Grafting Causes Physiological Changes and Promotes Adventitious Root Formation in Rejuvenated Soft Shoots of Taxodium hybrid ‘Zhongshanshan’
Abstract
1. Introduction
2. Results
2.1. Rooting of Mature and Rejuvenated Soft Shoots
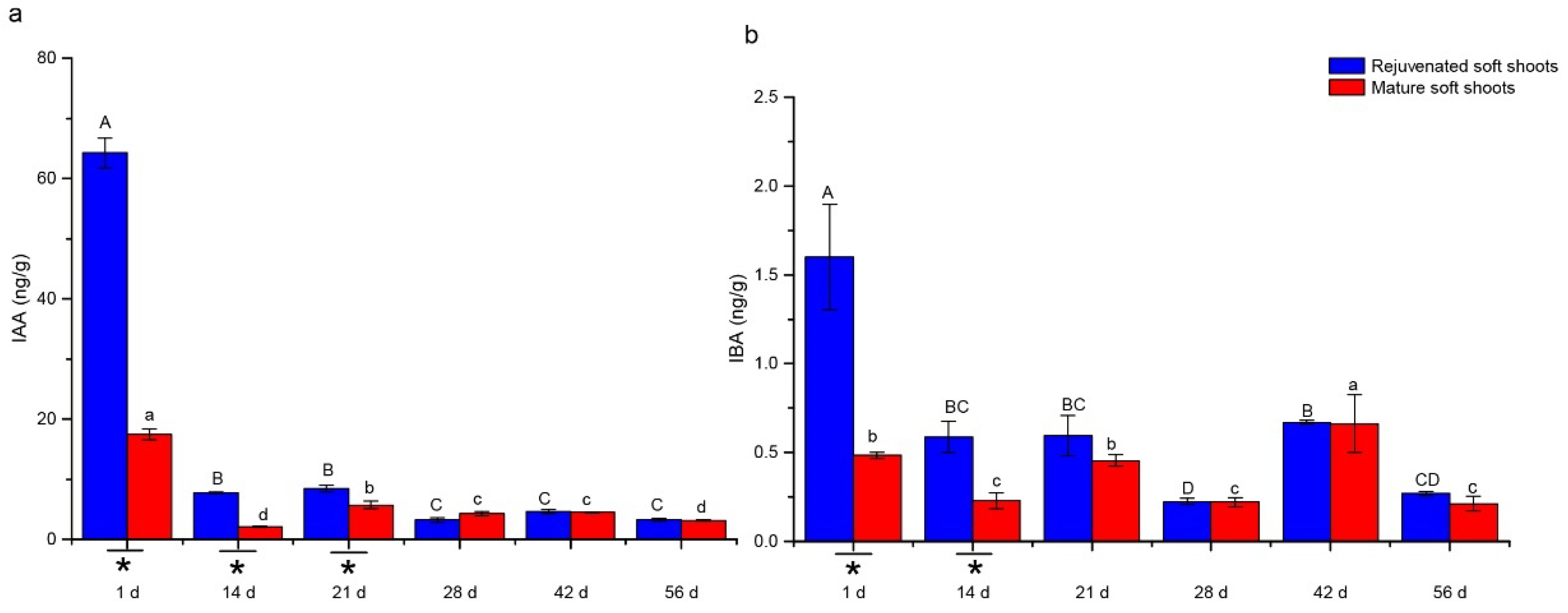
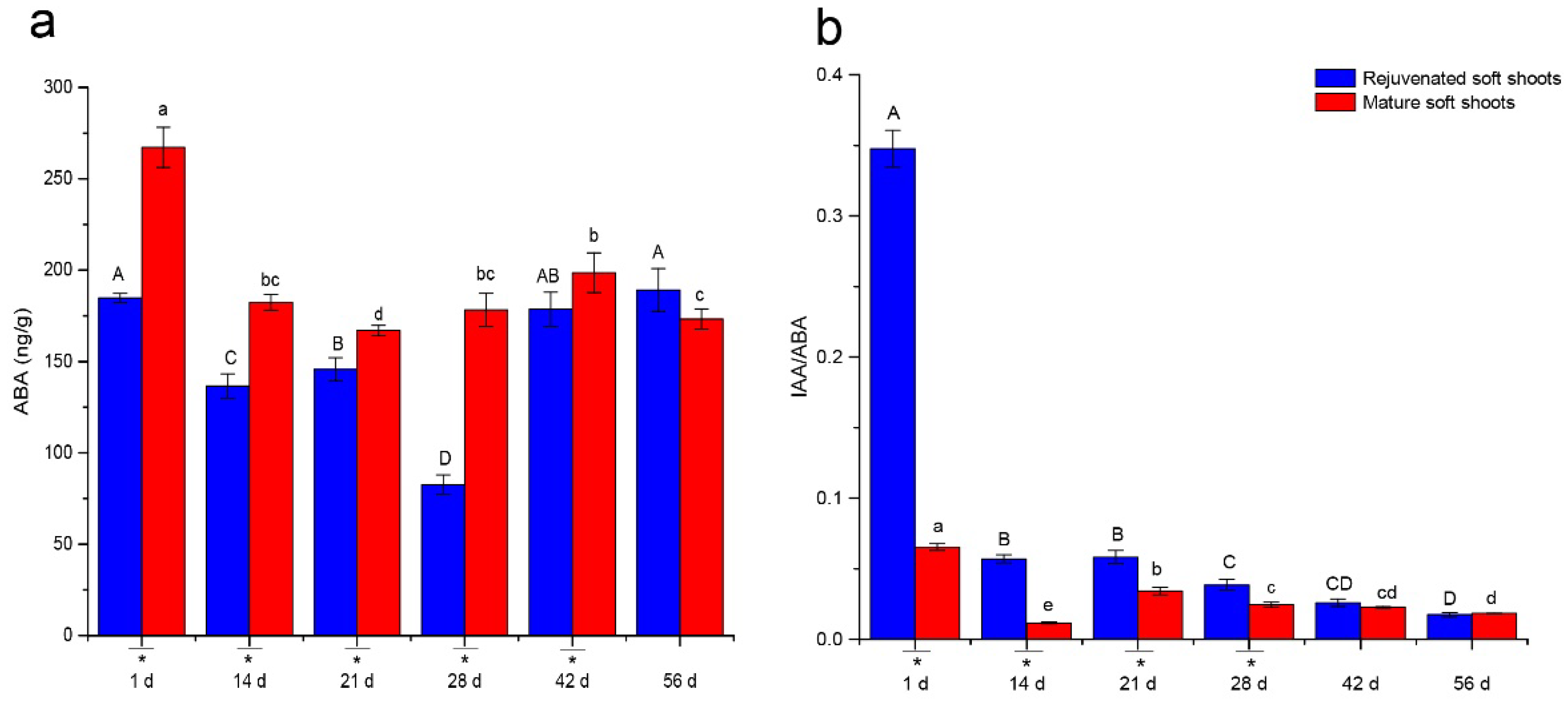
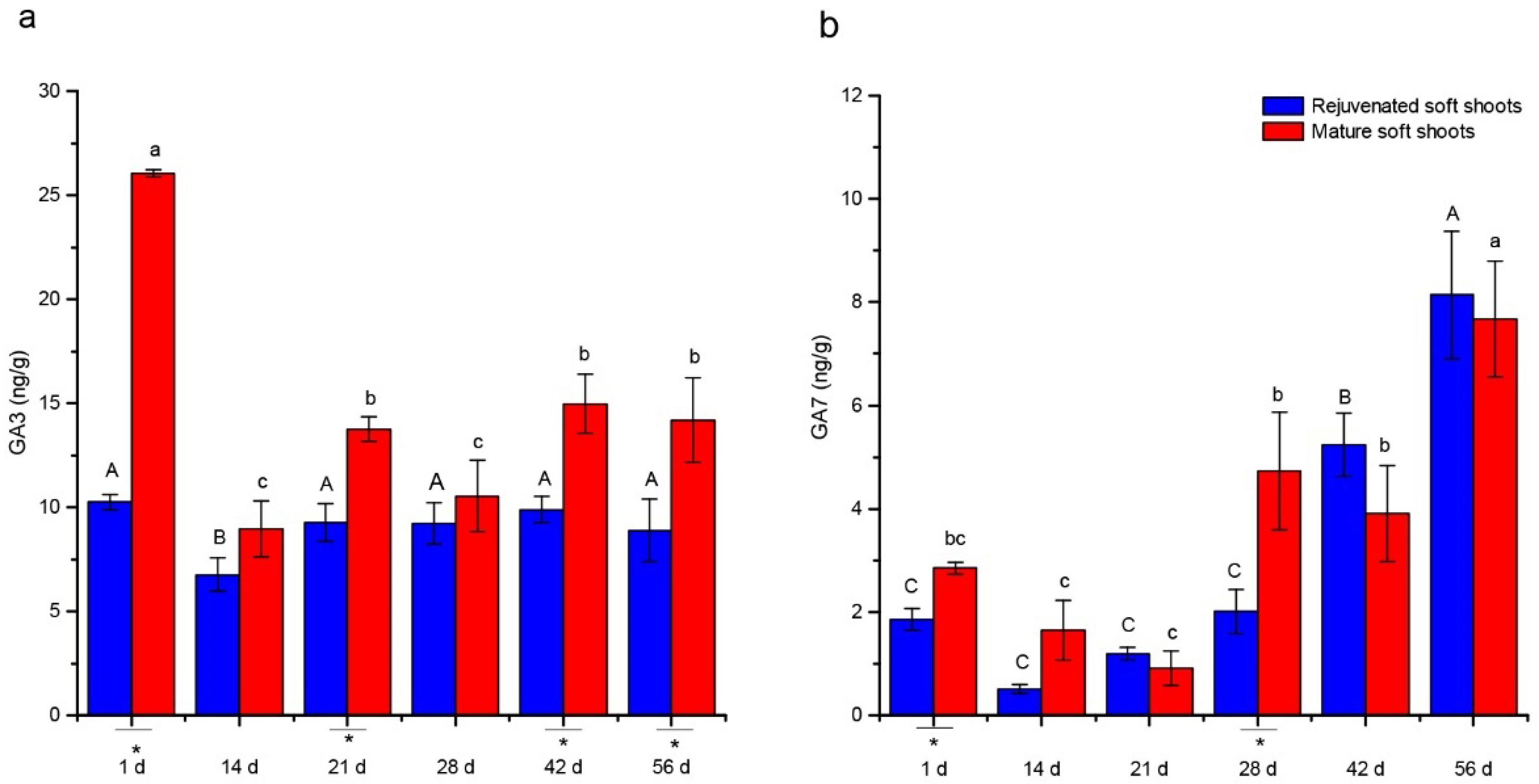
2.2. Quantitative Analyses of Endohormone Levels
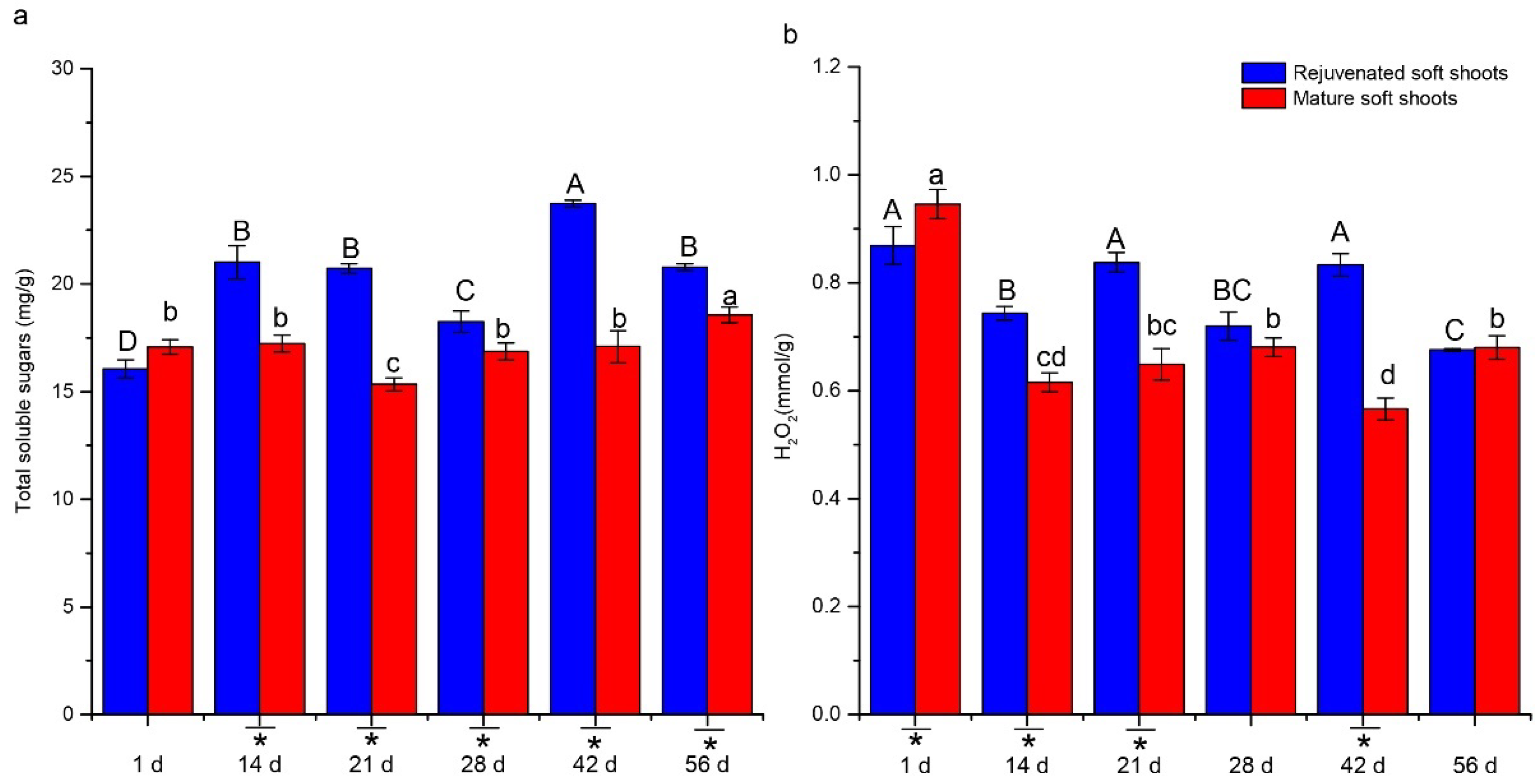
2.3. Analyses of H2O2 and Sugar
3. Discussion
4. Materials and Methods
4.1. Plant Material and Root Induction
4.2. Quantification of Endogenous Hormone Levels
4.3. Analyses of H2O2 and Soluble Sugar
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPLC | High performance liquid chromatography |
| iP | N6-(Δ2-isopentenyl)-adenine |
| iPA | N6-(Δ2-isopentenyl)-adenosine |
| tZR | Trans-zeatin riboside |
| ABA | Abscisic acid |
| GAs | Gibberellins |
| IAA | Indole-3-acetic acid |
| IBA | Indole-3-butyric acid |
| ROS | Reactive oxygen species |
| ARF | Adventitious root formation |
References
- Yu, C.; Xu, S.; Yin, Y. Transcriptome analysis of the Taxodium ‘Zhongshanshan 405’roots in response to salinity stress. Plant Physiol. Biochem. 2016, 100, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gu, C.; Xuan, L.; Hua, J.; Shi, Q.; Fan, W.; Yin, Y.; Yu, F. Identification of suitable reference genes in Taxodium ‘Zhongshanshan’ under abiotic stresses. Trees 2017, 31, 1519–1530. [Google Scholar] [CrossRef]
- Shi, Q.; Yin, Y.; Wang, Z.; Fan, W.; Hua, J. Physiological acclimation of Taxodium hybrid ‘Zhongshanshan 118’ plants to short-term drought stress and recovery. HortScience 2016, 51, 1159–1166. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Pan, B. The viscoelasticity and deformation mechanism of Taxodium hybrid ‘Zhongshanshan’ wood by dynamic mechanical analysis. BioResources 2021, 16, 6933–6942. [Google Scholar] [CrossRef]
