A Commercial Arbuscular Mycorrhizal Inoculum Alleviated the Effects of Acid Water on Lupinus angustifolius Grown in a Sterilized Mining Dump
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils and Water Characterization
2.2. Experimental Setting
2.3. Measurement of Soil Variables
2.4. Measurement of Plant Variables
2.5. Root Mycorrhizal Colonization
2.6. Data Processing
3. Results
3.1. Phosphorus, Potassium, and Other Elements
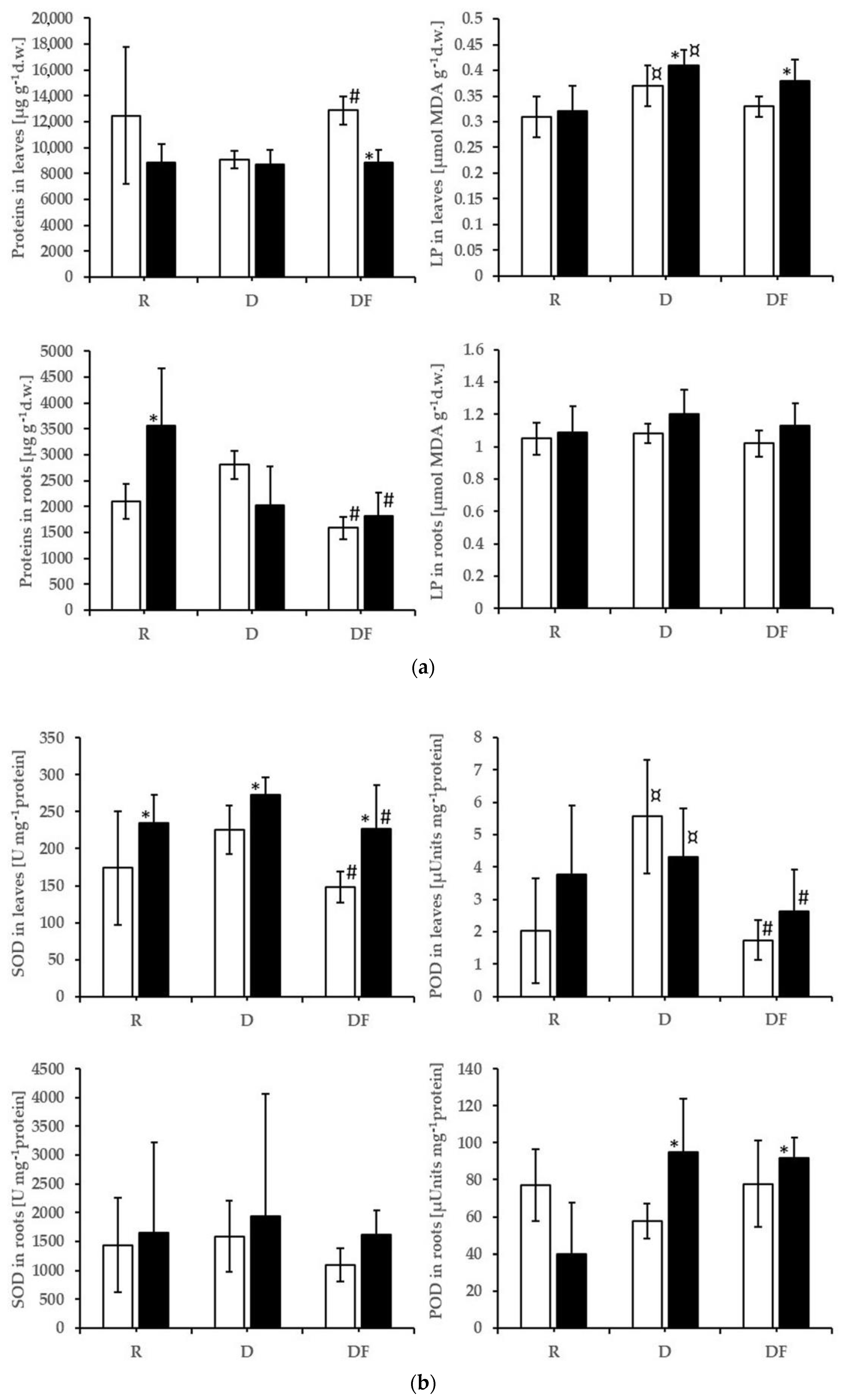
3.2. SOD, POD, LP, Protein, Assimilating Pigments
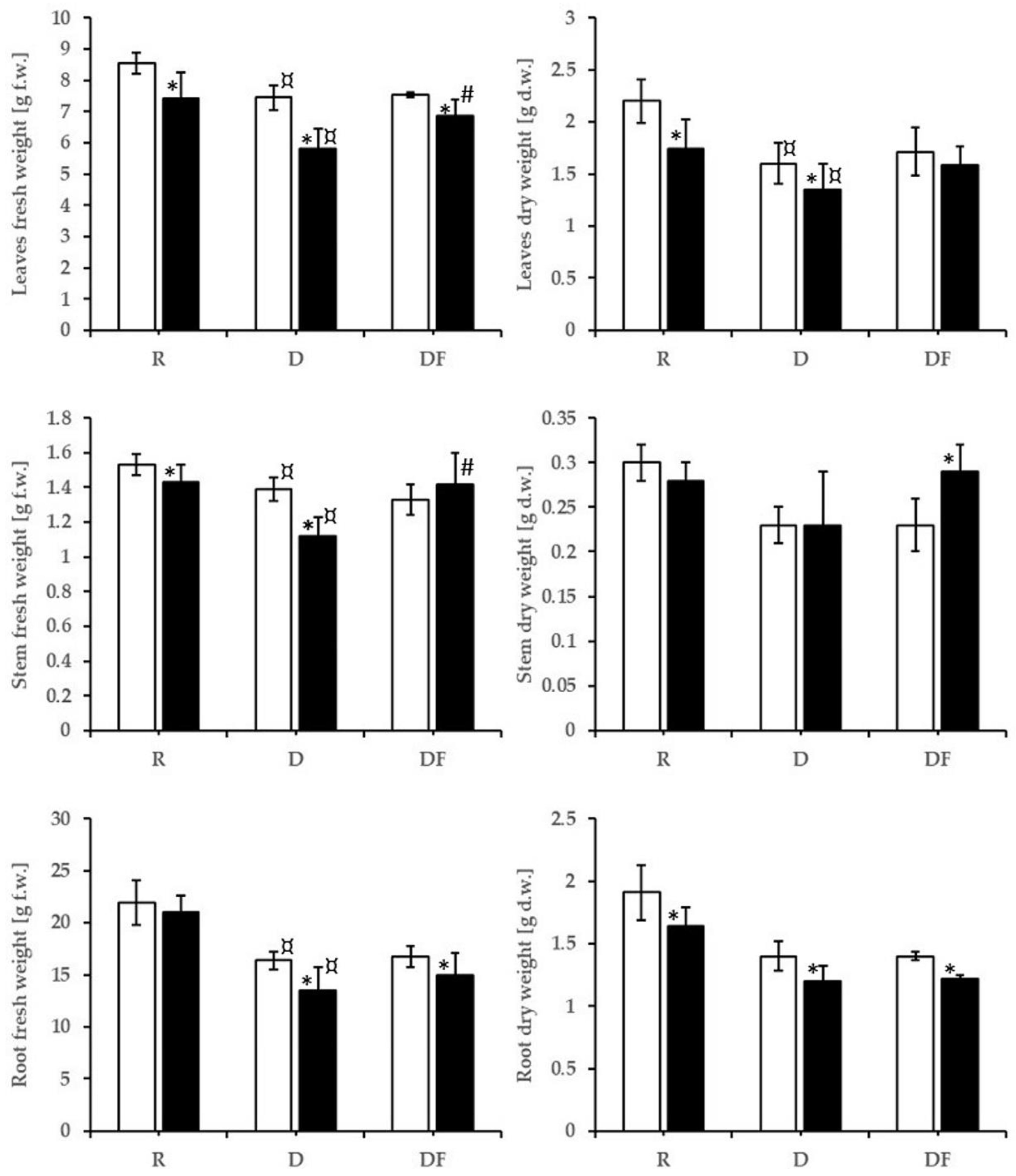
3.3. Plant Biomass
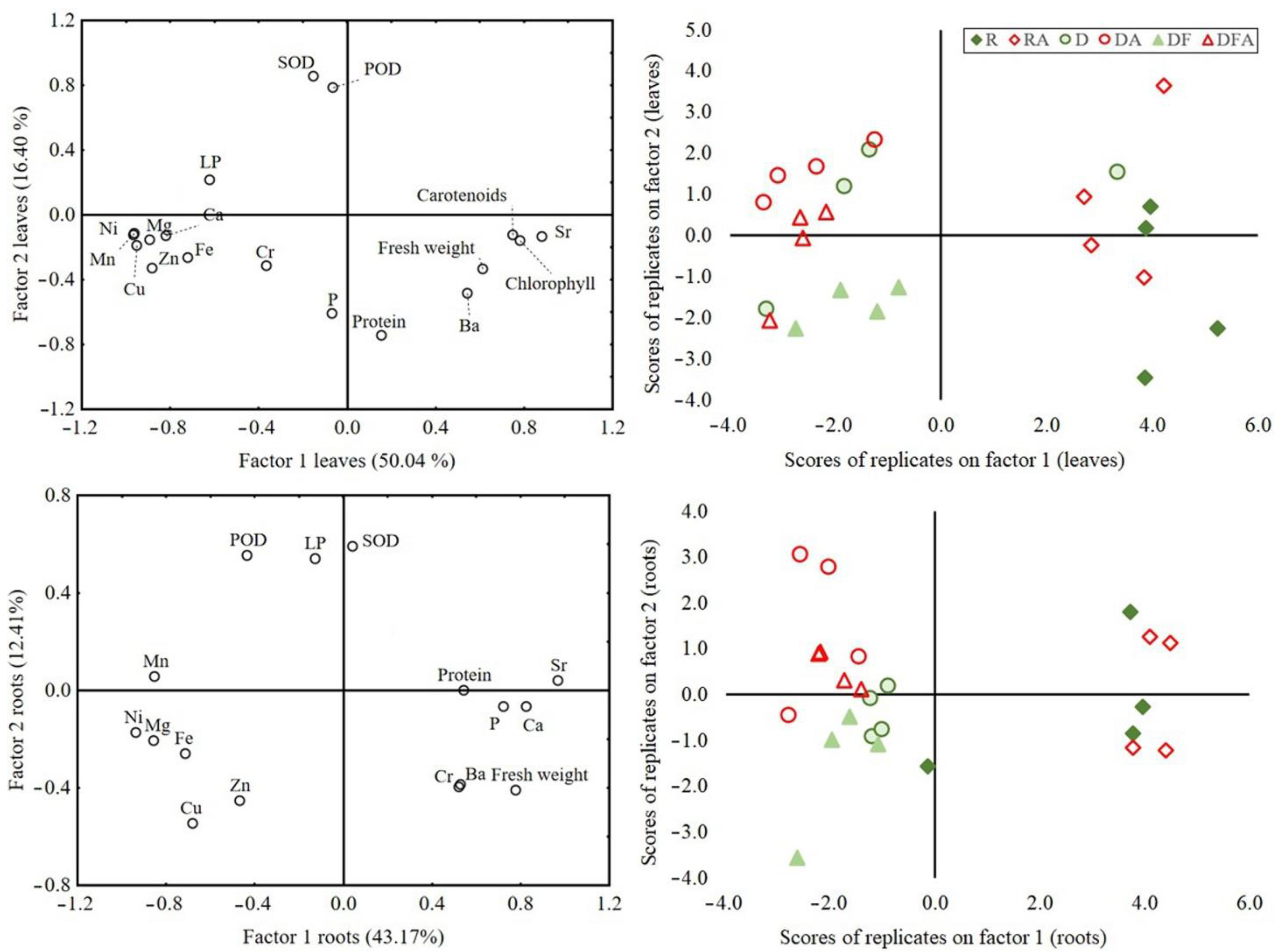
3.4. Principal Component Analysis of Plant Variables
3.5. Substrate Variables at the End of the Experiment
4. Discussions
4.1. Effects of Independent Variables: Substrate Type, Acid Water, and Commercial Inoculum
4.2. Effects on Dependent Variables: Elements, Oxidative Stress, and Biomass
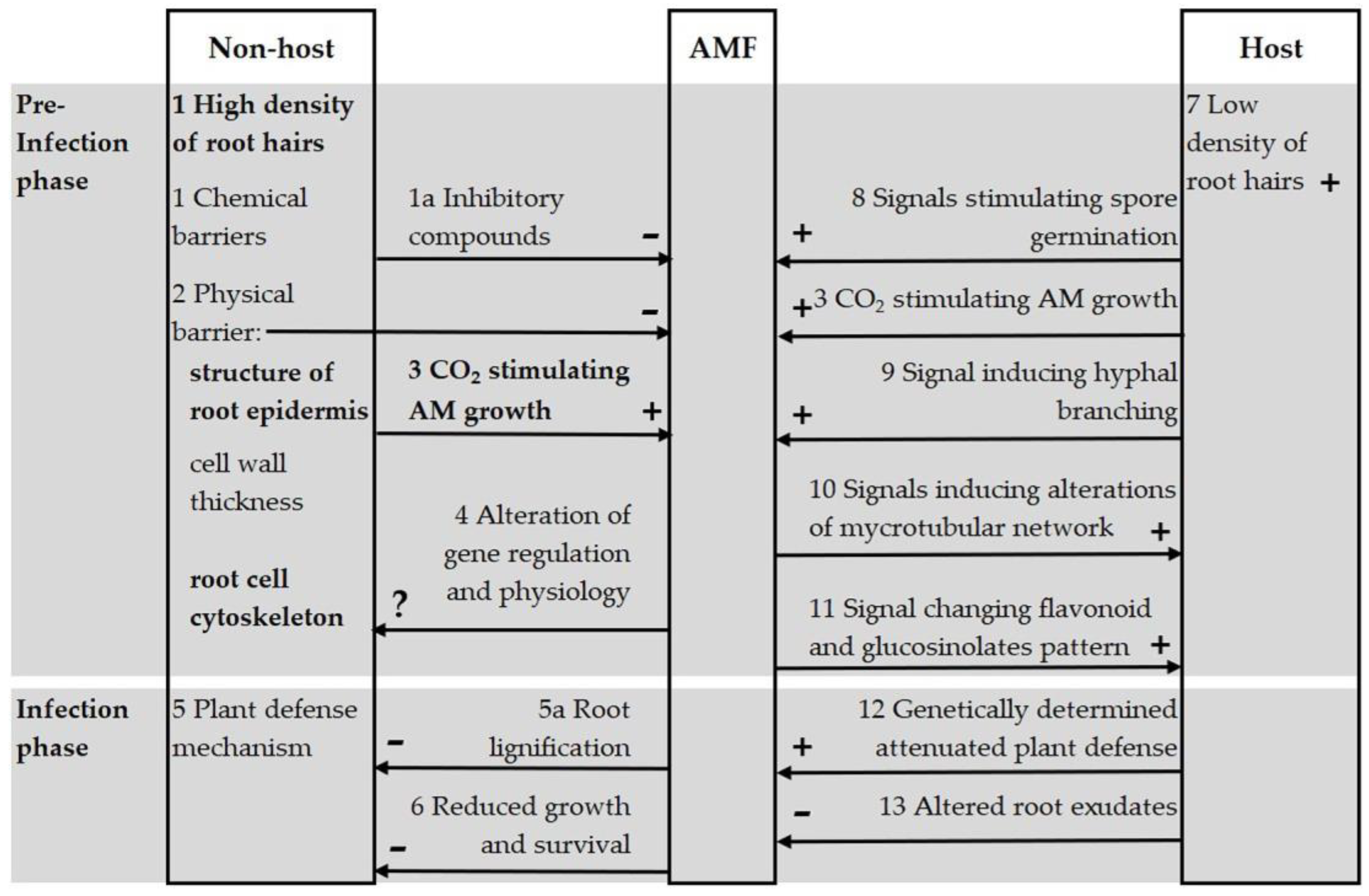
4.3. Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avio, L.; Sbrana, C.; Giovannetti, M. The response of different species of Lupinus to VAM endophytes. Symbiosis 1990, 9, 321–323. [Google Scholar]
- Oba, H.; Tawaraya, K.; Wagatsuma, T. Arbuscular mycorrhizal colonization in Lupinus and related genera. Soil Sci. Plant Nutr. 2001, 47, 685–694. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Zhang, X.L.; Su, S.X.; Lan, Z.J.; Li, K.; Wang, Y.M.; Wang, F.Y.; Chan, Y.L. Mycorrhizal relationship in lupines: A review. Legume Res.-Int. J. 2017, 40, 965–973. [Google Scholar] [CrossRef]
- Schreiner, R.P.; Koide, R.T. Stimulation of Vesicular-Arbuscular Mycorrhizal Fungi by Mycotrophic and Nonmycotrophic Plant-Root Systems. Appl. Environ. Microbiol. 1993, 59, 2750–2752. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Ehsan, M.; Santamaría-Delgado, K.; Vázquez-Alarcón, A.; Alderete-Chavez, A.; De la Cruz-Landero, N.; Jaén-Contreras, D.; Molumeli, P.A. Phytostabilization of cadmium contaminated soils by Lupinus uncinatus Schldl. Span. J. Agric. Res. 2009, 7, 390–397. [Google Scholar] [CrossRef]
- Dary, M.; Chamber-Perez, M.A.; Palomares, A.J.; Pajuelo, E. “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J. Hazard. Mater. 2010, 177, 323–330. [Google Scholar] [CrossRef]
- El Aafi, N.; Brhada, F.; Dary, M.; Maltouf, A.F.; Pajuelo, E. Rhizostabilization of metals in soils using Lupinus luteus inoculated with the metal resistant rhizobacterium Serratia sp. MSMC541. Int. J. Phytoremediation 2012, 14, 261–274. [Google Scholar] [CrossRef]
- Bishop, K.H.; Grip, H.; Oneill, A. The Origins of Acid Runoff in a Hillslope during Storm Events. J. Hydrol. 1990, 116, 35–61. [Google Scholar] [CrossRef]
- Hindar, A.; Nordstrom, D.K. Effects and quantification of acid runoff from sulfide-bearing rock deposited during construction of Highway E18, Norway. Appl. Geochem. 2015, 62, 150–163. [Google Scholar] [CrossRef]
- Wallin, J.; Karjalainen, A.K.; Schultz, E.; Jarvisto, J.; Leppanen, M.; Vuori, K.M. Weight-of-evidence approach in assessment of ecotoxicological risks of acid sulphate soils in the Baltic Sea river estuaries. Sci. Total Environ. 2015, 508, 452–461. [Google Scholar] [CrossRef]
- Lortzie, K.; Stylianou, M.; Dermatas, D.; Kostarelos, K. Long-term environmental impact at an abandoned gold–silver enrichment plant: A case study in Mitsero, Cyprus. Eng. Geol. 2015, 184, 119–125. [Google Scholar] [CrossRef]
- Ramchunder, S.J.; Voutchkova, D.D.; Estrada, E.S.; Chuah, C.J.; Evaristo, J.; Ng, D.; Cai, Y.; Koh, R.Y.T.; Ziegler, A.D. Flowpath influence on stream acid events in tropical urban streams in Singapore. Hydrol. Process. 2022, 36, e14467. [Google Scholar] [CrossRef]
