Abstract
Phytochemical investigations of leaves and twigs from Garcinia oligantha Merr. resulted in the isolation of five undescribed triterpene derivatives (1–5) and six known analogs (6–11). Their structures were determined based on extensive spectroscopic data and high-resolution mass spectra analyses. Compounds 1–11 were tested for their in vitro cytotoxicity against three human cancer cell lines (HeLa, HepG-2, and MCF-7). Compounds 1, 2, 8, and 11 exhibited broad and significant cytotoxicity against the tested cell lines with IC50 values ranging from 5.04 to 21.55 μM. Compounds 5 and 9 showed cytotoxicity against HeLa and MCF-7 with IC50 values ranging from 13.22 to 19.62 μM. The preliminary structure–activity relationship for the 11 isolated compounds is also discussed.
1. Introduction
The genus Garcinia, belonging to the family Clusiaceae or Guttiferae, has a rich source of compounds, including phloroglucinols [1,2,3], xanthones [4,5,6,7,8,9,10,11,12,13], and biflavonoids [14,15,16]. Dauphinol B, a phloroglucinol isolated from G. dauphinensis, showed potent antiplasmodial activity against the Dd2 drug-resistant strain of Plasmodium falciparum, with an IC50 of 0.8 μM [17]. 7-Epiclusianone from G. brasiliensis showed important biological effects, including anti-cancer [18], anti-inflammatory [19,20], and antianaphylactic activities [21]. α-Mangostin, a xanthone from G. mangostana, showed anti-biofilm [22,23], antimicrobial [24], and antidiabetic effects [25]. Kolaviron, a biflavonoid from G. kola, has antimalarial activities in P. berghei-infected mice [26]. In the last 20 years, our group’s focus on investigating Garcinia plants led to the isolation and characterization of many bioactive compounds, including depsidones, xanthones, biflavonoids, triterpenes, and biphenyl derivatives from G. paucinervis [27,28,29,30], G. bracteata [31,32,33], G. lancilimba [34], G. nujiangensis [35], G. multiflora [36], G. xanthochymus [37], and G. hanburyi [38,39].
G. oligantha, a 1–3 m tall shrub, has been used as a traditional Chinese herbal medicine to treat fevers, toothaches, and scalds [40]. Previous phytochemical investigations of the plant led to the isolation of many xanthone derivatives with anti-cancer and/or anti-inflammatory activities [4,10,41,42], but triterpenoids were rarely reported from it.
In our continuing search for biologically active and structurally unique compounds from the genus Garcinia, we investigated the chemical constituents of G. oligantha. Present phytochemical studies on the acetone extract of the leaves and twigs of G. oligantha afforded 11 triterpene derivatives (Figure 1), including five previously undescribed natural products, 2α,3β-diacetyl arjunolic acid (1), 3β,23-diacetyl arjunolic acid (2), 2α-acetyl arjunolic acid (3), 23-acetyl hovenic acid (4), and 2α,23-diacetyl hovenic acid (5), and six known triterpenoids, viz., betulinic acid (6) [43,44], betulin (7) [45], 3-hydroxy-23-acetoxy-lup-20(29)-en-28-oic acid (8) [46], 23-hydroxybetulinic acid (9) [47], tomentoid B (10) [48], and 2α,23-diacetoxy-3β-hydroxyolean-12-en-28-oic acid (11) [49,50]. These products’ in vitro cytotoxicities against three human cancer cell lines, HeLa (human cervical cancer), HepG-2 (human hepatocellular carcinoma), and MCF-7 (human breast cancer), were evaluated. In this paper, the isolation, structure elucidation of all compounds, and anti-cancer activities of 1–11 are reported.
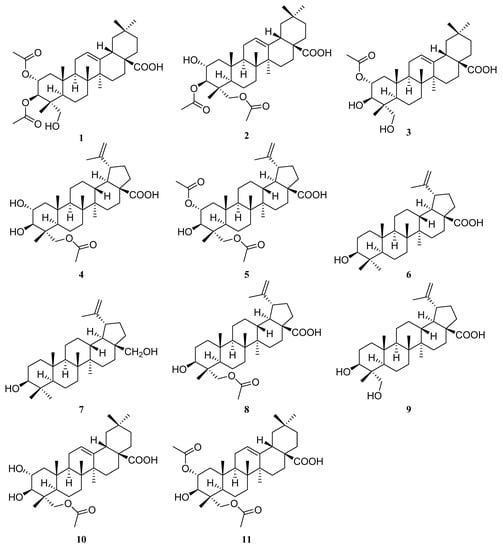
Figure 1.
Structures of 1–11.
2. Results
2.1. Isolation and Structure Elucidation of Triterpenoids
The dried leaves and twigs of G. oligantha were extracted with acetone (30 L × 72 h × 3) at room temperature. After removing the acetone under reduced pressure, the residue was extracted sequentially with petroleum ether (PE), dichloromethane (DCM), and methanol. The PE-soluble and DCM-soluble fractions were separated by silica gel, Sephadex LH-20 column chromatography, and semipreparative HPLC and purified by crystallization to yield 11 triterpene derivatives.