- Qi, B.; Yang, Y.; Yin, Y.; Xu, M.; Li, H. De novo sequencing, assembly, and analysis of the Taxodium ‘Zhongshansa’ roots and shoots transcriptome in response to short-term waterlogging. BMC Plant Biol. 2014, 14, 201. [Google Scholar] [CrossRef]
- Wang, Z.; Hua, J.; Yin, Y.; Gu, C.; Yu, C.; Shi, Q.; Guo, J.; Xuan, L.; Yu, F. An integrated transcriptome and proteome analysis reveals putative regulators of adventitious root formation in Taxodium ‘Zhongshanshan’. Int. J. Mol. Sci. 2019, 20, 1225. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, Y.; Hua, J.; Fan, W.; Yu, C.; Xuan, L.; Yu, F. Cloning and characterization of ThSHR s and ThSCR transcription factors in Taxodium hybrid ‘Zhongshanshan 406’. Genes 2017, 8, 185. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Y.; Li, Y. Plant rejuvenation: From phenotypes to mechanisms. Plant Cell Rep. 2020, 39, 1249–1262. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Song, X.; Ma, Q.; Zhang, J.; Pei, D. A novel rejuvenation approach to induce endohormones and improve rhizogenesis in mature juglans tree. Plant Methods 2018, 14, 13. [Google Scholar] [CrossRef]
- Onay, A.; Tilkat, E.; Süzerer, V.; Metin, Ö.K.; Çiftçi, Y.Ö.; Kilinc, F.M.; Koc, I.; Şakiroğlu, M.; Yildirim, H.; Uncuoğlu, A.A. Rejuvenation of mature lentisk by micrografting and evaluation of genetic stability. Turk. J. Biol. 2016, 40, 781–796. [Google Scholar] [CrossRef]
- Massoumi, M.; Krens, F.A.; Visser, R.G.; de Klerk, G.-J.M. Azacytidine and mir156 promote rooting in adult but not in juvenile Arabidopsis tissues. J. Plant Physiol. 2017, 208, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Hammatt, N.; Grant, N. Apparent rejuvenation of mature wild cherry (Prunus avium L.) during micropropagation. J. Plant Physiol. 1993, 141, 341–346. [Google Scholar] [CrossRef]