- Grennfelt, P.; Engleryd, A.; Forsius, M.; Hov, O.; Rodhe, H.; Cowling, E. Acid rain and air pollution: 50 years of progress in environmental science and policy. Ambio 2020, 49, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.S. Biological Effects of Acidity in Precipitation on Vegetation—A Review. Environ. Exp. Bot. 1982, 22, 155–169. [Google Scholar] [CrossRef]
- Yadav, D.S.; Jaiswal, B.; Gautam, M.; Agrawal, M. Soil acidification and its impact on plants. In Plant Responses to Soil Pollution; Singh, P., Singh, S.K., Prasad, S.M., Eds.; Springer Nature: Singapore, 2020. [Google Scholar] [CrossRef]
- French, R.J.; Sweetingham, M.W.; Shea, G.G. A comparison of the adaptation of yellow lupin (Lupinus luteus L.) and narrow-leafed lupin (L. angustifolius L.) to acid sandplain soils in low rainfall agricultural areas of Western Australia. Aust. J. Agric. Res. 2001, 52, 945–954. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Huynh, M.; Udall, J.A.; Kilian, A.; Adhikari, K.N.; Berger, J.D.; Erskine, W.; Nelson, M.N. The first genetic map for yellow lupin enables genetic dissection of adaptation traits in an orphan grain legume crop. BMC Genet. 2019, 20, 68. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Kim, J.-Y.; Ko, M.-S.; Kim, K.-W. Acid Rain Impact on Phytoavailability of Heavy Metals in Soils. Geosystem Eng. 2010, 13, 133–138. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhang, Y.; Wang, Y.; Pei, C. Response of soil chemical properties and enzyme activity of four species in the Three Gorges Reservoir area to simulated acid rain. Ecotoxicol. Environ. Saf. 2021, 208, 111457. [Google Scholar] [CrossRef]
- Ouyang, X.-J.; Zhou, G.-Y.; Huang, Z.-L.; Liu, J.-X.; Zhang, D.-Q.; Li, J. Effect of Simulated Acid Rain on Potential Carbon and Nitrogen Mineralization in Forest Soils. Pedosphere 2008, 18, 503–514. [Google Scholar] [CrossRef]
- Liao, B.H.; Liu, H.Y.; Zeng, Q.R.; Yu, P.Z.; Probst, A.; Probst, J.L. Complex toxic effects of Cd2+, Zn2+, and acid rain on growth of kidney bean (Phaseolus vulgaris L.). Environ. Int. 2005, 31, 891–895. [Google Scholar] [CrossRef]
- Xia, L.; Shao, C.; Zhang, N.; Wu, A.; Xie, J.; Qiu, Y.; He, X.; Pei, J.; Wang, X.; Wang, Y. Improved Tolerance of Mycorrhizal Torreya grandis Seedlings to Sulfuric Acid Rain Related to Phosphorus and Zinc Contents in Shoots. J. Fungi 2021, 7, 296. [Google Scholar] [CrossRef]
- He, X.; Shao, C.; Wu, A.; Xia, L.; Li, T.; Pei, J.; Zhang, N.; Wang, Y. Arbuscular mycorrhizal fungi enhance nutrient acquisition and reduce aluminum toxicity in Lespedeza formosa under acid rain. Environ. Sci. Pollut. Res. 2022, 29, 29904–29916. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Shao, C.; Wu, A.; He, X.; Xia, L.; Wang, X.; Qiu, Y.; Yu, S.; Pei, J.; et al. Enhancement of photosynthetic parameters and growth of Zelkova serrata by arbuscular mycorrhizal fungi under simulated sulfuric acid rain. Plant Ecol. 2021, 222, 1361–1374. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Pinto, A.P.; Teixeira, D.; Brito, I.; Carvalho, M. Diversity of Native Arbuscular Mycorrhiza Extraradical Mycelium Influences Antioxidant Enzyme Activity in Wheat Grown Under Mn Toxicity. Bull. Environ. Contam. Toxicol. 2022, 108, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Malcova, R.; Vosatka, M.; Albrechtova, J. Influence of arbuscular mycorrhizal fungi and simulated acid rain on the growth and coexistence of the grasses Calamagrostis villosa and Deschampsia flexuosa. Plant Soil 1999, 207, 45–57. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Carvalho, M. The Protective Biochemical Properties of Arbuscular Mycorrhiza Extraradical Mycelium in Acidic Soils Are Maintained throughout the Mediterranean Summer Conditions. Agronomy 2021, 11, 748. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Ma, F.; Yang, D.; You, Y. Earthworm and arbuscular mycorrhiza interactions: Strategies to motivate antioxidant responses and improve soil functionality. Environ. Pollut. 2021, 272, 115980. [Google Scholar] [CrossRef]
