Iron in the Symbiosis of Plants and Microorganisms
Abstract
1. Introduction
2. Iron Transport in Rhizobia–Legume Symbiosis
2.1. Iron Transport from Root to Nodule
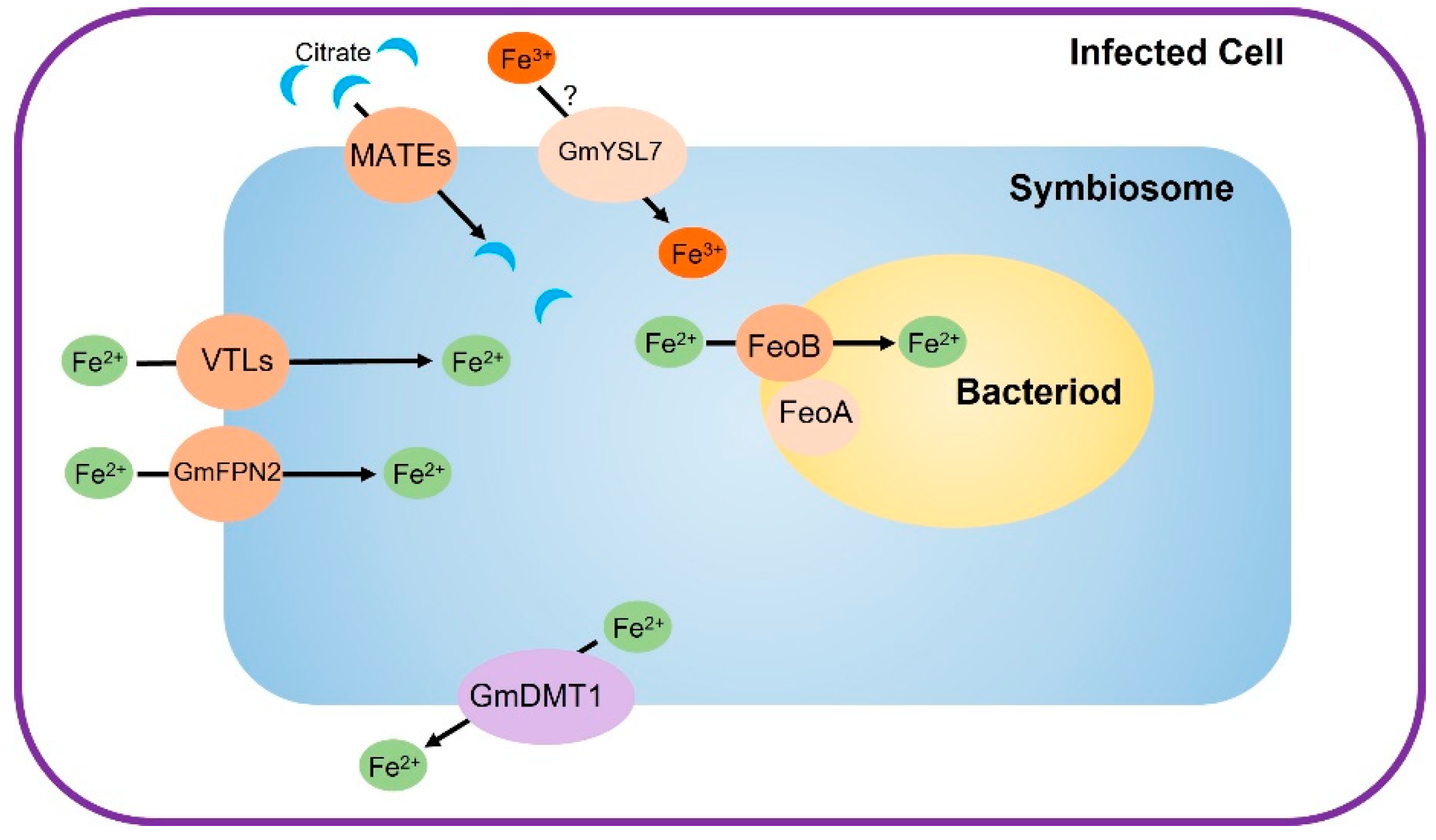
2.2. Iron Transport through the Symbiosome Membrane
2.3. Transporting of Iron into the Bacteroids
2.4. Iron Homeostasis in Rhizobia–Legume Symbiosis
3. Iron in Arbuscular Mycorrhizal Symbiosis
3.1. Iron Uptake in AM Fungi
3.2. Iron Uptake Regulation in AM Plants
3.3. Interactions of Iron and Other Nutrient Elements during AM Symbiosis
4. Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnson, L. Iron and siderophores in fungal-host interactions. Mycol. Res. 2008, 112 Pt 2, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kong, D.; Wu, H.L.; Ling, H.Q. Iron in plant-pathogen interactions. J. Exp. Bot. 2021, 72, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.C.; Garcia-Herrero, A.; Johanson, T.H.; Krewulak, K.D.; Lau, C.K.; Peacock, R.S.; Slavinskaya, Z.; Vogel, H.J. Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals 2010, 23, 601–611. [Google Scholar] [CrossRef]
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Greenshields, D.L.; Liu, G.; Wei, Y. Roles of iron in plant defence and fungal virulence. Plant Signal. Behav. 2007, 2, 300–302. [Google Scholar] [CrossRef]
- Liu, G.; Greenshields, D.L.; Sammynaiken, R.; Hirji, R.N.; Selvaraj, G.; Wei, Y. Targeted alterations in iron homeostasis underlie plant defense responses. J. Cell Sci. 2007, 120 Pt 4, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Aznar, A.; Chen, N.W.; Thomine, S.; Dellagi, A. Immunity to plant pathogens and iron homeostasis. Plant Sci. 2015, 240, 90–97. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef]
- Luginbuehl, L.H.; Menard, G.N.; Kurup, S.; Van Erp, H.; Radhakrishnan, G.V.; Breakspear, A.; Oldroyd, G.E.D.; Eastmond, P.J. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.K.; Day, D.A. Metabolite transport across symbiotic membranes of legume nodules. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 493–523. [Google Scholar] [CrossRef]
- Brear, E.M.; Day, D.A.; Smith, P.M. Iron: An essential micronutrient for the legume-rhizobium symbiosis. Front. Plant Sci. 2013, 4, 359. [Google Scholar] [CrossRef]
- Abreu, I.; Mihelj, P.; Raimunda, D. Transition metal transporters in rhizobia: Tuning the inorganic micronutrient requirements to different living styles. Metallomics 2019, 11, 735–755. [Google Scholar] [CrossRef]
- Vasse, J.; de Billy, F.; Camut, S.; Truchet, G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J. Bacteriol. 1990, 172, 4295–4306. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, L.F.; Day, D.A. The peribacteroid membrane. Physiol. Plant. 1997, 100, 30–44. [Google Scholar] [CrossRef]