- Giovannelli, A.; Giannini, R. Reinvigoration of mature chestnut (Castanea sativa) by repeated graftings and micropropagation. Tree Physiol. 2000, 20, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-C.; Lius, S.; Huang, B.-L.; Murashige, T.; Mahdi, E.F.M.; van Gundy, R. Rejuvenation of sequoia sempervirens by repeated grafting of shoot tips onto juvenile rootstocks in vitro: Model for phase reversal of trees. Plant Physiol. 1992, 98, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, H.; Li, S.; Yang, C.; Jiang, J.; Liu, G. The rooting of poplar cuttings: A review. New For. 2014, 45, 21–34. [Google Scholar] [CrossRef]
- Shang, C.; Yang, H.; Ma, S.; Shen, Q.; Liu, L.; Hou, C.; Cao, X.; Cheng, J. Physiological and transcriptomic changes during the early phases of adventitious root formation in mulberry stem hardwood cuttings. Int. J. Mol. Sci. 2019, 20, 3707. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef]
- Pacurar, D.I.; Perrone, I.; Bellini, C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant. 2014, 151, 83–96. [Google Scholar] [CrossRef]
- Quan, L.J.; Zhang, B.; Shi, W.W.; Li, H.Y. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Huang, Y.; Ji, K.-S.; Zhai, J.-R. Relationship between rooting ability and endogenous phytohormone changes in successive continuous generation cuttings of Buxus sinica var. Parvifolia, an endangered woody species in China. For. Stud. China 2007, 9, 189–197. [Google Scholar] [CrossRef]
- Considine, M.J.; Foyer, C.H. Redox regulation of plant development. Antioxid. Redox Signal. 2014, 21, 1305–1326. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.L.; Chen, Y.K.; Xu, X.Z.; Shen, F.; Zheng, Q.B.; Du, Z.; Wang, Y.; Wu, T.; Xu, X.F.; Han, Z.H. Mir156 switches on vegetative phase change under the regulation of redox signals in apple seedlings. Sci. Rep. 2017, 7, 14223. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-W.; Leng, Y.; Shi, R.