The molecular formula of compound 1 was determined as C34H52O7 based on the HRESIMS data at m/z 571.3633 [M − H]- (calcd 571.3640). The 1H NMR spectrum (Table 1) of 1 displayed signals corresponding to six tertiary methyls at δH 1.13 (3H, s), 1.08 (3H, s), 0.92 (3H, s), 0.90 (3H, s), 0.74 (3H, s), and 0.72 (3H, s), and two acetyl groups at δH 2.08 (3H, s) and 2.00 (3H, s), as well as one olefinic proton at δH 5.26 (1H, t, J = 3.8 Hz). The 13C NMR spectrum (Table 2) of 1 showed 34 carbon signals assigned to eight primary, ten secondary, six tertiary, and ten quaternary carbons, including two acetyl groups and the typical olefinic signals at δC 144.0 and 122.2 for the double bond at C-12 (13) of an oleanane-type triterpene. The 1H and 13C NMR spectra of 1 were similar to those of arjunolic acid except for the presence of two acetyl groups instead of two hydroxy groups in arjunolic acid [51]. The HMBC spectrum showed cross-peaks from H-2 and H3-32 to C-31 and from H-3 and H3-34 to C-33, confirming two acetyl groups located at C-2 and C-3, respectively (Figure 2). The stereochemistry of 1 was determined by analyzing the coupling constants and the NOESY experiment. The coupling constant (J = 10.7 Hz) between H-2 and H-3 indicated the orientation of two protons possessing dual-axial bonds. The NOESY spectrum showed the cross-peaks of H-2 with H3-24, H3-24 with H3-25, H3-25 with H3-26, and H-18 with H3-30, indicating the β orientation in these protons. The NOESY correlations of H-3 with H-5 and H2-23 and H-9 with H-5 and H3-27 suggested their α-orientation (Figure 3). After the hydrolysis of 1, compound 1a was obtained, and its 1D NMR data and specific rotation were the same as those of arjunolic acid, which further confirmed the above conclusion [51,52]. Thus, the structure of 1 was determined, as shown in Figure 1, and named 2α,3β-diacetyl arjunolic acid.

Table 1.
1H NMR spectroscopic data of 1–6 measured at 600 MHz [δH, mult. (J in Hz)].

Table 2.
13C NMR spectroscopic data of 1–5 measured at 125 MHz.
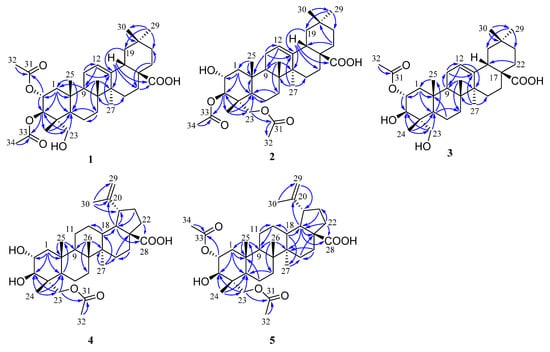
Figure 2.
Key HMBC ( ) correlations of 1–5.
) correlations of 1–5.
 ) correlations of 1–5.
) correlations of 1–5.
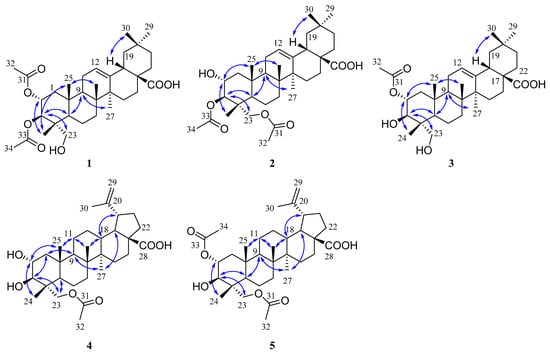
Figure 3.
Key NOESY ( ) correlations of 1–5.
) correlations of 1–5.
 ) correlations of 1–5.
) correlations of 1–5.
Compound 2 displayed an [M − H]− ion peak at m/z 571.3639 (calcd 571.3640) in its negative mode HRESIMS, corresponding to the molecular formula C34H52O7. The 1H NMR data (Table 1) showed eight methyls at δH 2.11 (3H, s), 2.08 (3H, s), 1.12 (3H, s), 1.01 (3H, s), 0.92 (3H, s), 0.90 (3H, s), 0.84 (3H, s), and 0.74 (3H, s), and one olefinic proton at δH 5.28 (1H, t, J = 3.4 Hz). The 13C NMR spectrum (Table 2) showed 34 carbon signals, including three carbonyls at δC 183.87, 172.32, and 171.03, and two olefinic carbons at δC 143.8 and 122.4. The 13C and 1H NMR data of 1 and 2 are very similar, with major differences being the chemical shifts of C-2, and H-2 shifted upfield from δC 69.6, and δH 5.23 to δC 67.6, and δH 3.86; C-23 and H2-23 were shifted downfield from δC 64.6, and δH 3.37, 2.89 to δC 65.5, and δH 3.82, 3.65, respectively, suggesting that the acetoxy at C-2 in 1 was instead located at C-23 in 2. This finding was further supported by the HMBC correlation from H2-23 to C-31. We determined the relative configuration of 2 according to the NOESY correlations (Figure 3) and the coupling constant (10.1 Hz) between H-2 and H-3. Hence, the structure of 2 was confirmed, as shown in Figure 1, and named 3β,23-diacetyl arjunolic acid.