- Farghaly, F.A.; Nafady, N.A.; Abdel-Wahab, D.A. The efficiency of arbuscular mycorrhiza in increasing tolerance of Triticum aestivum L. to alkaline stress. BMC Plant Biol. 2022, 22, 490. [Google Scholar] [CrossRef] [PubMed]
- Vosatka, M.; Batkhuugyin, E.; Albrechtova, J. Response of three arbuscular mycorrhizal fungi to simulated acid rain and aluminium stress. Biol. Plant. 1999, 42, 289–296. [Google Scholar] [CrossRef]
- Vosatka, M.; Dodd, J.C. The role of different arbuscular mycorrhizal fungi in the growth of Calamagrostis villosa and Deschampsia flexuosa, in experiments with simulated acid rain. Plant Soil 1998, 200, 251–263. [Google Scholar] [CrossRef]
- van Aarle, I.M.; Olsson, P.A.; Soderstrom, B. Arbuscular mycorrhizal fungi respond to the substrate pH of their extraradical mycelium by altered growth and root colonization. New Phytol. 2002, 155, 173–182. [Google Scholar] [CrossRef] [PubMed]
- An, G.-H.; Miyakawa, S.; Kawahara, A.; Osaki, M.; Ezawa, T. Community structure of arbuscular mycorrhizal fungi associated with pioneer grass species Miscanthus sinensis in acid sulfate soils: Habitat segregation along pH gradients. Soil Sci. Plant Nutr. 2008, 54, 517–528. [Google Scholar] [CrossRef]
- Ayut, K.; Bernard, D.; Benjavan, R. Alleviating acid soil stress in cowpea with a local population of arbuscular mycorrhizal fungi. Afr. J. Biotechnol. 2011, 10, 14410–14418. [Google Scholar] [CrossRef]
- He, L.; Xu, J.; Hu, L.; Ren, M.; Tang, J.; Chen, X. Nurse effects mediated by acid-tolerance of target species and arbuscular mycorrhizal colonization in an acid soil. Plant Soil 2019, 441, 161–172. [Google Scholar] [CrossRef]
- Foy, C. Tolerances of lupin species and genotypes to acid soil and coal mine spoil. J. Plant Nutr. 1997, 20, 1095–1118. [Google Scholar] [CrossRef]
- Neagoe, A.; Constantinescu, P.; Nicoara, A.; Onete, M.; Iordache, V. Data from experiments with tailing material and Agrostis capillaris at three scales: Pot, lysimeter and field plot. Data Brief 2020, 28, 104964. [Google Scholar] [CrossRef]
- Neagoe, A.; Ebenå, G.; Carlsson, E. The effect of soil amendments on plant performance in an area affected by acid mine drainage. Geochemistry 2005, 65, 115–129. [Google Scholar] [CrossRef]
- Neagoe, A.; Iordache, V.; Bergmann, H.; Kothe, E. Patterns of effects of arbuscular mycorrhizal fungi on plants grown in contaminated soil. J. Plant Nutr. Soil Sci. 2013, 176, 273–286. [Google Scholar] [CrossRef]
- FAO-UNESCO. Soil Map of the World; Volume 5: Europe; UNESCO: Paris, France, 1974. [Google Scholar]
- von Alten, H.; Blal, B.; Dood, J.C.; Feldmann, F.; Vosatka, M. Quality control of arbuscular mycorrhizal fungiinoculum in Europe. In Mycorrhizal Technology in Agriculture; From Genes to Bioproducts; Gianinazzi, S., Schüepp, H., Barea, J.M., Haselwandter, K., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2002. [Google Scholar]
- Zhang, T.; Yang, X.; Guo, R.; Guo, J. Response of AM fungi spore population to elevated temperature and nitrogen addition and their influence on the plant community composition and productivity. Sci. Rep. 2016, 6, 24749. [Google Scholar] [CrossRef]
- Hoffmann, G. Methodenbuch. Die Untersuchung von Böden; VDLUFA-Verlag: Darmstadt, Germany, 1991; Volume 1. [Google Scholar]
- Schopfer, P. Experimentelle Pflanzenphysiologie-Einführung in Die Anwendung; Band 2; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Lowry, O.H. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymatic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef] [PubMed]