-F. Transcriptomic profiling provides molecular insights into hydrogen peroxide-induced adventitious rooting in mung bean seedlings. BMC Genom. 2017, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Husen, A. Stock-plant etiolation causes drifts in total soluble sugars and anthraquinones, and promotes adventitious root formation in teak (Tectona grandis L. F.) coppice shoots. Plant Growth Regul. 2008, 54, 13–21. [Google Scholar] [CrossRef]
- Husen, A. Rejuvenation and adventitious rooting in coppice-shoot cuttings of Tectona grandis as affected by stock-plant etiolation. Am. J. Plant Sci. 2011, 2, 370. [Google Scholar] [CrossRef][Green Version]
- Graves, W.R. IBA, juvenility, and position on ortets influence propagation of Carolina buckthorn from softwood cuttings. J. Environ. Hortic. 2002, 20, 57–61. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Y.; Yin, Y.; Yu, C.; Yang, Y.; Shi, Q.; Hao, Z.; Li, H. Genetic linkage map construction and QTL mapping of seedling height, basal diameter and crown width of Taxodium ‘Zhongshanshan 302’ × T. mucronatum. Springerplus 2016, 5, 936. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Li, H.; Yu, C.; Yin, Y. Rooting capabilities for Taxodium ‘Zhongshanshan’302, 118, and 405. J. Zhejiang AF Univ. 2015, 32, 648–654. [Google Scholar]
- Vielba, J.; Vidal, N.; José, M.; Rico, S.; Sánchez, C. Recent advances in adventitious root formation in Chestnut. Plants 2020, 9, 1543. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, R. Increased endogenous indole-3-acetic acid: Abscisic acid ratio is a reliable marker of Pinus massoniana rejuvenation. Biotech. Histochem. 2019, 94, 546–553. [Google Scholar] [CrossRef]
- Caboni, E.; Tonelli, M.G.; Lauri, P.; Iacovacci, P.; Kevers, C.; Damiano, C.; Gaspar, T. Biochemical aspects of almond microcuttings related to in vitro rooting ability. Biol. Plant. 1997, 39, 91–97. [Google Scholar] [CrossRef]
