyner, A.L.; Park, J.H.Y. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am. J. Physiol. Liver Physiol. 2007, 292, G66–G75. [Google Scholar] [CrossRef]
- Tan, J.; Wang, B.; Zhu, L. Regulation of Survivin and Bcl-2 in HepG2 Cell Apoptosis Induced by Quercetin. Chem. Biodivers. 2009, 6, 1101–1110. [Google Scholar] [CrossRef]
- Selvendiran, K.; Koga, H.; Ueno, T.; Yoshida, T.; Maeyama, M.; Torimura, T.; Yano, H.; Kojiro, M.; Sata, M. Luteolin Promotes Degradation in Signal Transducer and Activator of Transcription 3 in Human Hepatoma Cells: An Implication for the Antitumor Potential of Flavonoids. Cancer Res. 2006, 66, 4826–4834. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, K.; Zhang, Q.; Mei, J.; Chen, C.-J.; Feng, Z.-Z.; Yu, D.-H. Effects of quercetin on the apoptosis of the human gastric carcinoma cells. Toxicol. Vitr. 2012, 26, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, Q.; Shen, W.; Zhu, J. Anti-proliferative and chemosensitizing effects of luteolin on human gastric cancer AGS cell line. Mol. Cell. Biochem. 2008, 313, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-C.; Yang, J.-S.; Lu, H.-F.; Ip, S.-W.; Lo, C.; Wu, C.-C.; Lin, J.-P.; Tang, N.-Y.; Chung, J.-G.; Chou, M.-J.; et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharmacal Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Woo, J.S.; Kwon, C.H.; Kim, J.H.; Kim, Y.K.; Kim, K.H. Luteolin induces apoptotic cell death through AIF nuclear translocation mediated by activation of ERK and p38 in human breast cancer cell lines. Cell Biol. Int. 2012, 36, 339–344. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Liu, H.-F.; Chao, J.-I. Survivin and p53 Modulate Quercetin-induced Cell Growth Inhibition and Apoptosis in Human Lung Carcinoma Cells. J. Biol. Chem. 2004, 279, 55875–55885. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.-S.; Liu, Y.-P.; Zhang, L.; Yan, L.-G.; Fan, F.-T.; Shen, C.-S.; Wang, A.-Y.; Zheng, S.-Z.; Wang, S.-M.; Lu, Y. Luteolin reduces the invasive potential of malignant melanoma cells by targeting β3 integrin and the epithelial-mesenchymal transition. Acta Pharmacol. Sin. 2012, 33, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, S.; Xu, Q. Quercetin inhibits the invasion of murine melanoma B16-BL6 cells by decreasing pro-MMP-9 via the PKC pathway. Cancer Chemother. Pharmacol. 2004, 53, 82–88. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, G.; Ren, N.; Zhang, X.; Yin, Q.; Sun, X. Luteolin suppresses growth and migration of human lung cancer cells. Mol. Biol. Rep. 2011, 38, 1115–1119. [Google Scholar] [CrossRef]
- De Souza, K.; Schapoval, E.; Bassani, V. LC determination of flavonoids: Separation of quercetin, luteolin and 3-O-methylquercetin in Achyrocline satureioides preparations. J. Pharm. Biomed. Anal. 2002, 28, 771–777. [Google Scholar] [CrossRef]
- Guss, K.L.; Pavanni, S.; Prati, B.; Dazzi, L.; de Oliveira, J.P.; Nogueira, B.V.; Pereira, T.M.; Fronza, M.; Endringer, D.C.; Scherer, R. Ultrasound-assisted extraction of Achyrocline satureioides prevents contrast-induced nephropathy in mice. Ultrason. Sonochem. 2017, 37, 368–374. [Google Scholar] [CrossRef]
- Balestrin, L.A.; Kreutz, T.; Fachel, F.N.S.; Bidone, J.; Gelsleichter, N.E.; Koester, L.S.; Bassani, V.L.; Braganhol, E.; Dora, C.L.; Teixeira, H.F. Achyrocline satureioides (Lam.) DC (Asteraceae) Extract-Loaded Nanoemulsions as a Promising Topical Wound Healing Delivery System: In Vitro Assessments in Human Keratinocytes (HaCaT) and HET-CAM Irritant Potential. Pharmaceutics 2021, 13, 1241. [Google Scholar] [CrossRef] [PubMed]