- Lagrimini, L.M. Wound-induced deposition of polyphenols in transgenic plants overexpressing peroxidase. Plant Physiol. 1991, 96, 577–583. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Mascher, R.; Nagy, E.; Lippmann, B.; Hörnlein, S.; Fischer, S.; Scheiding, W.; Neagoe, A.; Bergmann, H. Improvement of tolerance to paraquat and drought in barley (Hordeum vulgare L.) by exogenous 2-aminoethanol: Effects on superoxide dismutase activity and chloroplast ultrastructure. Plant Sci. 2005, 168, 691–698. [Google Scholar] [CrossRef]
- Schmitz, O.; Danneberg, G.; Hundeshagen, B.; Klingner, A.; Bothe, H. Quantification of Vesicular-Arbuscular Mycorrhiza by Biochemical Parameters. J. Plant Physiol. 1991, 139, 106–114. [Google Scholar] [CrossRef]
- Mains, D.; Craw, D.; Rufaut, C.G.; Smith, C.M.S. Phytostabilization of gold mine tailings from New Zealand. Part 2: Experimental evaluation of arsenic mobilization during revegetation. Int. J. Phytoremediation 2006, 8, 163–183. [Google Scholar] [CrossRef]
- Pszczolkowska, A.; Okorski, A.; Olszewski, J.; FordoŃSki, G.; Krzebietke, S.; ChareŃSka, A. Effects of pre-preceding leguminous crops on yield and chemical composition of winter wheat grain. Plant Soil Environ. 2018, 64, 592–596. [Google Scholar] [CrossRef]
- Zhong, H.; Lambers, H.; Wong, W.S.; Dixon, K.W.; Stevens, J.C.; Cross, A.T. Initiating pedogenesis of magnetite tailings using Lupinus angustifolius (narrow-leaf lupin) as an ecological engineer to promote native plant establishment. Sci. Total Environ. 2021, 788, 147622. [Google Scholar] [CrossRef]
- Shafer, S.R.; Schoeneberger, M.M.; Horton, S.J.; Davey, C.B.; Miller, J.E. Effects of rhizobium, arbuscular mycorrhizal fungi and anion content of simulated rain on subterranean clover. Environ. Pollut. 1996, 92, 55–66. [Google Scholar] [CrossRef]
- Shafer, S.; Bruck, R.; Heagle, A. Influence of simulated acidic rain on Phytophthora cinnamomi and Phytophthora root rot of blue lupine. Phytopathology 1985, 75, 996–1003. [Google Scholar] [CrossRef]
- Campbell, C.L.; Bruck, R.I.; Sinn, J.P.; Martin, S.B. Influence of acidity level in simulated rain on disease progress in four plant pathosystems. Environ. Pollut. 1988, 53, 219–234. [Google Scholar] [CrossRef]
- Shafer, S.R. Influence of ozone and simulated acidic rain on microorganisms in the rhizosphere of Sorghum. Environ. Pollut. 1988, 51, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Shafer, S.R. Responses of microbial populations in the rhizosphere to deposition of simulated acidic rain onto foliage and/or soil. Environ. Pollut. 1992, 76, 267–278. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Hua, Z.; Chen, H.; Liu, Y.; Li, Y.; Zhang, Z. Effects of simulated acid rain on rhizosphere microorganisms of invasive Alternanthera philoxeroides and native Alternanthera sessilis. Front. Microbiol. 2022, 13, 993147. [Google Scholar] [CrossRef]
- Moller, L.; Kessler, K.D.; Steyn, A.; Valentine, A.J.; Botha, A. The role of Cryptococcus laurentii and mycorrhizal fungi in the nutritional physiology of Lupinus angustifolius L. hosting N2-fixing nodules. Plant Soil 2016, 409, 345–360. [Google Scholar] [CrossRef]
- Elliott, A.J.; Daniell, T.J.; Cameron, D.D.; Field, K.J. A commercial arbuscular mycorrhizal inoculum increases root colonization across wheat cultivars but does not increase assimilation of mycorrhiza-acquired nutrients. Plants People Planet 2021, 3, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, E.; Turrini, A.; Gamper, H.A.; Cafa, G.; Bonari, E.; Young, J.P.W.; Giovannetti, M. Establishment, persistence and effectiveness of arbuscular mycorrhizal fungal inoculants in the field revealed using molecular genetic tracing and measurement of yield components. New Phytol. 2012, 194, 810–822. [Google Scholar] [CrossRef]
- Zhong, H.; Wong, W.S.; Zhou, J.; Cross, A.; Lambers, H. Growth of Native Plants on Mined Materials was not Improved by a Commercial Microbial Inoculant. Res. Sq. 2022; preprint. [Google Scholar] [CrossRef]