Compound 3 was isolated as a white amorphous powder. The pseudomolecular ion peak at m/z 531.3694 [M + H]+ in its HRESIMS suggested C32H50O6 as its molecular formula. The 1H NMR (Table 1) of 3 revealed the presence of seven methyls at δH 2.05 (3H, s), 1.17 (3H, s), 1.08 (3H, s), 0.94 (3H, s), 0.90 (3H, s), 0.84 (3H, s), and 0.73 (3H, s), and one olefinic proton at δH 5.23 (1H, br s). The 13C NMR spectrum (Table 2) showed 31 signals, including a carbonyl at δC 173.0 and two olefinic carbons at δC 145.7 and 123.1, respectively. Comparing the 1D NMR data of 3 to those of tomentoid B (10) [48] indicated that the two compounds were closely related. The obvious spectroscopic difference between them resulted from the presence of the acetoxy group at C-2 in 3, instead of C-23 in 10. The shielded chemical shifts of C-23 and H2-23 of 3 further supported the above assignment. Its similar NMR data and same molecular formula as tomentoid B (10) indicated that 3 also had a carboxyl group at C-17, of which the carbon signal was missing. Fortunately, the carboxyl carbon signal was observed at 181.1 in the 13C NMR spectrum measured in CDCl3 (Figure S25). The large proton spin-coupling constant (J = 10.8 Hz) of H-3 with H-2 suggests that the protons at C-2 and C-3 are trans-axial. Furthermore, the relative stereochemistry of 3 was established by the NOESY cross-peaks shown in Figure 3. Therefore, the structure of 3 was named 2α-acetyl arjunolic acid (Figure 1).
Compound 4 was obtained as a white amorphous powder. Its molecular formula was deduced as C32H50O6 based on a negative-ion at m/z 529.3528 [M − H]− (calcd for C32H49O6−, 529.3535). Signals of six methyl singlets at δH 2.06 (3H, s), 1.70 (3H, s), 1.01 (3H, s), 0.97 (3H, s), 0.96 (3H, s), 0.75 (3H, s), and two olefinic protons at δH 4.72 (1H, d, J = 2.2 Hz) and 4.60 (1H, br s) were observed in the 1H NMR spectrum (Table 1). The 13C NMR spectrum (Table 2) showed 32 carbon signals, including six methyls, two carbonyls, and two olefinic carbons. The 1H and 13C NMR spectra of 4 were similar to those of the known compound hovenic acid [53], with the most noticeable difference observed for the hydroxy at C-23 in hovenic acid replaced by an acetoxy in 4. The HMBC correlations from H2-23 and H3-32 to C-31 confirmed the above deduction. The stereochemistry of 4 was determined by analyzing the coupling constants and NOESY data. The large spin-coupling constant (JH-2, H-3 = 9.7 Hz) indicated that the 2,3-dihydroxyl groups should have a 2α,3β-orientation, further supported by the NOESY correlations of H-2 with H3-24, and H-3 with H2-23 (Figure 3). We can confirm the above conclusion by comparing the compound’s specific rotation and NOESY spectrum to hovenic acid [53,54]. Thus, we determined the structure of 4 as 23-acetyl hovenic acid (Figure 1).
Compound 5 was obtained as a white amorphous powder. Its molecular formula was determined as C34H52O7 based on the HRESIMS. The 1H NMR spectrum (Table 1) of 5 in CD3OD (600 MHz) displayed signals corresponding to five tertiary methyls at δH 1.70 (3H, s), 1.01 (6H, s), 0.97 (3H, s), 0.80 (3H, s), two acetoxy groups at δH 2.07 (3H, s), 2.04 (3H, s), oxygenated methylene at δH 3.95 (2H, s), and two olefinic protons at δH 4.71 (1H, s) and 4.59 (1H, s), respectively. The 13C NMR spectrum (Table 2) of 5 revealed signals corresponding to 34 carbon atoms, including seven methyls, three carbonyls, and a double bond. The 1H and 13C NMR data of 5 were identical to those of 4, except for the existence of one more acetoxyl group in 5 at δH 2.04 (3H, s) and δC 172.9 and 21.3. Compared to the 1H NMR spectrum of 4, the resonance of H-2 shifted downfield from δH 3.68 to 5.01, and the HMBC correlation of H-2 to C-33 in 5 suggested the acetoxy group located at C-2. The coupling constant (JH-2, H-3 = 10.1 Hz) established the 2α,3β-orientation of 2-OH and 3-OH, further confirmed by the NOESY correlations (Figure 3) of H-2 with H3-24 and H-3 with H2-23. Therefore, we determined the structure of 5 as 2α,23-diacetyl hovenic acid.
2.2. Evaluation of Biological Activity of Compounds 1–11
We tested the anti-tumor activities of compounds 1–11 against three cancer cell lines (HeLa, HepG-2, and MCF-7).
Compounds 1–3, 10, and 11 are oleanane-type triterpenoids. Compounds 1, 2, and 11 with two acetoxys had stronger inhibitory activity than compounds 3 and 10 with one acetoxy, indicating that the more acetylated the hydroxyl groups, the stronger the activity.