- O’Hara, G.W.; Dilworth, M.J.; Boonkerd, N.; Parkpian, P. Iron-deficiency specifically limits nodule development in peanut inoculated with Bradyrhizobium sp. New Phytol. 1988, 108, 51–57. [Google Scholar] [CrossRef]
- Tang, C.; Robson, A.D.; Dilworth, M.J. The role of iron in nodulation and nitrogen fixation in Lupinus angustifolius L. New Phytol. 1990, 114, 173–182. [Google Scholar] [CrossRef]
- Burton, J.W.; Harlow, C.; Theil, E.C. Evidence for reutilization of nodule iron in soybean seed development. J. Plant Nutr. 1998, 21, 913–927. [Google Scholar] [CrossRef]
- Orozco-Mosqueda Mdel, C.; Macias-Rodriguez, L.I.; Santoyo, G.; Farias-Rodriguez, R.; Valencia-Cantero, E. Medicago truncatula increases its iron-uptake mechanisms in response to volatile organic compounds produced by Sinorhizobium meliloti. Folia Microbiol. 2013, 58, 579–585. [Google Scholar] [CrossRef]
- Sankari, S.; Babu, V.M.P.; Bian, K.; Alhhazmi, A.; Andorfer, M.C.; Avalos, D.M.; Smith, T.A.; Yoon, K.; Drennan, C.L.; Yaffe, M.B.; et al. A haem-sequestering plant peptide promotes iron uptake in symbiotic bacteria. Nat. Microbiol. 2022, 7, 1453–1465. [Google Scholar] [CrossRef]
- Cline, G.R.; Powell, P.E.; Szaniszlo, P.J.; Reid, C.P.P. Comparison of the Abilities of Hydroxamic, Synthetic, and Other Natural Organic Acids to Chelate Iron and Other Ions in Nutrient Solution. Soil Sci. Soc. Am. J. 1982, 46, 1158–1164. [Google Scholar] [CrossRef]
- Guinel, F.C. Getting around the legume nodule: I. The structure of the peripheral zone in four nodule types. Botany 2009, 87, 1117–1138. [Google Scholar] [CrossRef]
- Day, D.A.; Smith, P.M.C. Iron Transport across Symbiotic Membranes of Nitrogen-Fixing Legumes. Int. J. Mol. Sci. 2021, 22, 432. [Google Scholar] [CrossRef] [PubMed]
- Kryvoruchko, I.S.; Routray, P.; Sinharoy, S.; Torres-Jerez, I.; Tejada-Jiménez, M.; Finney, L.A.; Nakashima, J.; Pislariu, C.I.; Benedito, V.A.; González-Guerrero, M.; et al. An Iron-Activated Citrate Transporter, MtMATE67, Is Required for Symbiotic Nitrogen Fixation. Plant Physiol. 2018, 176, 2315–2329. [Google Scholar] [CrossRef]
- Takanashi, K.; Yokosho, K.; Saeki, K.; Sugiyama, A.; Sato, S.; Tabata, S.; Ma, J.F.; Yazaki, K. LjMATE1: A citrate transporter responsible for iron supply to the nodule infection zone of Lotus japonicus. Plant Cell Physiol. 2013, 54, 585–594. [Google Scholar] [CrossRef]
- Tejada-Jimenez, M.; Castro-Rodriguez, R.; Kryvoruchko, I.; Lucas, M.M.; Udvardi, M.; Imperial, J.; Gonzalez-Guerrero, M. Medicago truncatula natural resistance-associated macrophage Protein1 is required for iron uptake by rhizobia-infected nodule cells. Plant Physiol. 2015, 168, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Y.; Ni, Q.; Li, H.; Wu, X.; Yuan, Z.; Xiao, R.; Ren, Z.; Lu, J.; Yun, J.; et al. GmYSL7 controls iron uptake, allocation, and cellular response of nodules in soybean. J. Integr. Plant Biol. 2022, 65, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, R.; Abreu, I.; Reguera, M.; Novoa-Aponte, L.; Mijovilovich, A.; Escudero, V.; Jimenez-Pastor, F.J.; Abadia, J.; Wen, J.; Mysore, K.S.; et al. The Medicago truncatula Yellow Stripe1-Like3 gene is involved in vascular delivery of transition metals to root nodules. J. Exp. Bot. 2020, 71, 7257–7269. [Google Scholar] [CrossRef] [PubMed]
