Influence of Experimental Warming on the Rate and Duration of Fruit Growth and Oil Accumulation in Young Olive Trees (cvs. Arbequina, Coratina)
Abstract
1. Introduction
2. Results
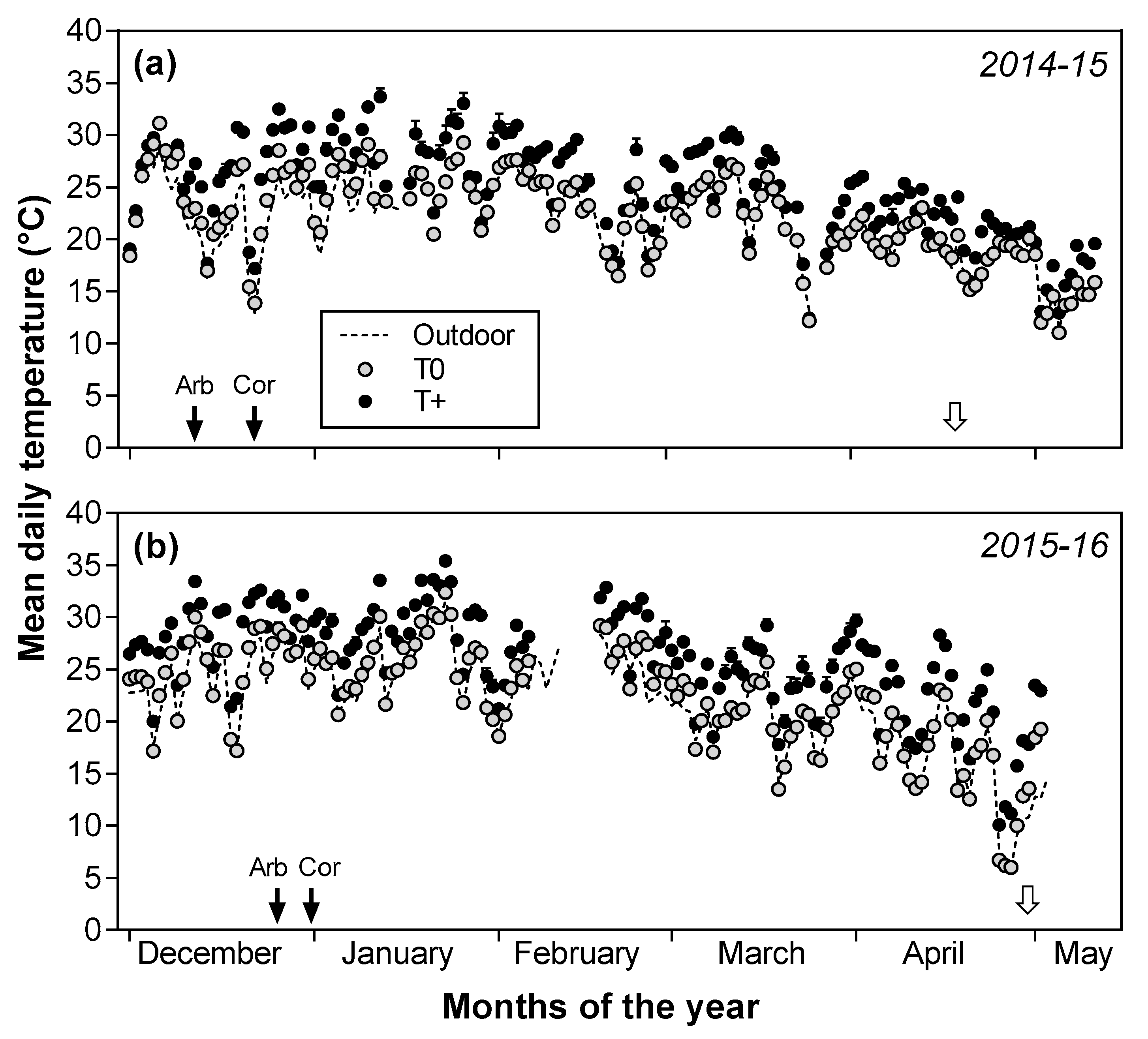
2.1. Air Temperature and Other Environmental Variables
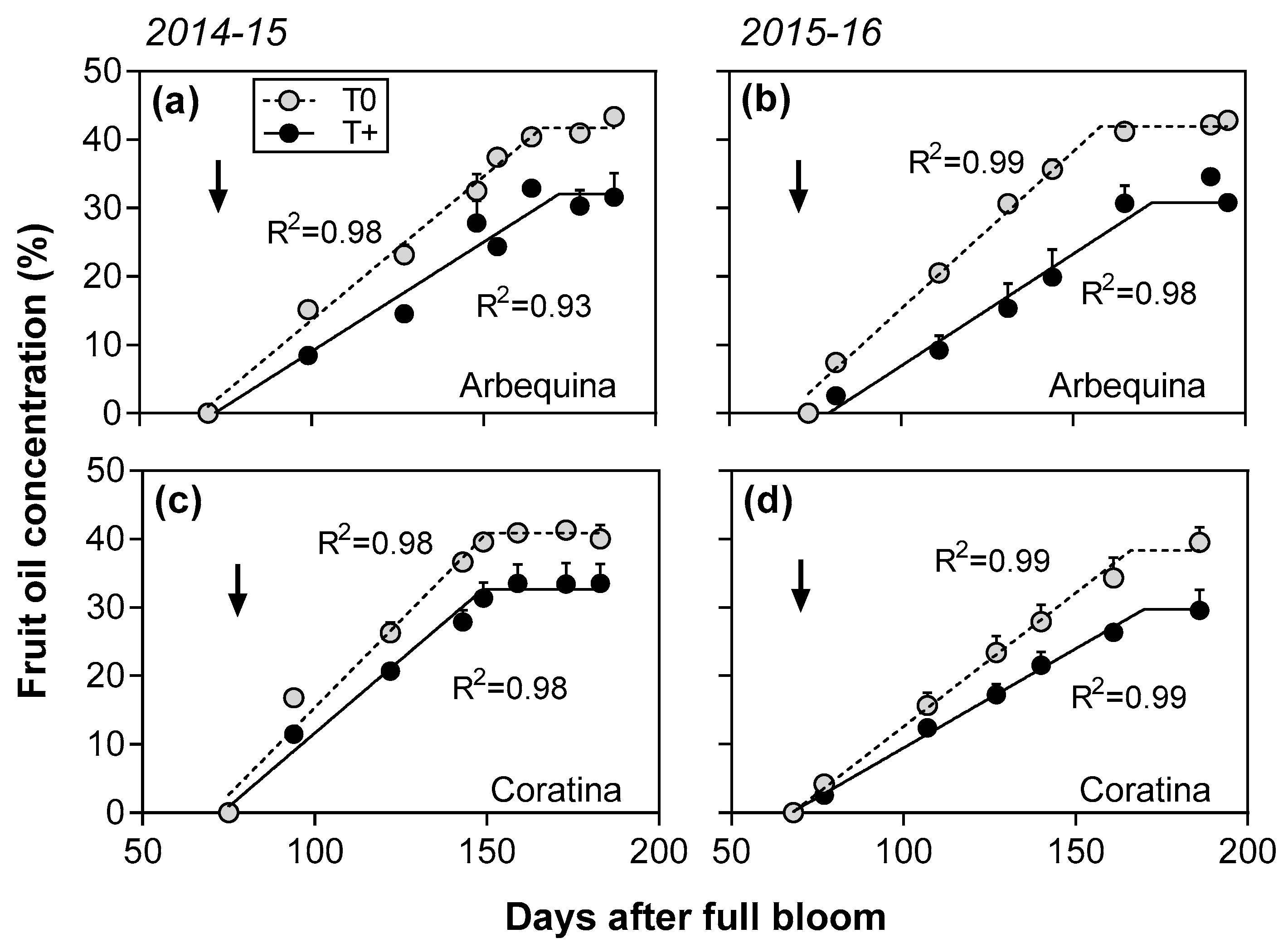
2.2. Seasonal Patterns of Fruit Growth and Oil Accumulation
| Season | Cultivar | Temp. Treatment | Fruit growth | Fruit Oil Accumulation | ||||
|---|---|---|---|---|---|---|---|---|
| Rate a (mg day−1) | Duration b (DAFB) | Maximum Weight c (g) | Rate a (% day−1) | Duration b (DAFB) | Maximum Oil c (%) | |||
| 2014–2015 | Arbequina | T0 | 6.2 ± 0.3 | 174 ± 7 | 0.91 ± 0.06 | 0.42 ± 0.01 | 167 ± 3 | 42 ± 1 |
| T+ | 4.7 ± 0.3 | 171 ± 13 | 0.72 ± 0.07 | 0.32 ± 0.02 | 172 ± 6 | 32 ± 3 | ||
| Coratina | T0 | 12.0 ± 0.7 | 148 ± 10 | 1.47 ± 0.07 | 0.51 ± 0.02 | 150 ± 5 | 41 ± 1 | |
| T+ | 9.6 ± 0.1 | 143 ± 6 | 1.16 ± 0.06 | 0.43 ± 0.01 | 149 ± 7 | 33 ± 3 | ||
| temp | ** | ns | ** | ** | ns | ** | ||
| cv | ** | * | ** | ** | ** | ns | ||
| int | ns | ns | ns | ns | ns | ns | ||
| 2015–2016 | Arbequina | T0 | 6.4 ± 0.3 b | 181 ± 6 a | 0.99 ± 0.06 | 0.46 ± 0.02 | 158 ± 4 | 42 ± 1 |
| T+ | 4.1 ± 0.6 c | 182 ± 12 a | 0.66 ± 0.04 | 0.33 ± 0.04 | 173 ± 9 | 33 ± 0 | ||
| Coratina | T0 | 13.2 ± 2.1 a | 136 ± 9 b | 1.36 ± 0.21 | 0.39 ± 0.03 | 166 ± 7 | 38 ± 2 | |
| T+ | 12.0 ± 1.0 a | 127 ± 6 c | 1.13 ± 0.03 | 0.29 ± 0.02 | 170 ± 10 | 30 ± 2 | ||
| temp | ns | ns | ** | ** | ns | ** | ||
| cv | ** | ** | * | * | ns | * | ||
| int | * | * | ns | ns | ns | ns | ||
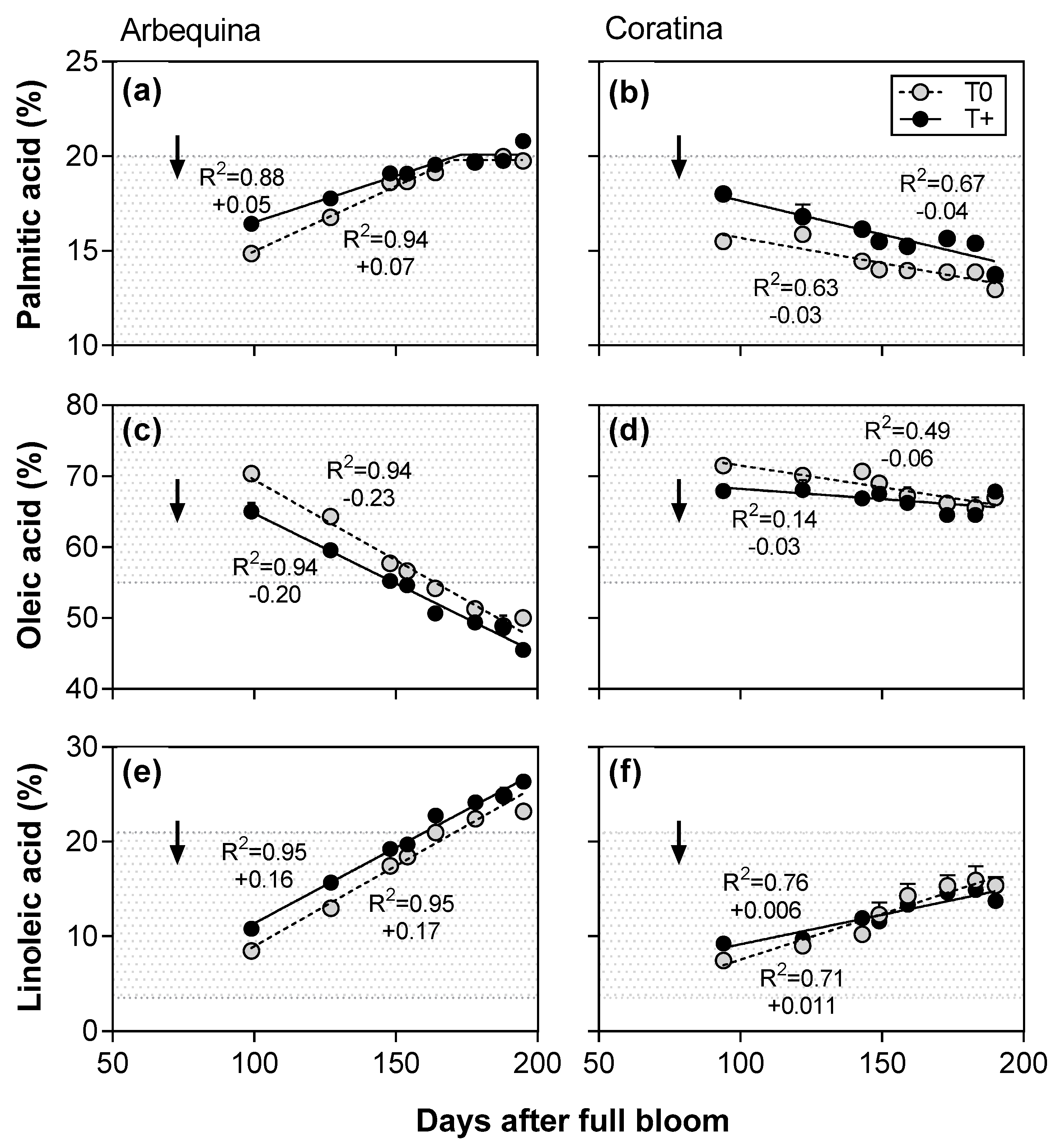
2.3. Patterns of the Main Fatty Acids during Oil Accumulation
2.4. Fruit Characteristics and Fatty Acid Composition at Harvest
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Temperature Treatments and Experimental Design
