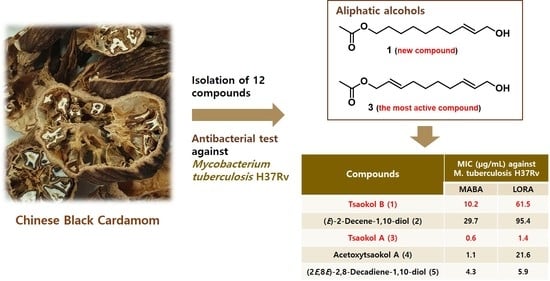
Anti-Microbial Activity of Aliphatic Alcohols from Chinese Black Cardamom (Amomum tsao-ko) against Mycobacterium tuberculosis H37Rv
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of Compound 1 and Identification of 2–12
2.2. Antimicrobial Activitiy of Isolated Compounds aganist M. tuberculosis H37Rv
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
(E)-1-Acetyl-8-decene-1,10-diol (1, Tsaokol B)
3.4. Synthesis of Tsaokol A
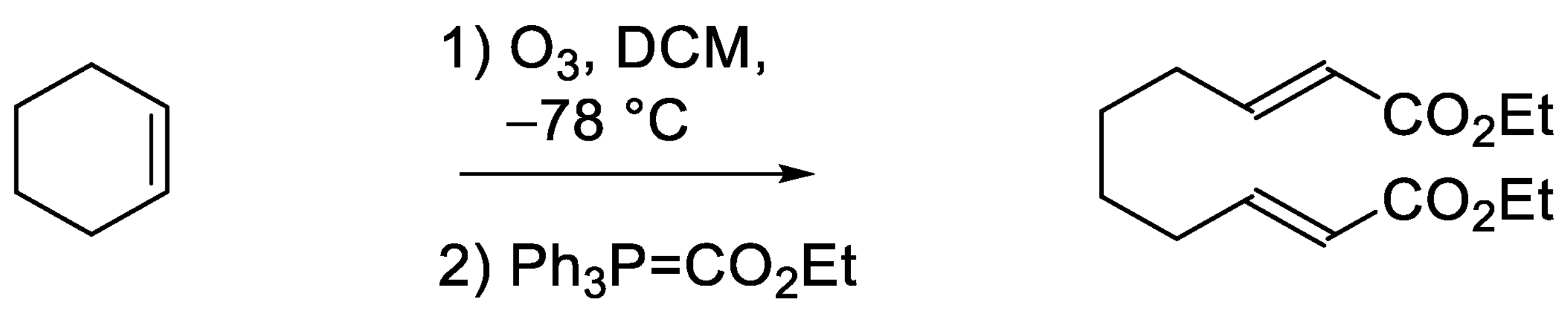
3.4.1. Diethyl (2E,8E)-Deca-2,8-dienoate (Scheme 1)
3.4.2. (2E,8E)-2,8-Decadiene-1,10-diol (5) (Scheme 2)

3.4.3. Tsaokol A (3) (Scheme 3)

3.5. MIC Testing against M. tuberculosis
3.6. MIC Testing against Non-Replicating M. tuberculosis
3.7. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parthasarathy, V.A.; Kandinnan, K.; Srinivasan, V. Fenugreek. In Organic Spices; New India Publishing Agencies: New Delhi, India, 2008. [Google Scholar]
- Nanasombat, S.; Wimuttigosol, P. Antimicrobial and antioxidant activity of spice essential oils. Food Sci Biotechnol. 2011, 20, 45–53. [Google Scholar] [CrossRef]
- Arun, K.T.; Shikha, U.; Mantu, B. A review on prospects of essential oils as biopesticide in insect-pest management. J. Pharmacogn. Phytother. 2009, 1, 52–63. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Yue, X.; Wang, Y.; Yang, Y.; Sun, D.; Li, H.; Chen, L. Chemistry and bioactivity of plants from the genus Amomum. J. Ethnopharmacol. 2021, 281, 114563. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, R.W.; Cai, X.Q.; Zheng, Z.L.; Zou, G.L. Chemical composition and antimicrobial activity of the essential oil of Amomum tsao-ko. J. Sci. Food Agric. 2008, 88, 2111–2116. [Google Scholar] [CrossRef]
- Min, D.; Cheng, P.; Fenghui, S. Anti-infectious efficacy of essential oil from Caoguo (Fructus Tsaoko). J. Tradit. Chin. Med. 2016, 36, 799–804. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Mdluli, K.; Kaneko, T.; Upton, A. The tuberculosis drug discovery and development pipeline and emerging drug targets. Cold Spring Harb. Perspect. Med. 2015, 5, a021154. [Google Scholar] [CrossRef]
- Shetye, G.S.; Choi, K.B.; Kim, C.Y.; Franzblau, S.G.; Cho, S. In Vitro Profiling of Antitubercular Compounds by Rapid, Efficient, and Nondestructive Assays Using Autoluminescent Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2021, 65, e00282-21. [Google Scholar] [CrossRef]
- Shetye, G.S.; Franzblau, S.G.; Cho, S. New tuberculosis drug targets, their inhibitors, and potential therapeutic impact. Trans. Res. 2020, 220, 68–97. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.C.; Subedi, L.; Cho, K.H.; Kim, S.Y.; Park, H.J.; Kim, K.H. Bioactive compounds from the seeds of Amomum tsaoko Crevost et Lemaire, a Chinese spice as inhibitors of sphingosine kinases, SPHK1/2. RSC Adv. 2019, 9, 33957–33968. [Google Scholar] [CrossRef]
- Chai, L. Chemical constituents from fruits of Amomum paratsao-ko. Zhong Cao Yao 2018, 49, 3217–3221. [Google Scholar]