Compounds 4–9 belong to lupane-type triterpenoids. Among them, compound 8 was the strongest inhibitor with IC50 values of 5.04–9.76 μM, whereas compounds 6 and 7 exhibited no cytotoxicity to the three cell lines, indicating that 23-hydroxyl or 23-acetoxy group can increase inhibitory activity. Compound 4 exhibited moderate activity against HeLa and HepG-2 cell lines, while 5 and 9 had moderate activity against HeLa and MCF-7 cell lines (Table 3).

Table 3.
Cytotoxic activities of compounds 1–11 against three cancer cell lines.
3. Discussion
In previous phytochemical investigations on the genus Garcinia, pentacyclic triterpenoids were rarely reported [55]. In this study, we isolated 11 pentacyclic triterpenoids, including five new natural products (1–5), from the leaves and twigs of G. oligantha. They belong to oleanane- and lupane-type triterpenes, most of which possess 23-acetoxyl or acetoxy groups. Compounds 1–11 were tested for their cytotoxic activity against HeLa, HepG-2, and MCF-7 cell lines. Compound 8 showed the highest anti-cancer activity against these three human cancer cell lines, with IC50 values ranging from 5.04 to 9.76 μM. Among the five oleanane-type triterpenoids, the derivatives with two acetoxy groups showed more potent activity than those with one acetoxy group. For lupane-type triterpenes, the ones possessing 23-hydroxyl or 23-acetoxy groups were more active. The above results may support future investigations into the anti-tumor drug design of triterpenoids.
4. Materials and Methods
4.1. General Experimental Procedure
The UV spectra were obtained by a Shimadzu UV-2600i spectrometer (Shimadzu, Kyoto, Japan). Optical rotations were measured by a JASCO P-2000 polarimeter (Anton Paar, Ostfildern, Germany). We recorded the HRESIMS data on Bruker microTOFQ-Q mass spectrometers (Billerica, MA, USA) and an Agilent 6550 Q-TOF (Agilent Technologies, Palo Alto, CA, USA). The NMR spectra were recorded on a Bruker AVANCE Ⅲ HD 600 MHz NMR spectrometer (Bruker BioSpin, Billerica, MA, USA) using TMS as an internal standard. The chromatographic silica gel (Qingdao Haiyang Chemical Factory, Qingdao, China), ODS (YMC Co., Ltd., Kyoto, Japan), and Sephadex LH-20 (GE Healthcare, Uppsala, Sweden). We recorded the analytical HPLC data using a Shimadzu SPD-M20A series machine equipped with a YMC C-18 column (250 mm × 4.6 mm, 5 μm). We conducted the semipreparative HPLC using a Shimadzu LC-6AD series pumping system equipped with an SPD-20A UV detector and C-18 column (20 mm × 250 mm, 5 μm; YMC Co., Ltd.). All the organic solvents were purchased from Yuwang and Laibo Chemicals Industries, Ltd., Shenyang, China.
4.2. Plant Material
The plant material was purchased from Kunming Plant Classification Biotechnology Co., Ltd., and collected from Diaoluo Mountain (GPS coordinates 109°41′38″~110°4′46″ E, 18°38′42″~18°50′22″ N), Lingshui County, Hainan Province, China, in March 2019, which was authenticated as G. oligantha by Mr. Jun Zhang of Kunming Plant Classification Biotechnology Co., Ltd. Kunming, China. A voucher specimen (DHSZZ-201903) was deposited in the Department of Natural Products Chemistry, Shenyang Pharmaceutical University, Shenyang, China.
4.3. Extraction and Isolation
The air-dried leaves and twigs of G. oligantha (3.07 kg) were extracted with acetone (3 × 30 L) at room temperature to produce 387 g of dried resin, which was then extracted by PE, DCM, and methanol, successively.
The PE-soluble part (50.2 g) was fractionated by column chromatography (CC) over silica gel using solvent mixtures of PE-acetone (100:0–0:100, v/v) to obtain nine fractions (P1–P9). Fraction P3 (1.9 g) was separated over a silica gel column and eluted with CH2Cl2-MeOH (0:1–1:0) to yield subfractions P3.1–P3.7. Fr. P3.3 (736.3 mg) was separated over a silica gel column and eluted with PE-DCM (0:1–1:0, v/v) to produce ten subfractions P3.3.1–P3.3.7. Fr. P3.3.6 (23.6 mg) was purified by Sephadex LH-20 (MeOH) and then isolated by semipreparative HPLC (93% MeOH-H2O) to yield 12 (tR = 29.9 min, 6.5 mg).
The DCM-soluble part (128.0 g) was subjected to silica gel CC with a PE-EtOAc gradient system to produce six fractions (D1–D6). Fraction D2 (3107.2 mg) was chromatographed on ODS with a MeOH-H2O gradient system to produce six subfractions (D2.1–D2.6). Fr. D2.4 (2214.4 mg) was subjected to a Sephadex LH-20 column (MeOH) to produce subfractions D2.4.1–D2.4.5. Fr. D2.4.3 (1167.7 mg) was further purified by semipreparative HPLC using a solvent of 80% MeOH-H2O from 0 to 100 min, and ending with 100% MeOH from 100 to 120 min. This process created three subfractions D2.4.3.1 (224.1 mg), D2.4.3.2 (195.2 mg), and D2.4.3.3 (543.9 mg). Fr. D2.4.3.2 and D2.4.3.3 were repeatedly recrystallized from MeOH to obtain 6 (10.3 mg) and 7 (8.9 mg), respectively.