- Balestrin, L.A.; Back, P.I.; Marques, M.D.S.; Araújo, G.D.M.S.; Carrasco, M.C.F.; Batista, M.M.; Silveira, T.; Rodrigues, J.L.; Fachel, F.N.S.; Koester, L.S.; et al. Effect of Hydrogel Containing Achyrocline satureioides (Asteraceae) Extract–Loaded Nanoemulsions on Wound Healing Activity. Pharmaceutics 2022, 14, 2726. [Google Scholar] [CrossRef]
- Goltz, C.; Ávila, S.; Barbieri, J.B.; Igarashi-Mafra, L.; Mafra, M.R. Ultrasound-assisted extraction of phenolic compounds from Macela (Achyrolcine satureioides) extracts. Ind. Crop. Prod. 2018, 115, 227–234. [Google Scholar] [CrossRef]
- Villa, C.; Gambaro, R.; Mariani, E.; Dorato, S. High-performance liquid chromatographic method for the simultaneous determination of 24 fragrance allergens to study scented products. J. Pharm. Biomed. Anal. 2007, 44, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Calvo, D.; Cariddi, L.N.; Grosso, M.; Demo, M.S.; Maldonado, A.M. Achyrocline satureioides (LAM.) DC (Marcela): Antimicrobial activity on Staphylococcus spp. and immunomodulating effects on human lymphocytes. Rev. Latinoam. Microbiol. 2006, 48, 247–255. [Google Scholar] [PubMed]
- Lemos, G.; Oliviera, L.; Eberli, B.; Motta, O.; Folly, M. Bactericidal activity of macela (Achyrocline satureioides (Lam.) DC.) and jaborandi-falso (Piper aduncum L.) against strains of Staphylococcus aureus isolated from subclinical bovine mastitis. Rev. Bras. Plantas Med. 2000, 3, 67–72. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically: Approved Standard M7-A11; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed.; CLSI Standard M02; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Moresco, K.S.; Silveira, A.K.; Zeidán-Chuliá, F.; Correa, A.P.F.; Oliveria, R.R.; Borges, A.G.; Grun, L.; Barbé-Tuana, F.; Zmozinski, A.; Brandelli, A.; et al. Effects of Achyrocline satureioides Inflorescence Extracts against Pathogenic Intestinal Bacteria: Chemical Characterization, In Vitro Tests, and In Vivo Evaluation. Evid.-Based Complement. Altern. Med. 2017, 2017, 4874865. [Google Scholar] [CrossRef]
- Aboki, M.; Mohammed, M.; Musa, S.; Zuru, B. Physicochemical and anti-microbial properties of sunflower (Helianthus annuus L.) seed oil. Int. J. Sci. Technol. 2012, 2, 151–154. [Google Scholar]
- Nogueira, C.; Mussi, L.; Baby, A.R.; Zupeli, R.; Magalhães, W.V. Xylityl Sesquicaprylate Efficacy as an Antiseptic Ingredient for Oral Care Products (Mouthwash): An In Vitro Screening Investigation against Eight Microorganisms. Molecules 2023, 28, 28. [Google Scholar] [CrossRef]
- Gratzl, G.; Paulik, C.; Hild, S.; Guggenbichler, J.P.; Lackner, M. Antimicrobial activity of poly(acrylic acid) block copolymers. Mater. Sci. Eng. C 2014, 38, 94–100. [Google Scholar] [CrossRef]
- Law, No. 13.123 of 20 May 2015: Access and Benefits Sharing of Genetic Resources and Associated Traditional Knowledge; Diário Oficial da União: Brasília, Brazil, 2015.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folinciocalteu reagent. Oxid. Antioxid. Part A 1999, 299, 152–178. [Google Scholar]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Bokrova, J.; Marova, I.; Matouskova, P.; Pavelkova, R. Fabrication of novel PHB-liposome nanoparticles and study of their toxicity in vitro. J. Nanopart. Res. 2019, 21, 49. [Google Scholar] [CrossRef]
- Li, X.; Turánek, J.; Knötigová, P.; Kudláčková, H.; Masek, J.; Parkin, S.; Rankin, S.; Knutson, B.L.; Lehmler, H.-J. Hydrophobic tail length, degree of fluorination and headgroup stereochemistry are determinants of the biocompatibility of (fluorinated) carbohydrate surfactants. Colloids Surf. B Biointerfaces 2009, 73, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, V.; Silva, C.; Nader-Macías, M.E. Antibiotic Susceptibility of Potentially Probiotic Vaginal Lactobacilli. Infect. Dis. Obstet. Gynecol. 2006, 2006, 18182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).