4.3. Seasonal Patterns of Fruit Growth, Oil Accumulation, and Fatty Acid Composition
4.4. Fruit Characteristics and Fatty Acid Composition at Harvest
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Besnard, G.; Terral, J.-F.; Cornille, A. On the Origins and Domestication of the Olive: A Review and Perspectives. Ann. Bot. 2018, 121, 385–403. [Google Scholar] [CrossRef]
- Gómez del Campo, M.; Morales-Sillero, A.; Vita Serman, F.; Rousseaux, M.C.; Searles, P.S. Olive Growing in the Arid Valleys of Northwest Argentina (Provinces of Catamarca, La Rioja and San Juan). Olivae 2010, 114, 23–45. [Google Scholar]
- Torres, M.; Pierantozzi, P.; Searles, P.; Rousseaux, M.C.; García-Inza, G.; Miserere, A.; Bodoira, R.; Contreras, C.; Maestri, D. Olive Cultivation in the Southern Hemisphere: Flowering, Water Requirements and Oil Quality Responses to New Crop Environments. Front. Plant. Sci. 2017, 8, 1830. [Google Scholar] [CrossRef] [PubMed]
- Rondanini, D.P.; Castro, D.N.; Searles, P.S.; Rousseaux, M.C. Contrasting Patterns of Fatty Acid Composition and Oil Accumulation during Fruit Growth in Several Olive Varieties and Locations in a Non-Mediterranean Region. Eur. J. Agron. 2014, 52, 237–246. [Google Scholar] [CrossRef]
- García-Inza, G.P.; Castro, D.N.; Hall, A.J.; Rousseaux, M.C. Responses to Temperature of Fruit Dry Weight, Oil Concentration, and Oil Fatty Acid Composition in Olive (Olea Europaea L. Var. ‘Arauco’). Eur. J. Agron. 2014, 54, 107–115. [Google Scholar] [CrossRef]
- Miserere, A.; Searles, P.S.; Rousseaux, M.C. Oil Yield Components and Biomass Production Responses to Warming during the Oil Accumulation Phase in Young Olive Trees. Sci. Hortic. 2022, 291, 110618. [Google Scholar] [CrossRef]