Fraction D4 (23.0 g) was purified by CC over ODS using solvent mixtures of MeOH-H2O (60:40–100:0, v/v) to produce five subfractions D4.1–D4.5. Fr. D4.3 was separated by a silica gel CC (PE/acetone) to obtain four subfractions D4.3.1–D4.3.4. Fr. D4.3.1 (1359.3 mg) was separated by Sephadex LH-20 eluted with MeOH to produce three subfractions D4.3.1.1–D4.3.1.3. Fr. D4.3.1.1 (1000.5 mg) was purified by semipreparative HPLC, eluted with 84% MeOH-H2O, and further separated by semipreparative HPLC (80% MeOH-H2O) to yield 8 (tR = 45.8 min, 7.2 mg).
Fraction D.5 (12.0 g) was chromatographed on an ODS CC and eluted with MeOH-H2O (55:45 to 100:0) in a step-gradient manner to produce four subfractions D5.1–D5.4. Fr. D5.2 (2308.5 mg) was separated by Sephadex LH-20 with MeOH to produce four subfractions D5.2.1–D5.2.4. Fr. D5.2.2 (183.4 mg) was subjected to a semipreparative HPLC (75% MeOH-H2O) to obtain three subfractions (D5.2.2.1–D5.2.2.3). Fr. D5.2.2.2 (14.4 mg) was purified by semipreparative HPLC (60% CH3CN-H2O) to obtain 4 (tR = 47.2 min, 5.3 mg). Subfraction D5.2.2.3 was separated by the same method to obtain 3 (tR = 48.5 min, 1.8 mg) and 10 (tR = 50.7 min, 3.1 mg). Fr. D5.3 (2042.7 mg) was separated over Sephadex LH-20 (MeOH), and then purified by semipreparative HPLC (83% MeOH-H2O, from 0 to 40 min; 100% MeOH, from 40 to 60 min) to yield 2 (tR = 17.1 min, 8.4 mg) and 11 (tR = 25.2 min, 40.2 mg) and subfraction D5.3.3.3 (tR = 40.0–60.0 min, 155.9 mg). Fr. D5.3.3.3 was subjected to semipreparative HPLC (76% MeOH-H2O) to obtain 1 (tR = 41.1 min, 10.9 mg) and 5 (tR = 50.7 min, 3.2 mg). Fr. D5.3.4 (1049.1 mg) was separated by semipreparative HPLC, using 80% MeOH-H2O as the mobile phase, producing the subfraction D5.3.4.3 (155.0 mg), which was subjected to semipreparative HPLC and eluted with 69% CH3CN-H2O to produce 9 (tR = 24.1 min, 11.9 mg).
4.3.1. 2α,3β-Diacetyl Arjunolic Acid (1)
4.3.2. 3β,23-Diacetyl Arjunolic Acid (2)
4.3.3. 2α-Acetyl Arjunolic Acid (3)
4.3.4. 23-Acetyl Hovenic Acid (4)
4.3.5. 2α,23-Diacetyl Hovenic Acid (5)
4.4. Hydrolysis of 2α,3β-Diacetyl Arjunolic Acid (1)
Compound 1 (1.5 mg) was dissolved in methanol, and 1.5 μL of NaOH-H2O saturated solution was added and stirred at room temperature for 4 h. Then, the reaction solution was adjusted to pH 5–6 with 10% HCl and filtered to obtain a white solid 1a.
Arjunolic Acid (1a): White amorphous powder; [α]20D +69° (c 0.1, EtOH); 1H NMR (CD3OD, 600 MHz) δH 0.70 (3H, s, H-24), 0.82 (3H, s, H-26), 0.91 (3H, s, H-29), 0.95 (3H, s, H-30), 1.03 (3H, s, H-25), 1.18 (3H, s, H-27), 2.86 (1H, dd, J = 12.8 and 3.4 Hz, H-18), 3.27 (1H, d, J = 9.0 Hz, H-3), 3.35 (1H, d, J = 12.0 Hz, H-23a), 3.50 (1H, d, J = 12.0 Hz, H-23b), 3.69 (1H, m, H-2), 5.26 (1H, brs, H-5). 13C NMR (CD3OD, 150 MHz): δC 47.6 (C-1), 69.7 (C-2), 78.2 (C-3), 44.1 (C-4), 48.2 (C-5), 19.1 (C-6), 33.8 (C-7), 40.6 (C-8), 47.9 (C-9), 39.0 (C-10), 24.0 (C-11), 123.4 (C-12), 145.4 (C-13), 43.0 (C-14), 28.8 (C-15), 24.6 (C-16), 47.2 (C-17), 42.7 (C-18), 48.9 (C-19), 31.6 (C-20), 34.9 (C-21), 33.3 (C-22), 66.3 (C-23), 13.9 (C-24), 17.8 (C-25), 17.6 (C-26), 26.5 (C-27), 181.9 (C-28), 33.6 (C-29), 24.0 (C-30).