- Xiao, Z.; Ji, N.; Zhang, X.; Zhang, Y.; Wang, Y.; Wu, T.; Han, Z. The lose of juvenility elicits adventitious rooting recalcitrance in apple rootstocks. Plant Cell Tissue Organ Cult. 2014, 119, 51–63. [Google Scholar] [CrossRef]
- Chang, I.-F.; Chen, P.-J.; Shen, C.-H.; Hsieh, T.-J.; Hsu, Y.-W.; Huang, B.-L.; Kuo, C.-I.; Chen, Y.-T.; Chu, H.-A.; Yeh, K.-W. Proteomic profiling of proteins associated with the rejuvenation of Sequoia sempervirens (d. Don) endl. Proteome Sci. 2010, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Kelen, M.; Ozkan, G. Relationships between rooting ability and changes of endogenous IAA and ABA during the rooting of hardwood cuttings of some grapevine rootstocks. Eur. J. Hortic. Sci. 2003, 68, 8–13. [Google Scholar]
- Materán, M.E.; Fernández, M.; Valenzuela, S.; Sáez, K.; Seemann, P.; Sánchez-Olate, M.; Ríos, D. Abscisic acid and 3-indolacetic acid levels during the reinvigoration process of Pinus radiata d. Don adult material. Plant Growth Regul. 2009, 59, 171–177. [Google Scholar] [CrossRef]
- Wolters, H.; Jürgens, G. Survival of the flexible: Hormonal growth control and adaptation in plant development. Nat. Rev. Genet. 2009, 10, 305–317. [Google Scholar] [CrossRef]
- Rasmussen, A.; Hosseini, S.A.; Hajirezaei, M.-R.; Druege, U.; Geelen, D. Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis. J. Exp. Bot. 2015, 66, 1437–1452. [Google Scholar] [CrossRef]
- Da Costa, C.T.; de Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef]
- Perrin, Y.; Doumas, P.; Lardets, L.; Carrons, M.P. Endogenous cytokinins as biochemical markers of rubber-tree (Hevea brasiliensis) clone rejuvenation. Plant Cell Tissue Organ Cult. 1997, 47, 239–245. [Google Scholar] [CrossRef]
- Chang, X.Y.; Zhang, K.; Yuan, Y.; Ni, P.; Ma, J.; Liu, H.; Gong, S.; Bai, M. A simple, rapid, and quantifiable system for studying adventitious root formation in grapevine. Plant Growth Regul. 2022, 98, 117–126. [Google Scholar] [CrossRef]