- Moreau, S.; Day, D.A.; Puppo, A. Ferrous iron is transported across the peribacteroid membrane of soybean nodules. Planta 1998, 207, 83–87. [Google Scholar] [CrossRef]
- Moreau, S.; Meyer, J.M.; Puppo, A. Uptake of iron by symbiosomes and bacteroids from soybean nodules. FEBS Lett. 1995, 361, 225–228. [Google Scholar] [CrossRef]
- LeVier, K.; Day, D.A.; Guerinot, M.L. Iron Uptake by Symbiosomes from Soybean Root Nodules. Plant Physiol. 1996, 111, 893–900. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Moreau, S.; Castelli, J.; Thomson, R.; Lambert, A.; Bogliolo, S.; Puppo, A.; Day, D.A. The soybean NRAMP homologue, GmDMT1, is a symbiotic divalent metal transporter capable of ferrous iron transport. Plant J. 2003, 35, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Mellor, R.B. Bacteroids in the Rhizobium-Legume Symbiosis Inhabit a Plant Internal Lytic Compartment: Implications for other Microbial Endosymbioses. J. Exp. Bot. 1989, 40, 831–839. [Google Scholar] [CrossRef]
- Kim, S.A.; Punshon, T.; Lanzirotti, A.; Li, L.; Alonso, J.M.; Ecker, J.R.; Kaplan, J.; Guerinot, M.L. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 2006, 314, 1295–1298. [Google Scholar] [CrossRef]
- Gollhofer, J.; Timofeev, R.; Lan, P.; Schmidt, W.; Buckhout, T.J. Vacuolar-Iron-Transporter1-Like proteins mediate iron homeostasis in Arabidopsis. PLoS ONE 2014, 9, e110468. [Google Scholar] [CrossRef] [PubMed]
- Connorton, J.M.; Jones, E.R.; Rodríguez-Ramiro, I.; Fairweather-Tait, S.; Uauy, C.; Balk, J. Wheat Vacuolar Iron Transporter TaVIT2 Transports Fe and Mn and Is Effective for Biofortification. Plant Physiol. 2017, 174, 2434–2444. [Google Scholar] [CrossRef]
- Liu, S.; Liao, L.L.; Nie, M.M.; Peng, W.T.; Zhang, M.S.; Lei, J.N.; Zhong, Y.J.; Liao, H.; Chen, Z.C. A VIT-like transporter facilitates iron transport into nodule symbiosomes for nitrogen fixation in soybean. New Phytol. 2020, 226, 1413–1428. [Google Scholar] [CrossRef]
- Brear, E.M.; Bedon, F.; Gavrin, A.; Kryvoruchko, I.S.; Torres-Jerez, I.; Udvardi, M.K.; Day, D.A.; Smith, P.M.C. GmVTL1a is an iron transporter on the symbiosome membrane of soybean with an important role in nitrogen fixation. New Phytol. 2020, 228, 667–681. [Google Scholar] [CrossRef]
- Libault, M.; Farmer, A.; Joshi, T.; Takahashi, K.; Langley, R.J.; Franklin, L.D.; He, J.; Xu, D.; May, G.; Stacey, G. An integrated transcriptome atlas of the crop model Glycine max, and its use in comparative analyses in plants. Plant J. 2010, 63, 86–99. [Google Scholar] [CrossRef]