- Wisłocka, M.; Krawczyk, J.; Klink, A.; Morrison, L. Bioaccumulation of Heavy Metals by Selected Plant Species from Uranium Mining Dumps in the Sudety Mts., Poland. Pol. J. Environ. Stud. 2006, 15, 811–818. [Google Scholar]
- Menon, M.; Hermle, S.; Günthardt-Goerg, M.S.; Schulin, R. Effects of heavy metal soil pollution and acid rain on growth and water use efficiency of a young model forest ecosystem. Plant Soil 2007, 297, 171–183. [Google Scholar] [CrossRef]
- Tamás, J.; Kovács, E. Vegetation pattern and heavy metal accumulation at a mine tailing at Gyöngyösoroszi, Hungary. Z. Für Nat. C 2005, 60, 362–368. [Google Scholar] [CrossRef]
- Drab, M.; Greinert, A. Calcium content in post-mining grounds in the Łęknica region. Civ. Environ. Eng. Rep. 2010, 4, 46–57. [Google Scholar]
- Spychalski, W.; Głowacki, A.; Woszczyk, M. Potassium contents in post-mining soils after 35-year-long field experiment: The effect of physical-chemical composition of soils. J. Res. Appl. Agric. Eng. 2016, 61, 160–163. [Google Scholar]
- Brennan, R.; Bolland, M.; Bowden, J. Potassium deficiency, and molybdenum deficiency and aluminium toxicity due to soil acidification, have become problems for cropping sandy soils in south-western Australia. Aust. J. Exp. Agric. 2004, 44, 1031–1039. [Google Scholar] [CrossRef]
- Paramisparam, P.; Ahmed, O.H.; Omar, L.; Ch’ng, H.Y.; Johan, P.D.; Hamidi, N.H. Co-Application of Charcoal and Wood Ash to Improve Potassium Availability in Tropical Mineral Acid Soils. Agronomy 2021, 11, 2081. [Google Scholar] [CrossRef]
- Chen, J.-H.; Barber, S.A. Soil pH and Phosphorus and Potassium Uptake by Maize Evaluated with an Uptake Model. Soil Sci. Soc. Am. J. 1990, 54, 1032–1036. [Google Scholar] [CrossRef]
- Prajapati, K.; Modi, H. The importance of potassium in plant growth—A review. Indian J. Plant Sci. 2012, 1, 177–186. [Google Scholar]
- Kleeberg, A.; Schapp, A.; Biemelt, D. Phosphorus and iron erosion from non-vegetated sites in a post-mining landscape, Lusatia, Germany: Impact on aborning mining lakes. Catena 2008, 72, 315–324. [Google Scholar] [CrossRef]
- Lambers, H.; Clements, J.C.; Nelson, M.N. How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am. J. Bot. 2013, 100, 263–288. [Google Scholar] [CrossRef]
- Akay, A.; Yorgancilar, M.; Atalay, E. Effects of different types of mycorrhiza on the development and the elemental content of lupin (Lupinus albus L.). J. Elem. 2016, 21, 327–335. [Google Scholar]
- Rahman, M.A.; Lee, S.H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.W. Importance of Mineral Nutrition for Mitigating Aluminum Toxicity in Plants on Acidic Soils: Current Status and Opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef] [PubMed]
- Bouray, M.; Moir, J.L.; Lehto, N.J.; Condron, L.M.; Touhami, D.; Hummel, C. Soil pH effects on phosphorus mobilization in the rhizosphere of Lupinus angustifolius. Plant Soil 2021, 469, 387–407. [Google Scholar] [CrossRef]
- Bouray, M.; Moir, J.L.; Condron, L.M.; Lehto, N.J. Lime-Induced pH Elevation Influences Phosphorus Biochemical Processes and Dynamics in the Rhizosphere of Lupinus polyphyllus and Lupinus angustifolius. J. Soil Sci. Plant Nutr. 2021, 21, 1978–1992. [Google Scholar] [CrossRef]
- He, S.-Y.; Gao, Y.-J.; Shentu, J.-L.; Chen, K.-B. Combined effects of copper and simulated acid rain on copper accumulation, growth, and antioxidant enzyme activities of Rumex acetosa. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2011, 22, 481–487. [Google Scholar]
- O’Dell, T.; Trappe, J. Root endophytes of lupin and some other legumes in Northwestern USA. New Phytol. 1992, 122, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Harrower, J.T.; Gilbert, G.S. Parasitism to mutualism continuum for Joshua trees inoculated with different communities of arbuscular mycorrhizal fungi from a desert elevation gradient. PLoS ONE 2021, 16, e0256068. [Google Scholar] [CrossRef]