- Nissim, Y.; Shlosberg, M.; Biton, I.; Many, Y.; Doron-Faigenboim, A.; Hovav, R.; Kerem, Z.; Avidan, B.; Ben-Ari, G. A High Temperature Environment Regulates the Olive Oil Biosynthesis Network. Plants 2020, 9, 1135. [Google Scholar] [CrossRef]
- Garcia-Tejera, O.; Bonada, M.; Petrie, P.R.; Nieto, H.; Bellvert, J.; Sadras, V.O. Viticulture Adaptation to Global Warming: Modelling Gas Exchange, Water Status and Leaf Temperature to Probe for Practices Manipulating Water Supply, Canopy Reflectance and Radiation Load. Agric. For. Meteorol. 2023, 331, 109351. [Google Scholar] [CrossRef]
- Fraga, H.; Pinto, J.G.; Santos, J.A. Olive Tree Irrigation as a Climate Change Adaptation Measure in Alentejo, Portugal. Agric. Water Manag. 2020, 237, 106193. [Google Scholar] [CrossRef]
- Rotondi, A.; Bertazza, G.; Faccini, B.; Ferretti, G.; Morrone, L. Effect of Different Foliar Particle Films (Kaolin and Zeolitite) on Chemical and Sensory Properties of Olive Oil. Agronomy 2022, 12, 3088. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Puertas, C.M.; Sadras, V.O. Modelling the Intraspecific Variation in the Dynamics of Fruit Growth, Oil and Water Concentration in Olive (Olea Europaea L.). Eur. J. Agron. 2012, 38, 83–93. [Google Scholar] [CrossRef]
- Mousavi, S.; de la Rosa, R.; Moukhli, A.; El Riachy, M.; Mariotti, R.; Torres, M.; Pierantozzi, P.; Stanzione, V.; Mastio, V.; Zaher, H.; et al. Plasticity of Fruit and Oil Traits in Olive among Different Environments. Sci. Rep. 2019, 9, 16968. [Google Scholar] [CrossRef] [PubMed]
- Navas-Lopez, J.F.; León, L.; Trentacoste, E.R.; de la Rosa, R. Multi-Environment Evaluation of Oil Accumulation Pattern Parameters in Olive. Plant. Physiol. Biochem. 2019, 139, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Ben Rouina, Y.; Zouari, M.; Zouari, N.; Ben Rouina, B.; Bouaziz, M. Olive Tree (Olea Europaea L. Cv. Zelmati) Grown in Hot Desert Climate: Physio-Biochemical Responses and Olive Oil Quality. Sci. Hortic. 2020, 261, 108915. [Google Scholar] [CrossRef]