- Kim, M.S.; Ahn, E.K.; Hong, S.S.; Oh, J.S. 2, 8-Decadiene-1, 10-diol inhibits lipopolysaccharide-induced inflammatory responses through inactivation of mitogen-activated protein kinase and nuclear factor-κB signaling pathway. Inflammation 2016, 39, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Kon, Y.; Yazawa, H.; Usui, Y.; Sato, K. Chemoselective Oxidation of Alcohols by a H2O2–Pt Black System under Organic Solvent-and Halide-Free Conditions. Chem. Asian J. 2008, 3, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- De Frémont, P.; Marion, N.; Nolan, S.P. Cationic NHC–gold (I) complexes: Synthesis, isolation, and catalytic activity. J. Organomet Chem. 2009, 694, 551–560. [Google Scholar] [CrossRef]
- Hong, S.S.; Lee, J.H.; Choi, Y.H.; Jeong, W.; Ahn, E.K.; Lym, S.H.; Oh, J.S. Amotsaokonal A–C, benzaldehyde and cycloterpenal from Amomum tsao-ko. Tetrahedron Lett. 2015, 56, 6681–6684. [Google Scholar] [CrossRef]
- Guerrini, A.; Rossi, D.; Paganetto, G.; Tognolini, M.; Muzzoli, M.; Romagnoli, C.; Antognoni, F.; Vertuani, S.; Medici, A.; Bruni, A. Chemical characterization (GC/MS and NMR fingerprinting) and bioactivities of South-African Pelargonium capitatum (L.) L’her.(Geraniaceae) essential oil. Chem. Biodivers 2011, 8, 624–642. [Google Scholar] [CrossRef]
- Mino, T.; Hasegawa, T.; Shirae, Y.; Sakamoto, M.; Fujita, T. N, O-ligand accelerated zinc-catalyzed transesterification of alcohols with vinyl esters. J. Organomet. Chem. 2007, 692, 4389–4396. [Google Scholar] [CrossRef]
- Blanc, M.C.; Bradesi, P.; Casanova, J. Enantiomeric differentiation of acyclic terpenes by 13C NMR spectroscopy using a chiral lanthanide shift reagent. Magn. Reason. Chem. 2005, 43, 176–179. [Google Scholar] [CrossRef]
- Cane, D.E.; Ha, H.J.; McIlwaine, D.B.; Pascoe, K.O. The synthesis of (3R)-nerolidol. Tetrahedron Lett. 1990, 31, 7553–7554. [Google Scholar] [CrossRef]
- Barrero, A.F.; Quílez del Moral, J.F.; Sánchez, E.M.; Arteaga, J.F. Regio-and diastereoselective reductive coupling of vinylepoxides catalyzed by titanocene chloride. Org. Lett. 2006, 8, 669–672. [Google Scholar] [CrossRef]
- Cho, S.; Lee, H.S.; Franzblau, S. Microplate alamar blue assay (MABA) and low oxygen recovery assay (LORA) for Mycobacterium tuberculosis. Methods Mol. Biol. 2015, 1285, 281–292. [Google Scholar] [PubMed]
- Cho, S.H.; Warit, S.; Wan, B.; Hwang, C.H.; Pauli, G.F.; Franzblau, S.G. Low-oxygen-recovery assay for high-throughput screening of compounds against nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2007, 51, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Andreu, N.; Zelmer, A.; Fletcher, T.; Elkington, P.T.; Ward, T.H.; Ripoll, J.; Parish, T.; Bancroft, G.J.; Schaible, U.; Robertson, B.D. Optimisation of bioluminescent reporters for use with mycobacteria. PLoS ONE 2010, 5, e10777. [Google Scholar] [CrossRef] [PubMed]
- Choules, M.P.; Wolf, N.M.; Lee, H.; Anderson, J.R.; Grzelak, E.M.; Wang, Y.; Ma, R.; Gao, W.; McAlpine, J.B.; Jin, Y.Y. Rufomycin targets ClpC1 proteolysis in Mycobacterium tuberculosis and M. abscessus. Antimicrob. Agents Chemother. 2019, 63, e02204-18. [Google Scholar] [CrossRef] [PubMed]