- Severin, A.J.; Woody, J.L.; Bolon, Y.T.; Joseph, B.; Diers, B.W.; Farmer, A.D.; Muehlbauer, G.J.; Nelson, R.T.; Grant, D.; Specht, J.E.; et al. RNA-Seq Atlas of Glycine max: A guide to the soybean transcriptome. BMC Plant Biol. 2010, 10, 160. [Google Scholar] [CrossRef]
- Cao, J. Molecular Evolution of the Vacuolar Iron Transporter (VIT) Family Genes in 14 Plant Species. Genes 2019, 10, 144. [Google Scholar] [CrossRef]
- Walton, J.H.; Kontra-Kovats, G.; Green, R.T.; Domonkos, A.; Horvath, B.; Brear, E.M.; Franceschetti, M.; Kalo, P.; Balk, J. The Medicago truncatula Vacuolar iron Transporter-Like proteins VTL4 and VTL8 deliver iron to symbiotic bacteria at different stages of the infection process. New Phytol. 2020, 228, 651–666. [Google Scholar] [CrossRef]
- Suganuma, N.; Nakamura, Y.; Yamamoto, M.; Ohta, T.; Koiwa, H.; Akao, S.; Kawaguchi, M. The Lotus japonicus Sen1 gene controls rhizobial differentiation into nitrogen-fixing bacteroids in nodules. Mol. Genet. Genom. 2003, 269, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Escudero, V.; Abreu, I.; Tejada-Jiménez, M.; Rosa-Núñez, E.; Quintana, J.; Prieto, R.I.; Larue, C.; Wen, J.; Villanova, J.; Mysore, K.S.; et al. Medicago truncatula Ferroportin2 mediates iron import into nodule symbiosomes. New Phytol. 2020, 228, 194–209. [Google Scholar] [CrossRef]
- Curie, C.; Panaviene, Z.; Loulergue, C.; Dellaporta, S.L.; Briat, J.F.; Walker, E.L. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 2001, 409, 346–349. [Google Scholar] [CrossRef]
- Gavrin, A.; Loughlin, P.C.; Brear, E.; Griffith, O.W.; Bedon, F.; Suter Grotemeyer, M.; Escudero, V.; Reguera, M.; Qu, Y.; Mohd-Noor, S.N.; et al. Soybean Yellow Stripe-like 7 is a symbiosome membrane peptide transporter important for nitrogen fixation. Plant Physiol. 2021, 186, 581–598. [Google Scholar] [CrossRef]
- Wexler, M.; Todd, J.D.; Kolade, O.; Bellini, D.; Hemmings, A.M.; Sawers, G.; Johnston, A.W.B. Fur is not the global regulator of iron uptake genes in Rhizobium leguminosarum. Microbiology 2003, 149 Pt 5, 1357–1365. [Google Scholar] [CrossRef]
- Wexler, M.; Yeoman, K.H.; Stevens, J.B.; de Luca, N.G.; Sawers, G.; Johnston, A.W. The Rhizobium leguminosarum tonB gene is required for the uptake of siderophore and haem as sources of iron. Mol. Microbiol. 2001, 41, 801–816. [Google Scholar] [CrossRef]
- Lynch, D.; O’Brien, J.; Welch, T.; Clarke, P.; Cuív, P.O.; Crosa, J.H.; O’Connell, M. Genetic organization of the region encoding regulation, biosynthesis, and transport of rhizobactin 1021, a siderophore produced by Sinorhizobium meliloti. J. Bacteriol. 2001, 183, 2576–2585. [Google Scholar] [CrossRef] [PubMed]
- Nienaber, A.; Hennecke, H.; Fischer, H.M. Discovery of a haem uptake system in the soil bacterium Bradyrhizobium japonicum. Mol. Microbiol. 2001, 41, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Sestok, A.E.; Linkous, R.O.; Smith, A.T. Toward a mechanistic understanding of Feo-mediated ferrous iron uptake. Metallomics 2018, 10, 887–898. [Google Scholar] [CrossRef]