- Nissim, Y.; Shloberg, M.; Biton, I.; Many, Y.; Doron-Faigenboim, A.; Zemach, H.; Hovav, R.; Kerem, Z.; Avidan, B.; Ben-Ari, G. High Temperature Environment Reduces Olive Oil Yield and Quality. PLoS ONE 2020, 15, e0231956. [Google Scholar] [CrossRef]
- Benlloch-González, M.; Sánchez-Lucas, R.; Benlloch, M.; Ricardo, F.E. An Approach to Global Warming Effects on Flowering and Fruit Set of Olive Trees Growing under Field Conditions. Sci. Hortic. 2018, 240, 405–410. [Google Scholar] [CrossRef]
- Benlloch-González, M.; Sánchez-Lucas, R.; Bejaoui, M.A.; Benlloch, M.; Fernández-Escobar, R. Global Warming Effects on Yield and Fruit Maturation of Olive Trees Growing under Field Conditions. Sci. Hortic. 2019, 249, 162–167. [Google Scholar] [CrossRef]
- Miserere, A.; Searles, P.S.; García-Inza, G.P.; Rousseaux, M.C. Elevated Temperature Affects Vegetative Growth and Fruit Oil Concentration in Olive Trees (Olea Europaea). Acta Hortic. 2018, 1199, 523–528. [Google Scholar] [CrossRef]
- Miserere, A.; Searles, P.S.; Hall, A.J.; García-Inza, G.P.; Rousseaux, M.C. Complementary Active Heating Methods for Evaluating the Responses of Young Olive Trees to Warming. Sci. Hortic. 2019, 257, 108754. [Google Scholar] [CrossRef]
- Miserere, A.; Searles, P.S.; Manchó, G.; Maseda, P.H.; Rousseaux, M.C. Sap Flow Responses to Warming and Fruit Load in Young Olive Trees. Front. Plant Sci. 2019, 10, 1199. [Google Scholar] [CrossRef]
- Miserere, A.; Rousseaux, M.C.; Ploschuk, E.L.; Brizuela, M.M.; Curcio, M.H.; Zabaleta, R.; Searles, P.S. Effects of Prolonged Elevated Temperature on Leaf Gas Exchange and Other Leaf Traits in Young Olive Trees. Tree Physiol. 2021, 41, 254–268. [Google Scholar] [CrossRef]