4.5. Cytotoxic Activity
4.5.1. Cell Lines and Tested Compounds
Three kinds of human cancer cell lines (HeLa, MCF-7, HepG2) were cultured in Dulbecco’s Modified Eagle medium (DMEM) (HyClone, Logan, UT, USA) with 10% fetal bovine serum (FBS, ExCell Bio, Shanghai, China) in a humidified atmosphere containing 5% CO2 at 37 °C.
The tested compounds were solubilized in DMSO and stored at 4 °C.
4.5.2. Cell Cultures
We determined the anti-cancer effects of compounds 1–11 using a CCK8 assay, as reported earlier [56]. The cells were seeded in a 96-well plate as 8 × 104 cells/mL (100 μL/well). In total, 100 μL/well of the serial dilutions of the tested compounds (50, 25, 12.5, 6.25, 3.125 µM) and adriamycin (2.5, 1.25, 0.625, 0.3125, 0.15625 µM) were added to the plate after the overnight incubation of the cells at 37 °C and 5% CO2. The cells were incubated for 48 h. Subsequently, 10 μL of CCK8 was added to each well, the plate was incubated for 1 h, and the absorbance of the wells was measured at 450 nm using a Biotech plate reader. Each experiment was repeated three times, and the standard deviation was calculated (±). The concentration that caused a 50% inhibition of cell growth (IC50) was calculated for each compound.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12010192/s1, Figures S1–S51: The 1H, 13C NMR, HSQC, HMBC, NOESY and HRMS spectra of compounds 1–5; Figures S52–S78: The 1H, 13C NMR and HRMS spectra of compounds 6–12.
Author Contributions
Conceptualization, D.L. and H.H.; methodology, X.P., C.W., Y.H. and J.T.; validation, X.P., C.W., Y.H. and J.T.; formal analysis, X.P.; investigation, X.P. and X.F.; resources, D.L. and H.H.; writing—original draft preparation, X.P.; writing—review and editing, X.P., D.L. and H.H.; supervision, D.L. and H.H.; project administration, H.H.; funding acquisition, H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (31570350).
Data Availability Statement
The data presented in this study are available in the Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheng, L.-Y.; Tsai, Y.-C.; Fu, S.-L.; Cheng, M.-J.; Sung, P.-J.; Chung, M.-I.; Chen, J.-J. Acylphloroglucinol derivatives from Garcinia multiflora with anti-inflammatory effect in LPS-Induced RAW264.7 Macrophages. Molecules 2018, 23, 2587. [Google Scholar] [CrossRef] [PubMed]
- Chaturonrutsamee, S.; Kuhakarn, C.; Surawatanawong, P.; Prabpai, S.; Kongsaeree, P.; Jaipetch, T.; Piyachaturawat, P.; Jariyawat, S.; Akkarawongsapat, R.; Suksen, K.; et al. Polycyclic polyprenylated acylphloroglucinols and biphenyl derivatives from the roots of Garcinia nuntasaenii Ngerns. & Suddee. Phytochemistry 2017, 146, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Lu, L.; Zhou, X.; Shen, J.; Song, W.; Tang, Y.; Xia, Z. A new cytotoxic polycyclic polyprenylated acylphloroglucinol from Garcinia nujiangensis screened by the LC-PDA and LC-MS. Nat. Prod. Res. 2019, 34, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zheng, H.; Wang, X.; Zhou, L.; Wang, S.; Shen, T.; Ren, D. Cytotoxic new caged-polyprenylated xanthonoids from Garcinia oligantha. Fitoterapia 2021, 156, 105092. [Google Scholar] [CrossRef]
- Gao, X.-M.; Cui, M.-Z.; Yu, T.; Hu, Q.-F.; Pu, J.-X.; Du, X.; Liu, T.-C.; Luo, K.Q. A novel xanthone from Garcinia oligantha. Helvetica Chim. Acta 2013, 96, 494–498. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, Y.; Qin, Y.; Xia, C.; Ye, Y.; Hu, Q.; Li, Y. A new xanthone from the stems of Garcinia oligantha and their anti-tobacco mosaic virus activity. Asian J. Chem. 2014, 26, 6685–6686. [Google Scholar] [CrossRef]
- Gao, X.-M.; Yu, T.; Cui, M.-Z.; Pu, J.-X.; Du, X.; Han, Q.-B.; Hu, Q.-F.; Liu, T.-C.; Luo, K.Q.; Xu, H.-X. Identification and evaluation of apoptotic compounds from Garcinia oligantha. Bioorganic Med. Chem. Lett. 2012, 22, 2350–2353. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Zhao, W.; Xia, Z.-Y.; Kong, G.-H.; Lu, X.-P.; Hu, Q.-F.; Gao, X.-M. Three new xanthones from the stems of Garcinia oligantha and their anti-TMV activity. Phytochem. Lett. 2013, 6, 629–632. [Google Scholar] [CrossRef]