- Johnson, N.C.; Gehring, C.A. Mycorrhizas: Symbiotic mediators of rhizosphere and ecosystem processes. In The Rhizosphere; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Trinick, M. Vesicular-arbuscular infection and soil phosphorus utilization in Lupinus spp. New Phytol. 1977, 78, 297–304. [Google Scholar] [CrossRef]
- Vierheilig, H.; Bago, B. Host and non-host impact on the physiology of the AM symbiosis. In In Vitro Culture of Mycorrhizas; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Hinsinger, P.; Plassard, C.; Tang, C.X.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Gardner, W.K.; Parbery, D.G.; Barber, D.A. Proteoid root morphology and function in Lupinus albus. Plant Soil 1981, 60, 143–147. [Google Scholar] [CrossRef]
- Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Giovannetti, M.; Sbrana, C. Meeting a non-host: The behaviour of AM fungi. Mycorrhiza 1998, 8, 123–130. [Google Scholar] [CrossRef]
- Harrison, M.J. Molecular and cellular aspects of the arbuscular mycorrhizal symbiosis. Annu. Rev. Plant Biol. 1999, 50, 361–389. [Google Scholar] [CrossRef]
- Giovannetti, M. Survival strategies in arbuscular mycorrhizal symbionts. In Symbiosis: Mechanisms and Model Systems; Seckbach, J., Ed.; Kluwer Academic Press: Dordrecht, The Netherlands, 2002. [Google Scholar]
- McGonigle, T.P.; Miller, M.H. Winter survival of extraradical hyphae and spores of arbuscular mycorrhizal fungi in the field. Appl. Soil Ecol. 1999, 12, 41–50. [Google Scholar] [CrossRef]
- Joner, E.J.; van Aarle, I.M.; Vosatka, M. Phosphatase activity of extra-radical arbuscular mycorrhizal hyphae: A review. Plant Soil 2000, 226, 199–210. [Google Scholar] [CrossRef]
- Villegas, J.; Fortin, J. Phosphorus solubilization and pH changes as a result of the interactions between soil bacteria and arbuscular mycorrhizal fungi on a medium containing NO3-as nitrogen source. Can. J. Bot. 2002, 80, 571–576. [Google Scholar] [CrossRef]
- Artursson, V.; Finlay, R.D.; Jansson, J.K. Combined bromodeoxyuridine immunocapture and terminal-restriction fragment length polymorphism analysis highlights differences in the active soil bacterial metagenome due to Glomus mosseae inoculation or plant species. Environ. Microbiol. 2005, 7, 1952–1966. [Google Scholar] [CrossRef]
- Bianciotto, V.; Minerdi, D.; Perotto, S.; Bonfante, P. Cellular interactions between arbuscular mycorrhizal fungi and rhizosphere bacteria. Protoplasma 1996, 193, 123–131. [Google Scholar] [CrossRef]
- Kabir, Z.; OHalloran, I.P.; Hamel, C. The proliferation of fungal hyphae in soils supporting mycorrhizal and non-mycorrhizal plants. Mycorrhiza 1996, 6, 477–480. [Google Scholar] [CrossRef]
- Ocampo, J.A.; Martin, J.; Hayman, D.S. Influence of Plant Interactions on Vesicular-Arbuscular Mycorrhizal Infections .I. Host and Non-Host Plants Grown Together. New Phytol. 1980, 84, 27–35. [Google Scholar] [CrossRef]
- Vierheilig, H.; Lerat, S.; Piche, Y. Systemic inhibition of arbuscular mycorrhiza development by root exudates of cucumber plants colonized by Glomus mosseae. Mycorrhiza 2003, 13, 167–170. [Google Scholar] [CrossRef]
- Pueyo, J.J.; Quiñones, M.A.; Coba de la Peña, T.; Fedorova, E.E.; Lucas, M.M. Nitrogen and phosphorus interplay in lupin root nodules and cluster roots. Front. Plant Sci. 2021, 12, 644218. [Google Scholar] [CrossRef] [PubMed]
- Dunbabin, V.M.; McDermott, S.; Bengough, A.G. Upscaling from Rhizosphere to Whole Root System: Modelling the Effects of Phospholipid Surfactants on Water and Nutrient Uptake. Plant Soil 2006, 283, 57–72. [Google Scholar] [CrossRef]