- Beltrán, G.; del Rio, C.; Sánchez, S.; Martínez, L. Influence of Harvest Date and Crop Yield on the Fatty Acid Composition of Virgin Olive Oils from Cv. Picual. J. Agric. Food Chem. 2004, 52, 3434–3440. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, F.; Bonofiglio, T.; Romano, B.; Fornaciari, M. Qualitative and Quantitative Aspects of Olive Production in Relation to Climate in Southern Italy. Sci. Hortic. 2012, 138, 151–158. [Google Scholar] [CrossRef]
- Navas-López, J.F.; Cano, J.; de la Rosa, R.; Velasco, L.; León, L. Genotype by Environment Interaction for Oil Quality Components in Olive Tree. Sci. Hortic. 2020, 119, 126115. [Google Scholar] [CrossRef]
- Tous, J.; Romero, A.; Plana, J.; Guerrero, L.; Díaz, I.; Hermoso, J.F. Características Químico-Sensoriales de Los Aceites de Oliva «Arbequina» Obtenidos En Distintas Zonas de España. Grasas Aceites 1997, 48, 415–424. [Google Scholar] [CrossRef]
- Ceci, L.N.; Carelli, A.A. Compositional Data Review of Monovarietal Argentinean Olive Oils. In Advances in Fats and Oil Research; Tomas, M.B., Ed.; Transworld Research Network: Kerala, India, 2010; pp. 71–97. [Google Scholar]
- Mailer, R.J.; Ayton, J.; Graham, K. The Influence of Growing Region, Cultivar and Harvest Timing on the Diversity of Australian Olive Oil. J. Am. Oil Chem. Soc. 2010, 87, 877–884. [Google Scholar] [CrossRef]
- Rondanini, D.P.; Castro, D.N.; Searles, P.S.; Rousseaux, M.C. Fatty Acid Profiles of Varietal Virgin Olive Oils (Olea Europaea L.) from Mature Orchards in Warm Arid Valleys of Northwestern Argentina (La Rioja). Grasas Aceites 2011, 62, 399–409. [Google Scholar] [CrossRef]
- Zaninelli, P.G.; Menéndez, C.G.; Falco, M.; López-Franca, N.; Carril, A.F. Future Hydroclimatological Changes in South America Based on an Ensemble of Regional Climate Models. Clim. Dyn. 2019, 52, 819–830. [Google Scholar] [CrossRef]