- Tang, Y.-X.; Fu, W.-W.; Wu, R.; Tan, H.-S.; Shen, Z.-W.; Xu, H.-X. Bioassay-guided isolation of prenylated xanthone derivatives from the leaves of Garcinia oligantha. J. Nat. Prod. 2016, 79, 1752–1761. [Google Scholar] [CrossRef]
- Tang, Y.-X.; Fu, W.-W.; Xi, Z.-C.; Yang, J.-L.; Zheng, C.-W.; Lu, Y.; Shen, Z.-W.; Xu, H.-X. Xanthone derivatives from the leaves of Garcinia oligantha. Eur. J. Med. Chem. 2019, 181, 111536. [Google Scholar] [CrossRef]
- Sukandar, E.R.; Kaennakam, S.; Rassamee, K.; Ersam, T.; Siripong, P.; Tip-pyang, S. Tetrandraxanthones A-I, prenylated and geranylated xanthones from the stem bark of Garcinia tetrandra. J. Nat. Prod. 2019, 82, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Raksat, A.; Phukhatmuen, P.; Yang, J.X.; Maneerat, W.; Charoensup, R.; Andersen, R.J.; Wang, Y.A.; Pyne, S.G.; Laphookhieo, S. Phloroglucinol benzophenones and xanthones from the leaves of Garcinia cowa and their nitric oxide production and alpha-glucosidase inhibitory activities. J. Nat. Prod. 2020, 83, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhang, H.; Jiang, J.-M.; Chen, Y.-Y.; Wan, S.-J.; Lin, Z.-X.; Xu, H.-X. Prenylated xanthones and biphenyls from Garcinia esculenta with antistaphylococcal activity. Nat. Prod. Res. 2019, 35, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Abderamane, B.; Tih, A.E.; Ghogomu, R.T.; Blond, A.; Bodo, B. New flavonoid C–O–C dimers and other chemical constituents from Garcinia brevipedicellata stem heartwood. Z. Naturforsch. C J. Biosci. 2016, 71, 233–241. [Google Scholar] [CrossRef]
- Akongwi, M.; Tih, A.E.; Nyongbela, K.D.; Samje, M.; Ghogomu, R.T. Brevipedicelones D and E, two C-O-C flavonoid dimers from the leaves of Garcinia brevipedicellata and anti-onchocercal activity. Nat. Prod. Bioprospect. 2019, 9, 61–68. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Nguyen, V.T.; Van, C.P.; Nguyen, T.T.; Le, T.T.A.; Mai, H.D.T.; Truong, B.N.; Litaudon, M.; Ninh, T.S. A new flavonoid from the leaves of Garcinia mckeaniana Craib and alpha-glucosidase and acetylcholinesterase inhibitory activities. Nat. Prod. Res. 2022, 36, 5074–5080. [Google Scholar] [CrossRef]
- Fuentes, R.G.; Pearce, K.C.; Du, Y.; Rakotondrafara, A.; Valenciano, A.L.; Cassera, M.B.; Rasamison, V.E.; Crawford, T.D.; Kingston, D.G.I. Phloroglucinols from the roots of Garcinia dauphinensis and their antiproliferative and antiplasmodial activities. J. Nat. Prod. 2018, 82, 431–439. [Google Scholar] [CrossRef]
- Sales, L.; Pezuk, J.A.; Borges, K.S.; Brassesco, M.S.; Scrideli, C.A.; Tone, L.G.; dos Santos, M.H.; Ionta, M.; de Oliveira, J.C. Anticancer activity of 7-epiclusianone, a benzophenone from Garcinia brasiliensis, in glioblastoma. BMC Complement. Altern. Med. 2015, 15, 393. [Google Scholar] [CrossRef]
- Santa-Cecília, F.V.; Freitas, L.A.; Vilela, F.C.; Veloso, C.D.C.; da Rocha, C.Q.; Moreira, M.E.; Dias, D.F.; Giusti-Paiva, A.; dos Santos, M.H. Antinociceptive and anti-inflammatory properties of 7-epiclusianone, a prenylated benzophenone from Garcinia brasiliensis. Eur. J. Pharmacol. 2011, 670, 280–285. [Google Scholar] [CrossRef]
- Santa-Cecília, F.V.; Santos, G.B.; Fuzissaki, C.N.; Derogis, P.B.M.C.; Freitas, L.A.; Gontijo, V.S.; Stringheta, P.C.; Nagem, T.J.; Brigagão, M.R.; Dos Santos, M.H. 7-Epiclusianone, the natural prenylated benzophenone, inhibits superoxide anions in the neutrophil respiratory burst. J. Med. Food 2012, 15, 200–205. [Google Scholar] [CrossRef]
- Neves, J.S.; Coelho, L.P.; Cordeiro, R.S.B.; Veloso, M.P.; Silva, P.M.R.; Santos, M.H.; Martins, M.A. Antianaphylactic properties of 7-epiclusianone, a tetraprenylated benzophenone isolated from Garcinia brasiliensis. Planta Med. 2007, 73, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Vo, B.H.; Tran, N.T.; Van, Q.D. Anti-biofilm activity of α-mangostin isolated from Garcinia mangostana L. Z. Naturforsch. C J. Biosci. 2015, 70, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.T.M.; Quang, N.V.; Mai, T.T.; Anh, N.V.; Kuhakarn, C.; Reutrakul, V.; Bolhuis, A. Antibiofilm activity of alpha-mangostin extracted from Garcinia mangostana L. against Staphylococcus aureus. Asian Pac. J. Trop. Med. 2017, 10, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.M.; Marquis, R.E. Antimicrobial actions of α-mangostin against oral streptococci. Can. J. Microbiol. 