- Cartron, M.L.; Maddocks, S.; Gillingham, P.; Craven, C.J.; Andrews, S.C. Feo—Transport of ferrous iron into bacteria. Biometals 2006, 19, 143–157. [Google Scholar] [CrossRef]
- Sankari, S.; O’Brian, M.R. The Bradyrhizobium japonicum Ferrous Iron Transporter FeoAB Is Required for Ferric Iron Utilization in Free Living Aerobic Cells and for Symbiosis. J. Biol. Chem. 2016, 291, 15653–15662. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Sha, Z.; Maruyama, H.; Yang, L.; Pan, G.; Xue, L.; Watanabe, T. Metabolic reprogramming in nodules, roots, and leaves of symbiotic soybean in response to iron deficiency. Plant Cell Environ. 2019, 42, 3027–3043. [Google Scholar] [CrossRef]
- Jin, C.W.; Ye, Y.Q.; Zheng, S.J. An underground tale: Contribution of microbial activity to plant iron acquisition via ecological processes. Ann. Bot. 2014, 113, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Soerensen, K.U.; Terry, R.E.; Jolley, V.D.; Brown, J.C.; Vargas, M.E. The interaction of iron-stress response and root nodules in iron efficient and inefficient soybeans. J. Plant Nutr. 1988, 11, 853–862. [Google Scholar] [CrossRef]
- Terry, R.E.; Hartzook, A.; Jolley, V.D.; Brown, J.C. Interactions of iron nutrition and symbiotic nitrogen fixation in peanuts. J. Plant Nutr. 1988, 11, 811–820. [Google Scholar] [CrossRef]
- Derylo, M.; Skorupska, A. Rhizobial siderophore as an iron source for clover. Physiol. Plant. 1992, 85, 549–553. [Google Scholar] [CrossRef]
- Jin, C.W.; You, G.Y.; He, Y.F.; Tang, C.; Wu, P.; Zheng, S.J. Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol. 2007, 144, 278–285. [Google Scholar] [CrossRef]
- Slatni, T.; Dell’Orto, M.; Ben Salah, I.; Vigani, G.; Smaoui, A.; Gouia, H.; Zocchi, G.; Abdelly, C. Immunolocalization of H(+)-ATPase and IRT1 enzymes in N(2)-fixing common bean nodules subjected to iron deficiency. J. Plant Physiol. 2012, 169, 242–248. [Google Scholar] [CrossRef]
- Ling, H.Q.; Bauer, P.; Bereczky, Z.; Keller, B.; Ganal, M. The tomato fer gene encoding a bHLH protein controls iron-uptake responses in roots. Proc. Natl. Acad. Sci. USA 2002, 99, 13938–13943. [Google Scholar] [CrossRef]
- Yuan, Y.X.; Zhang, J.; Wang, D.W.; Ling, H.Q. AtbHLH29 of Arabidopsis thaliana is a functional ortholog of tomato FER involved in controlling iron acquisition in strategy I plants. Cell Res. 2005, 15, 613–621. [Google Scholar] [CrossRef]
- Du, J.; Huang, Z.; Wang, B.; Sun, H.; Chen, C.; Ling, H.Q.; Wu, H. SlbHLH068 interacts with FER to regulate the iron-deficiency response in tomato. Ann. Bot. 2015, 116, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, H.; Wang, N.; Li, J.; Zhao, W.; Du, J.; Wang, D.; Ling, H.Q. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res. 2008, 18, 385–397. [Google Scholar] [CrossRef]