- Barone, E.; Gullo, G.; Zappia, R.; Inglese, P. Effect of Crop Load on Fruit Ripening and Olive Oil (Olea Europea L.) Quality. J. Hortic. Sc. 1994, 69, 67–73. [Google Scholar] [CrossRef]
- Salas, J.J.; Sánchez, J.; Ramli, U.S.; Manaf, A.M.; Williams, M.; Harwood, J.L. Biochemistry of Lipid Metabolism in Olive and Other Oil Fruits. Prog. Lipid Res. 2000, 39, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Conde, C.; Delrot, S.; Gerós, H. Physiological, Biochemical and Molecular Changes Occurring during Olive Development and Ripening. J. Plant Physiol. 2008, 165, 1545–1562. [Google Scholar] [CrossRef]
- Proietti, P.; Nasini, L.; Famiani, F. Effect of Different Leaf-to-Fruit Ratios on Photosynthesis and Fruit Growth in Olive (Olea Europaea L.). Photosynthetica 2006, 44, 275–285. [Google Scholar] [CrossRef]
- Fernández, F.J.; Ladux, J.L.; Hammami, S.B.M.; Rapoport, H.F.; Searles, P.S. Fruit, Mesocarp, and Endocarp Responses to Crop Load and to Different Estimates of Source: Sink Ratio in Olive (Cv. Arauco) at Final Harvest. Sci. Hortic. 2018, 234, 49–57. [Google Scholar] [CrossRef]
- Rosati, A.; Caporali, S.; Paoletti, A. Fertilization with N and K Increases Oil and Water Content in Olive (Olea Europaea L.) Fruit via Increased Proportion of Pulp. Sci. Hortic. 2015, 192, 381–386. [Google Scholar] [CrossRef]
- Rousseaux, M.C.; Cherbiy-Hoffmann, S.U.; Hall, A.J.; Searles, P.S. Fatty Acid Composition of Olive Oil in Response to Fruit Canopy Position and Artificial Shading. Sci. Hortic. 2020, 271, 109477. [Google Scholar] [CrossRef]