- Egle, K.; Soliman, M.F.; Romer, W.; Gerke, J. Effect of citrate on the uptake of copper and cadmium by Lupinus albus, Lupinus luteus and Lupinus angustifolius. In Plant Nutrition—Food Security and Sustainability of Agro-Ecosystems; Horst, W.J., Ed.; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2001. [Google Scholar]
- Romer, W.; Kang, D.K.; Egle, K.; Gerke, J.; Keller, H. The acquisition of cadmium by Lupinus albus L., Lupinus angustifolius L., and Lolium multiflorum Lam. J. Plant Nutr. Soil Sci. 2000, 163, 623–628. [Google Scholar] [CrossRef]
- Dessureault-Rompre, J.; Nowack, B.; Schulin, R.; Tercier-Waeber, M.L.; Luster, J. Metal solubility and speciation in the rhizosphere of Lupinus albus cluster roots. Environ. Sci. Technol. 2008, 42, 7146–7151. [Google Scholar] [CrossRef]
- Tang, C. Factors affecting soil acidification under legumes I. Effect of potassium supply. Plant Soil 1998, 199, 275–282. [Google Scholar] [CrossRef]
- Reay, P.F.; Waugh, C. Mineral-Element Composition of Lupinus albus and Lupinus angustifolius in Relation to Manganese Accumulation. Plant Soil 1981, 60, 435–444. [Google Scholar] [CrossRef]
- Mattina, M.I.; Lannucci-Berger, W.; Musante, C.; White, J.C. Concurrent plant uptake of heavy metals and persistent organic pollutants from soil. Environ. Pollut. 2003, 124, 375–378. [Google Scholar] [CrossRef]
- Vázquez, S.; Agha, R.; Granado, A.; Sarro, M.J.; Esteban, E.; Peñalosa, J.M.; Carpena, R.O. Use of White Lupin Plant for Phytostabilization of Cd and As Polluted Acid Soil. Water Air Soil Pollut. 2006, 177, 349–365. [Google Scholar] [CrossRef]
- Martínez-Alcalá, I.; Clemente, R.; Bernal, M.P. Metal Availability and Chemical Properties in the Rhizosphere of Lupinus albus L. Growing in a High-Metal Calcareous Soil. Water Air Soil Pollut. 2008, 201, 283–293. [Google Scholar] [CrossRef]
- De Haro, A.; Pujadas, A.; Polonio, A.; Font, R.; Velez, D.; Montoro, R.; Del Rio, M. Phytoremediation of the polluted soils after the toxic spill of the Aznalcollar mine by using wild species collected in situ. Fresenius Environ. Bull. 2000, 9, 275–280. [Google Scholar]
- Brennan, R.F.; Bolland, M.D.A. Lupinas luteus cv. Wodjil takes up more phosphorus and cadmium than Lupinas angustifolius cv. Kalya. Plant Soil 2003, 248, 167–185. [Google Scholar] [CrossRef]
- Brennan, R.F.; Bolland, M.D.A.; Shea, G. Comparing how Lupinus angustifolius and Lupinus luteus use zinc fertilizer for seed production. Nutr. Cycl. Agroecosystems 2001, 59, 209–217. [Google Scholar] [CrossRef]
- Brennan, R.F.; Mann, S.S. Accumulation of cadmium by lupin species as affected by Cd application to acidic yellow sand. Water Air Soil Pollut. 2005, 167, 243–258. [Google Scholar] [CrossRef]
- Herridge, D.; Doyle, A. The narrow-leafed lupin (Lupinus angustifolius L.) as a nitrogen-fixing rotation crop for cereal production. II. Estimates of fixation by field-grown crops. Aust. J. Agric. Res. 1988, 39, 1017–1028. [Google Scholar] [CrossRef]
- McNeill, A.M.; Fillery, I.R.P. Field measurement of lupin belowground nitrogen accumulation and recovery in the subsequent cereal-soil system in a semi-arid Mediterranean-type climate. Plant Soil 2007, 302, 297–316. [Google Scholar] [CrossRef]
- Dodd, J.; Arias, I.; Koomen, I.; Hayman, D. The management of populations of vesicular-arbuscular mycorrhizal fungi in acid-infertile soils of a savanna ecosystem. Plant Soil 1990, 122, 229–240. [Google Scholar] [CrossRef]
- Stark, C.; Condron, L.M.; Stewart, A.; Di, H.J.; O’Callaghan, M. Effects of past and current crop management on soil microbial biomass and activity. Biol. Fertil. Soils 2006, 43, 531–540. [Google Scholar] [CrossRef]
- Fernández-Pascual, M.; Pueyo, J.J.; Felipe, M.; Golvano, M.P.; Lucas, M.M. Singular features of the Bradyrhizobium-Lupinus symbiosis. In Dynamic Soil, Dynamic Plant; Shima, K., Teixeira da Silva, J.A., Eds.; Global Science Books: Miki, Japan, 2007. [Google Scholar]