- Harrison, M.J. Cellular programs for arbuscular mycorrhizal symbiosis. Curr. Opin. Plant Biol. 2012, 15, 691–698. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. 1—The symbionts forming arbuscular mycorrhizas. In Mycorrhizal Symbiosis, 3rd ed.; Smith, S.E., Read, D., Eds.; Academic Press: London, UK, 2008; pp. 13–41. [Google Scholar]
- Ho-Plágaro, T.; García-Garrido, J.M. Molecular Regulation of Arbuscular Mycorrhizal Symbiosis. Int. J. Mol. Sci. 2022, 23, 5960. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Ren, Y.; Chen, A.; Yang, C.; Zheng, Q.; Chen, J.; Wang, D.; Li, Y.; Hu, S.; Xu, G. Plant nitrogen nutrition: The roles of arbuscular mycorrhizal fungi. J. Plant Physiol. 2022, 269, 153591. [Google Scholar] [CrossRef] [PubMed]
- Luginbuehl, L.H.; Oldroyd, G.E.D. Understanding the Arbuscule at the Heart of Endomycorrhizal Symbioses in Plants. Curr. Biol. 2017, 27, R952–R963. [Google Scholar] [CrossRef] [PubMed]
- Bago, B.; Pfeffer, P.E.; Abubaker, J.; Jun, J.; Allen, J.W.; Brouillette, J.; Douds, D.D.; Lammers, P.J.; Shachar-Hill, Y. Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiol. 2003, 131, 1496–1507. [Google Scholar] [CrossRef]
- Clark, R.B.; Zeto, S.K. Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 2000, 23, 867–902. [Google Scholar] [CrossRef]
- Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops—A meta-analysis. Soil Biol. Biochem. 2015, 81, 147–158. [Google Scholar] [CrossRef]
- Caris, C.; Hördt, W.; Hawkins, H.-J.; Römheld, V.; George, E. Studies of iron transport by arbuscular mycorrhizal hyphae from soil to peanut and sorghum plants. Mycorrhiza 1998, 8, 35–39. [Google Scholar] [CrossRef]
- Kabir, A.H.; Debnath, T.; Das, U.; Prity, S.A.; Haque, A.; Rahman, M.M.; Parvez, M.S. Arbuscular mycorrhizal fungi alleviate Fe-deficiency symptoms in sunflower by increasing iron uptake and its availability along with antioxidant defense. Plant Physiol. Biochem. 2020, 150, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.A.B.; Clark, R.B.; Ellis, J.R. Effects of mes [2(n-morpholino)-ethanesulfonic acid] and ph on mineral nutrient uptake by mycor-rhizal and nonmycorrhizal maize. J. Plant Nutr. 1993, 16, 2255–2272. [Google Scholar] [CrossRef]
- Medeiros, C.A.B.; Clark, R.B.; Ellis, J.R. Growth and nutrient uptake of sorghum cultivated with vesicular-arbuscular mycorrhiza isolates at varying pH. Mycorrhiza 1994, 4, 185–191. [Google Scholar] [CrossRef]
- Raju, P.S.; Clark, R.B.; Ellis, J.R.; Maranville, J.W. Effects of species of VA-mycorrhizal fungi on growth and mineral uptake of sorghum at different temperatures. Plant Soil 1990, 121, 165–170. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Clark, R.B. Growth, mineral acquisition, and water use by mycorrhizal wheat grown under water stress. J. Plant Nutr. 1998, 21, 263–276. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Al-Raddad, A.; Clark, R.B. Water stress and mycorrhizal isolate effects on growth and nutrient acquisition of wheat. J. Plant Nutr. 1998, 21, 891–902. [Google Scholar] [CrossRef]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef]