- Contreras, C.; Pierantozzi, P.; Maestri, D.; Tivani, M.; Searles, P.; Brizuela, M.; Fernández, F.; Toro, A.; Puertas, C.; Trentacoste, E.R.; et al. How Temperatures May Affect the Synthesis of Fatty Acids during Olive Fruit Ripening: Genes at Work in the Field. Plants 2022, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Cortés, F.; Martínez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llácer, G.; Meier, U. Phenological Growth Stages of Olive Trees (Olea Europaea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- del Río, C.; Romero, A.M. Whole, Unmilled Olives Can Be Used to Determine Their Oil Content by Nuclear Magnetic Resonance. HortTechnology 1999, 9, 675–680. [Google Scholar] [CrossRef]
- Uceda, M.; Hermoso, M. La Calidad Del Aceite de Oliva. In El cultivo del olivo; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Junta de Andalucía-Ediciones Mundi-Prensa: Madrid, Spain, 2001; pp. 589–614. [Google Scholar]
- García-Inza, G.P.; Castro, D.N.; Hall, A.J.; Rousseaux, M.C. Opposite Oleic Acid Responses to Temperature in Oils from the Seed and Mesocarp of the Olive Fruit. Eur. J. Agron. 2016, 76, 138–147. [Google Scholar] [CrossRef]


| Cultivar | Temp. Treatment | Fruit Dry Weight (g) | Fruit Oil Content (g fruit−1) | Fruit Moisture (%) | Maturity Index (0–7) | ||
|---|---|---|---|---|---|---|---|
| Pulp | Pit | Pulp/Pit | |||||
| Arbequina | T0 | 0.71 ± 0.06 | 0.28 ± 0.01 c | 2.48 ± 0.15 a | 0.41 ± 0.03 | 58.5 ± 0.6 | 3.8 ± 0.6 |
| T+ | 0.37 ± 0.03 | 0.26 ± 0.01 c | 1.41 ± 0.10 c | 0.20 ± 0.02 | 61.7 ± 2.4 | 3.3 ± 0.3 | |
| Coratina | T0 | 1.20 ± 0.12 | 0.57 ± 0.03 a | 2.09 ± 0.12 b | 0.77 ± 0.08 | 50.2 ± 2.0 | 0.5 ± 0.1 |
| T+ | 0.81 ± 0.04 | 0.47 ± 0.03 b | 1.73 ± 0.03 bc | 0.44 ± 0.02 | 55.9 ± 2.3 | 1.0 ± 0.3 | |
| temp | ** | ** | ** | ** | ns | ns | |
| cv | ** | ** | ** | ** | ** | ** | |
| int | ns | ** | ** | ns | ns | ns | |
| Season | Cultivar | Temp. Treatment | Major Fatty Acids (%) | ||