- Mauriat, M.; Petterle, A.; Bellini, C.; Moritz, T. Gibberellins inhibit adventitious rooting in hybrid Aspen and Arabidopsis by affecting auxin transport. Plant J. 2014, 78, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Kracke, H.; Cristoferi, G.; Marangoni, B. Hormonal changes during the rooting of hardwood cuttings of grapevine rootstocks. Am. J. Enol. Vitic. 1981, 32, 135–137. [Google Scholar]
- Ibáñez, S.; Ruiz-Cano, H.; Fernández, M.Á.; Sánchez-García, A.B.; Villanova, J.; Micol, J.L.; Pérez-Pérez, J.M. A network-guided genetic approach to identify novel regulators of adventitious root formation in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Pirdastan, M.; Aboutalebi Jahromi, A.; Hassanzadeh Khankahdani, H. Effect of hydrogen peroxide, ascorbic acid and indolic-3-butyric acid on root induction and development in cuttings of Bougainvillea spectabilis. J. Ornam. Plants 2020, 10, 145–154. [Google Scholar]
- Maynard, B.; Bassuk, N. Etiolation and banding effects on adventitious root formation. Adv. Plant Sci. Ser. 1988, 2, 29–46. [Google Scholar]
- Zang, Y.; Xu, C.; Xuan, L.; Ding, L.; Zhu, J.; Si, Z.; Zhang, T.; Hu, Y. Identification and characteristics of a novel gland-forming gene in cotton. Plant J. 2021, 108, 781–792. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Q.; Wu, R.; Wang, X.; Wang, H.; Wang, Z.; Shi, C.; Bi, Y. Role of hydrogen peroxide in regulating glucose-6-phosphate dehydrogenase activity under salt stress. Biol. Plant. 2012, 56, 313–320. [Google Scholar] [CrossRef]


| Treatments | Calli Formation Rate (%) | Rooting Rate (%) | ||
|---|---|---|---|---|
| Induction stage | 1st day | Rejuvenated soft shoots | 0 | 0 |
| Mature soft shoots | 0 | 0 | ||
| 7th day | Rejuvenated soft shoots | 0 | 0 | |
| Mature soft shoots | 0 | 0 | ||