- Kobae, Y.; Tomioka, R.; Tanoi, K.; Kobayashi, N.I.; Ohmori, Y.; Nishida, S.; Fujiwara, T. Selective induction of putative iron transporters, OPT8a and OPT8b, in maize by mycorrhizal colonization. Soil Sci. Plant Nutr. 2014, 60, 843–847. [Google Scholar] [CrossRef]
- Tamayo, E.; Gómez-Gallego, T.; Azcón-Aguilar, C.; Ferrol, N. Genome-wide analysis of copper, iron and zinc transporters in the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front. Plant Sci. 2014, 5, 547. [Google Scholar] [CrossRef] [PubMed]
- López-Lorca, V.M.; Molina-Luzón, M.J.; Ferrol, N. Characterization of the NRAMP Gene Family in the Arbuscular Mycorrhizal Fungus Rhizophagus irregularis. J. Fungi 2022, 8, 592. [Google Scholar] [CrossRef]
- Li, M.; Wang, R.; Tian, H.; Gao, Y. Transcriptome responses in wheat roots to colonization by the arbuscular mycorrhizal fungus Rhizophagus irregularis. Mycorrhiza 2018, 28, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhong, X.; Li, M.; Yang, X.; Abou Elwafa, S.F.; Albaqami, M.; Tian, H. Genome-wide analyses of the Nodulin-like gene family in bread wheat revealed its potential roles during arbuscular mycorrhizal symbiosis. Int. J. Biol. Macromol. 2022, 201, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Haselwandter, K.; Haas, H.; Haninger, G.; Winkelmann, G. Siderophores in plant root tissue: Tagetes patula nana colonized by the arbuscular mycorrhizal fungus Gigaspora margarita. Biometals 2020, 33, 137–146. [Google Scholar] [CrossRef]
- Rahman, M.A.; Parvin, M.; Das, U.; Ela, E.J.; Lee, S.H.; Lee, K.W.; Kabir, A.H. Arbuscular Mycorrhizal Symbiosis Mitigates Iron (Fe)-Deficiency Retardation in Alfalfa (Medicago sativa L.) through the Enhancement of Fe Accumulation and Sulfur-Assisted Antioxidant Defense. Int. J. Mol. Sci. 2020, 21, 2219. [Google Scholar] [CrossRef]
- Prity, S.A.; Sajib, S.A.; Das, U.; Rahman, M.M.; Haider, S.A.; Kabir, A.H. Arbuscular mycorrhizal fungi mitigate Fe deficiency symptoms in sorghum through phytosiderophore-mediated Fe mobilization and restoration of redox status. Protoplasma 2020, 257, 1373–1385. [Google Scholar] [CrossRef]
- Chorianopoulou, S.N.; Saridis, Y.I.; Dimou, M.; Katinakis, P.; Bouranis, D.L. Arbuscular mycorrhizal symbiosis alters the expression patterns of three key iron homeostasis genes, ZmNAS1, ZmNAS3, and ZmYS1, in S deprived maize plants. Front. Plant Sci. 2015, 6, 257. [Google Scholar] [CrossRef]
- Vasconcelos, M.W.; Li, G.W.; Lubkowitz, M.A.; Grusak, M.A. Characterization of the PT Clade of Oligopeptide Transporters in Rice. Plant Genome 2008, 1, 77–88. [Google Scholar] [CrossRef]