| Position a | 1 | 3 | ||
|---|---|---|---|---|
| δH Multi (J in Hz) | δc | δH Multi (J in Hz) | δc | |
| 1 | 4.03 t (7.0) | 64.8 | 4.49 dd (6.5, 1.0) | 65.4 |
| 2 | 1.59 m | 28.7 | 5.55 dt (15.0, 6.5) | 124.0 |
| 3 | 1.20–1.39 b | 26.0 | 5.74 dtt (15.0, 6.5, 1.0) | 136.5 |
| 4 | 29.3 | 2.04 m b | 32.1 | |
| 5 | 29.9 | 1.38 m b | 28.7 | |
| 6 | 29.2 | 1.38 m b | 28.4 | |
| 7 | 2.02 m b | 32.4 | 2.04 m b | 32.2 |
| 8 | 5.67 dt (15.5, 6.5) | 133.6 | 5.66 dt (15.0, 6.5) | 133.2 |
| 9 | 5.61 dt (15.5, 5.5) | 129.1 | 5.63 dt (15.0, 5.0) | 129.2 |
| 10 | 4.07 d (5.5) | 64.1 | 4.07 d (5.0) | 63.9 |
| 1-OCOCH3 | 171.5 | 171.1 | ||
| 1-OCOCH3 | 2.02 s b | 21.2 | 2.05 s b | 21.2 |
| Extract or Compounds | MIC (µg/mL) against M. tuberculosis H37Rv | Cytotoxicity against Vero Cells | ||
|---|---|---|---|---|
| MABA | LORA | IC50 | ||
| - | Acetone extract | 9.7 | 86.8 | 92.8 |
| 1 | Tsaokol B | 10.2 | 61.5 | >200 (10%) |
| 2 | (E)-2-Decene-1,10-diol | 29.7 | 95.4 | >200 (25%) |
| 3 | Tsaokol A | 0.6 | 1.4 | >200 (7%) |
| 4 | Acetoxytsaokol A | 1.1 | 21.6 | 143.6 |
| 5 | (2E,8E)-2,8-Decadiene-1,10-diol | 4.3 | 5.9 | 72.9 |
| 6 | (2E,6E)-1,8-Diacetoxy-2,6-octadiene | 26.1 | >100 (49%) | 76.8 |
| 7 | (E)-Decenal | >100 (68%) | >100 (67%) | |
| 8 | (E)-Dec 2-enyl acetate | >100 (48%) | >100 (14%) | |
| 9 | (E)-2-Dodecen-1-yl acetate | >100 (81%) | >100 (50%) | |
| 10 | Geraniol | >100 (16%) | >100 (16%) | |
| 11 | Geranyl acetate | 88.6 | >100 (8%) | >200 (21%) |
| 12 | (3R)-(E)-Nerolidol | >100 (71%) | >100 (71%) | |
| Synthetic tsaokol A | 1.4 | >200 (10%) | ||
| Rifampicin | 0.025 | 0.14 | >100 (50%) | |
| Isoniazid | 0.03 | >18 (85%) | ||
| Linezolid | 0.16 | 0.62 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Shetye, G.S.; Son, S.-R.; Lee, H.; Klein, L.L.; Yoshihara, J.K.; Ma, R.; Franzblau, S.G.; Cho, S.; Jang, D.S. Anti-Microbial Activity of Aliphatic Alcohols from Chinese Black Cardamom (Amomum tsao-ko) against Mycobacterium tuberculosis H37Rv. Plants 2023, 12, 34. https://doi.org/10.3390/plants12010034
Lee SY, Shetye GS, Son S-R, Lee H, Klein LL, Yoshihara JK, Ma R, Franzblau SG, Cho S, Jang DS. Anti-Microbial Activity of Aliphatic Alcohols from Chinese Black Cardamom (Amomum tsao-ko) against Mycobacterium tuberculosis H37Rv. Plants. 2023; 12(1):34. https://doi.org/10.3390/plants12010034
Chicago/Turabian StyleLee, So Young, Gauri S. Shetye, So-Ri Son, Hyun Lee, Larry L. Klein, Jeffrey K. Yoshihara, Rui Ma, Scott G. Franzblau, Sanghyun Cho, and Dae Sik Jang. 2023. "Anti-Microbial Activity of Aliphatic Alcohols from Chinese Black Cardamom (Amomum tsao-ko) against Mycobacterium tuberculosis H37Rv" Plants 12, no. 1: 34. https://doi.org/10.3390/plants12010034
APA StyleLee, S. Y., Shetye, G. S., Son, S.-R., Lee, H., Klein, L. L., Yoshihara, J. K., Ma, R., Franzblau, S. G., Cho, S., & Jang, D. S. (2023). Anti-Microbial Activity of Aliphatic Alcohols from Chinese Black Cardamom (Amomum tsao-ko) against Mycobacterium tuberculosis H37Rv. Plants, 12(1), 34. https://doi.org/10.3390/plants12010034