| 14th day | Rejuvenated soft shoots | 0 | 0 | |
| Mature soft shoots | 0 | 0 | ||
| 21st day | Rejuvenated soft shoots | 61.0 | 0 | |
| Mature soft shoots | 34.9 | 0 | ||
| Initiation stage | 28th day | Rejuvenated soft shoots | 67.5 | 0 |
| Mature soft shoots | 33.3 | 0 | ||
| 35th day | Rejuvenated soft shoots | 76.4 | 0 | |
| Mature soft shoots | 38.1 | 0 | ||
| Expression stage | 42nd day | Rejuvenated soft shoots | 69.6 | 4 |
| Mature soft shoots | 31.4 | 0 | ||
| 49th day | Rejuvenated soft shoots | 69.3 | 16.8 | |
| Mature soft shoots | 42.6 | 0 | ||
| 56th day | Rejuvenated soft shoots | 75.6 | 51.1 | |
| Mature soft shoots | 25.2 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shi, Q.; Chen, P.; Sun, F.; Creech, D.; Lu, Z.; Yin, Y.; Yu, C. Grafting Causes Physiological Changes and Promotes Adventitious Root Formation in Rejuvenated Soft Shoots of Taxodium hybrid ‘Zhongshanshan’. Plants 2023, 12, 201. https://doi.org/10.3390/plants12010201
Wang Z, Shi Q, Chen P, Sun F, Creech D, Lu Z, Yin Y, Yu C. Grafting Causes Physiological Changes and Promotes Adventitious Root Formation in Rejuvenated Soft Shoots of Taxodium hybrid ‘Zhongshanshan’. Plants. 2023; 12(1):201. https://doi.org/10.3390/plants12010201
Chicago/Turabian StyleWang, Zhiquan, Qin Shi, Peipei Chen, Feng Sun, David Creech, Zhiguo Lu, Yunlong Yin, and Chaoguang Yu. 2023. "Grafting Causes Physiological Changes and Promotes Adventitious Root Formation in Rejuvenated Soft Shoots of Taxodium hybrid ‘Zhongshanshan’" Plants 12, no. 1: 201. https://doi.org/10.3390/plants12010201
APA StyleWang, Z., Shi, Q., Chen, P., Sun, F., Creech, D., Lu, Z., Yin, Y., & Yu, C. (2023). Grafting Causes Physiological Changes and Promotes Adventitious Root Formation in Rejuvenated Soft Shoots of Taxodium hybrid ‘Zhongshanshan’. Plants, 12(1), 201. https://doi.org/10.3390/plants12010201