- Xie, X.; Hu, W.; Fan, X.; Chen, H.; Tang, M. Interactions Between Phosphorus, Zinc, and Iron Homeostasis in Nonmycorrhizal and Mycorrhizal Plants. Front. Plant Sci. 2019, 10, 1172. [Google Scholar] [CrossRef]
- Azcón, R.; Ambrosano, E.; Charest, C. Nutrient acquisition in mycorrhizal lettuce plants under different phosphorus and nitrogen concentration. Plant Sci. 2003, 165, 1137–1145. [Google Scholar] [CrossRef]
- Hoseinzade, H.; Ardakani, M.R.; Shahdi, A.; Rahmani, H.A.; Noormohammadi, G.; Miransari, M. Rice (Oryza sativa L.) nutrient management using mycorrhizal fungi and endophytic Herbaspirillum seropedicae. J. Integr. Agric. 2016, 15, 1385–1394. [Google Scholar] [CrossRef]
- Andrino, A.; Guggenberger, G.; Kernchen, S.; Mikutta, R.; Sauheitl, L.; Boy, J. Production of Organic Acids by Arbuscular Mycorrhizal Fungi and Their Contribution in the Mobilization of Phosphorus Bound to Iron Oxides. Front. Plant Sci. 2021, 12, 661842. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Ryan, M.H.; Wen, Z.; Lambers, H.; Liu, Y.; Zhang, Y.; Tueux, G.; Jenkins, S.; Mickan, B.; Wong, W.S.; et al. Enhanced nodulation and phosphorus acquisition from sparingly-soluble iron phosphate upon treatment with arbuscular mycorrhizal fungi in chickpea. Physiol. Plant. 2023, 175, e13873. [Google Scholar] [CrossRef]
- Ibiang, Y.B.; Mitsumoto, H.; Sakamoto, K. Bradyrhizobia and arbuscular mycorrhizal fungi modulate manganese, iron, phosphorus, and polyphenols in soybean (Glycine max (L.) Merr.) under excess zinc. Environ. Exp. Bot. 2017, 137, 1–13. [Google Scholar] [CrossRef]
- Ibiang, Y.B.; Innami, H.; Sakamoto, K. Effect of excess zinc and arbuscular mycorrhizal fungus on bioproduction and trace element nutrition of Tomato (Solanum lycopersicum L. cv. Micro-Tom). Soil Sci. Plant Nutr. 2018, 64, 342–351. [Google Scholar] [CrossRef]
- Wittenberg, J.B.; Wittenberg, B.A.; Day, D.A.; Udvardi, M.K.; Appleby, C.A. Siderophore-bound iron in the peribacteriod space of soybean root nodules. Plant Soil 1996, 178, 161–169. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xiong, Z.; Wu, W.; Ling, H.-Q.; Kong, D. Iron in the Symbiosis of Plants and Microorganisms. Plants 2023, 12, 1958. https://doi.org/10.3390/plants12101958
Liu Y, Xiong Z, Wu W, Ling H-Q, Kong D. Iron in the Symbiosis of Plants and Microorganisms. Plants. 2023; 12(10):1958. https://doi.org/10.3390/plants12101958
Chicago/Turabian StyleLiu, Yi, Zimo Xiong, Weifeng Wu, Hong-Qing Ling, and Danyu Kong. 2023. "Iron in the Symbiosis of Plants and Microorganisms" Plants 12, no. 10: 1958. https://doi.org/10.3390/plants12101958
APA StyleLiu, Y., Xiong, Z., Wu, W., Ling, H.-Q., & Kong, D. (2023). Iron in the Symbiosis of Plants and Microorganisms. Plants, 12(10), 1958. https://doi.org/10.3390/plants12101958