|---|---|---|---|---|---|
| Palmitic | Oleic | Linoleic | |||
| 2014–2015 | Arbequina | T0 | 19.8 ± 0.1 | 50.0 ± 0.8 b | 23.2 ± 0.5 b |
| T+ | 20.8 ± 0.2 | 45.5 ± 0.9 c | 26.4 ± 0.5 a | ||
| Coratina | T0 | 12.9 ± 0.4 | 67.0 ± 1.1 a | 15.4 ± 0.9 c | |
| T+ | 13.7 ± 0.2 | 67.8 ± 0.6 a | 13.7 ± 0.4 c | ||
| temp | ** | * | ** | ||
| cv | ** | ** | ** | ||
| int | ns | ** | ** | ||
| 2015–2016 | Arbequina | T0 | 19.5 ± 0.7 | 54.7 ± 1.6 | 19.1 ± 0.5 |
| T+ | 22.3 ± 0.9 | 51.6 ± 2.4 | 22.7 ± 0.7 | ||
| Coratina | T0 | 12.7 ± 0.3 | 74.7 ± 0.8 | 9.3 ± 0.8 | |
| T+ | 15.5 ± 0.5 | 68.5 ± 0.4 | 13.8 ± 1.4 | ||
| temp | ** | * | ** | ||
| cv | ** | ** | ** | ||
| int | ns | ns | ns | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miserere, A.; Searles, P.S.; Rousseaux, M.C. Influence of Experimental Warming on the Rate and Duration of Fruit Growth and Oil Accumulation in Young Olive Trees (cvs. Arbequina, Coratina). Plants 2023, 12, 1942. https://doi.org/10.3390/plants12101942
Miserere A, Searles PS, Rousseaux MC. Influence of Experimental Warming on the Rate and Duration of Fruit Growth and Oil Accumulation in Young Olive Trees (cvs. Arbequina, Coratina). Plants. 2023; 12(10):1942. https://doi.org/10.3390/plants12101942
Chicago/Turabian StyleMiserere, Andrea, Peter S. Searles, and M. Cecilia Rousseaux. 2023. "Influence of Experimental Warming on the Rate and Duration of Fruit Growth and Oil Accumulation in Young Olive Trees (cvs. Arbequina, Coratina)" Plants 12, no. 10: 1942. https://doi.org/10.3390/plants12101942
APA StyleMiserere, A., Searles, P. S., & Rousseaux, M. C. (2023). Influence of Experimental Warming on the Rate and Duration of Fruit Growth and Oil Accumulation in Young Olive Trees (cvs. Arbequina, Coratina). Plants, 12(10), 1942. https://doi.org/10.3390/plants12101942