| Soil Variables | R | D | DF | |||
|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | |
| Soil texture | Loamy sand | Sandy loam soil (10–30% loam) | ||||
| pH (H2O) | 6.58 (composite) | 4.88 (composite) | ||||
| N-NH4+ [mg kg−1] | 38.7 (composite) | 4.58 | 2.69 | 7.03 | 3.46 | |
| N-N03− [mg kg−1] | 48.4 (composite) | 0.27 | 0.12 | 0.30 | 0.16 | |
| LOI [%] | 2.08 | 0.33 | 2.48 * | 0.20 | 2.57 | 0.13 |
| Elements [mg kg−1] | ||||||
| Al | 6908 | 265.1 | 11193 * | 177.7 | 12,443 # | 93.06 |
| As | 13.12 | 1.29 | 19.36 * | 1.26 | 20.37 | 1.64 |
| Ba | 35.15 | 2.21 | 92.79 * | 3.10 | 95.94 | 5.69 |
| Ca | 1810 | 88.49 | 2021 * | 152.0 | 1986 | 283.7 |
| Cd | 1.75 | 0.48 | 3.24 * | 0.20 | 3.05 | 0.45 |
| Co | 4.06 | 0.42 | 15.17 * | 0.49 | 14.77 | 0.38 |
| Cr | 14.74 | 0.54 | 32.42 * | 2.53 | 30.81 | 1.16 |
| Cu | 21.36 | 1.30 | 46.89 * | 0.75 | 46.02 | 1.30 |
| Fe | 7152 | 253.3 | 42272 * | 732.9 | 41549 | 730.5 |
| K | 1042 | 64.51 | 1261 * | 41.49 | 1417 # | 173.9 |
| Mg | 1395 | 51.29 | 3077 * | 43.61 | 2959 # | 29.35 |
| Mn | 246.4 | 5.94 | 713.7 * | 10.13 | 712.3 | 16.30 |
| Mo | 0.41 | 0.11 | 2.05 * | 0.26 | 2.17 | 0.24 |
| Ni | 8.55 | 1.01 | 46.06 * | 1.73 | 44.08 # | 1.48 |
| P | 376.8 | 10.01 | 489.5 * | 12.98 | 491.4 | 7.16 |
| Pb | 27.91 | 8.38 | 24.27 | 7.47 | 20.62 | 4.38 |
| Sr | 21.61 | 0.75 | 18.94 * | 1.05 | 19.35 | 1.06 |
| Ti | 159.1 | 9.18 | 410.3 * | 9.49 | 416.8 | 16.33 |
| V | 16.70 | 0.49 | 40.55 * | 0.86 | 41.11 | 1.02 |
| Zn | 93.15 | 26.29 | 77.56 | 4.94 | 60.75 # | 3.07 |
| Soil Type | Soil Code | Water Type | Number of Pots | Amount of Soil [g] | Amount of Clay Plus Fungi [g] |
|---|---|---|---|---|---|
| Reference | R | Neutral | 4 | 355 | 0 |
| R | Acidic | 4 | 355 | 0 | |
| Dump material without fungi | D | Neutral | 4 | 355 | 0 |
| D | Acidic | 4 | 355 | 0 | |
| Dump material with fungi | DF | Neutral | 4 | 320 | 35 |
| DF | Acidic | 4 | 320 | 35 |
| Treatments | Al | As | Ca | Cr | Cu | K | Mg | Mn | P | Sr | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R | 0.95 | 0.26 | 0.85 | 0.89 | 0.95 | 0.93 | 0.92 | 0.26 | |||
| RA | 0.27 | 0.80 | 0.86 | 0.93 | 0.90 | 0.88 | 0.36 | ||||
| D | 0.84 | 0.74 | 0.87 | 0.93 | 0.94 | 0.66 | |||||
| DA | 0.73 | 0.86 | 0.92 | 0.96 | 0.92 | 0.90 | 0.72 | ||||
| DF | 0.97 | 0.81 | 0.94 | 0.93 | 0.88 | ||||||
| DFA | 0.97 | 0.87 | 0.78 | 0.93 | 0.92 | 0.85 |
| Start | End of the Experiment | |||
|---|---|---|---|---|
| Substrate | Substrate | Plants | ||
| Roots | Leaves | |||
| Al | Increase | Increase | ||
| Cu | Increase | |||
| K | Increase | Increase | ||
| Mg | Decrease | Decrease | ||
| Ni | Decrease | Decrease | ||
| P | Increase | Increase | ||
| Sr | Increase | Increase | ||
| Zn | Decrease | Increase | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagoe, A.; Iordache, V. A Commercial Arbuscular Mycorrhizal Inoculum Alleviated the Effects of Acid Water on Lupinus angustifolius Grown in a Sterilized Mining Dump. Plants 2023, 12, 1983. https://doi.org/10.3390/plants12101983
Neagoe A, Iordache V. A Commercial Arbuscular Mycorrhizal Inoculum Alleviated the Effects of Acid Water on Lupinus angustifolius Grown in a Sterilized Mining Dump. Plants. 2023; 12(10):1983. https://doi.org/10.3390/plants12101983
Chicago/Turabian StyleNeagoe, Aurora, and Virgil Iordache. 2023. "A Commercial Arbuscular Mycorrhizal Inoculum Alleviated the Effects of Acid Water on Lupinus angustifolius Grown in a Sterilized Mining Dump" Plants 12, no. 10: 1983. https://doi.org/10.3390/plants12101983
APA StyleNeagoe, A., & Iordache, V. (2023). A Commercial Arbuscular Mycorrhizal Inoculum Alleviated the Effects of Acid Water on Lupinus angustifolius Grown in a Sterilized Mining Dump. Plants, 12(10), 1983. https://doi.org/10.3390/plants12101983