2011, 57, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Nelli, G.B.; Kilari, A.S.; Kilari, E.K. Antidiabetic effect of α-mangostin and its protective role in sexual dysfunction of streptozotocin induced diabetic male rats. Syst. Biol. Reprod. Med. 2013, 59, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Oluwatosin, A.; Tolulope, A.; Ayokulehin, K.; Patricia, O.; Aderemi, K.; Catherine, F.; Olusegun, A. Antimalarial potential of kolaviron, a biflavonoid from Garcinia kola seeds, against Plasmodium berghei infection in Swiss albino mice. Asian Pac. J. Trop. Med. 2014, 7, 97–104. [Google Scholar] [CrossRef]
- Li, D.-H.; Li, C.-X.; Jia, C.-C.; Sun, Y.-T.; Xue, C.-M.; Bai, J.; Hua, H.-M.; Liu, X.-Q.; Li, Z.-L. Xanthones from Garcinia paucinervis with in vitro anti-proliferative activity against HL-60 cells. Arch. Pharmacal Res. 2015, 39, 172–177. [Google Scholar] [CrossRef]
- Jia, C.; Xue, J.; Li, X.; Li, D.; Li, Z.; Hua, H. New depsidone and dichromone from the stems of Garcinia paucinervis with antiproliferative activity. J. Nat. Med. 2018, 73, 278–282. [Google Scholar] [CrossRef]
- Jia, C.; Han, T.; Xu, J.; Li, S.; Sun, Y.; Li, D.; Li, Z.; Hua, H. A new biflavonoid and a new triterpene from the leaves of Garcinia paucinervis and their biological activities. J. Nat. Med. 2017, 71, 642–649. [Google Scholar] [CrossRef]
- Jia, C.-C.; Xue, J.-J.; Gong, C.; Li, X.-Y.; Li, D.-H.; Li, Z.-L.; Hua, H.-M. Chiral resolution and anticancer effect of xanthones from Garcinia paucinervis. Fitoterapia 2018, 127, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.-L.; Li, Z.-L.; Ji, F.; Liu, G.-Y.; Zhao, N.; Liu, X.-Q.; Jing, Y.-K.; Hua, H.-M. Xanthones from the stem bark of Garcinia bracteata with growth inhibitory effects against HL-60 cells. Phytochemistry 2012, 77, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.-L.; Li, D.-H.; Li, X.-Y.; Wang, Y.-T.; Li, S.-G.; Bai, J.; Pei, Y.-H.; Jing, Y.-K.; Li, Z.-L.; Hua, H.-M. Bioassay- and chemistry-guided isolation of scalemic caged prenylxanthones from the leaves of Garcinia bracteata. J. Nat. Prod. 2018, 81, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.-L.; Li, D.-H.; Wang, Y.-T.; Wang, K.-B.; Lin, B.; Jing, Y.-K.; Hua, H.-M.; Bai, J.; Li, Z.-L. Neobraclactones A–C, three unprecedented chaise longue-shaped xanthones from Garcinia bracteata. Org. Biomol. Chem. 2017, 15, 4901–4906. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, D.; Jia, C.; Xue, C.; Bai, J.; Li, Z.; Hua, H. Three new xanthones from the leaves of Garcinia lancilimba. J. Nat. Med. 2015, 70, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Hu, X.; Peng, X.-H.; Wang, Y.-T.; Huang, X.-F.; Zan, Y.-H.; Li, D.-H.; Li, Z.-L.; Hua, H.-M. Polyprenylated xanthones from the twigs and leaves of Garcinia nujiangensis and their cytotoxic evaluation. Bioorganic Chem. 2019, 94, 103370. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.-Y.; Jiang, C.; Ji, F.; Hua, H.-M.; Li, Z.-L. Chemical constituents from the stem barks of Garcinia multiflora. J. Asian Nat. Prod. Res. 2013, 15, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Li, Z.; Liu, G.; Niu, S.; Zhao, N.; Liu, X.; Hua, H. Xanthones with antiproliferative effects on prostate cancer cells from the stem bark of Garcinia xanthochymus. Nat. Prod. Commun. 2012, 7, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Li, Z.-L.; Song, D.-D.; Sun, L.; Pei, Y.-H.; Jing, Y.-K.; Hua, H.-M. Two novel triterpenoids with antiproliferative and apoptotic activities in human leukemia cells isolated from the resin of Garcinia hanburyi. Planta Medica 2008, 74, 1735–1740. [Google Scholar] [CrossRef]
- Wang, L.L.; Li, Z.L.; Xu, Y.P.; Liu, X.Q.; Pei, Y.H.; Jing, Y.K.; Hua, H.M. A new cytotoxic caged polyprenylated xanthone from the resin of Garcinia hanburyi. Chin. Chem. Lett. 2008, 19, 1221–1223. [Google Scholar] [CrossRef]
- Yang, J.; Fu, W.; Xiang, Q.; Tang, Y.; Wu, R.; Zheng, C.; Lu, Y.; Zhou, H.; Xu, H. Cytotoxic xanthone derivatives from the twigs of Garcinia oligantha. Phytochemistry 2020, 174, 112329. [Google Scholar] [CrossRef]
- Yang, J.; Fu, W.; Xiang, Q.; Wu, R.; Tang, Y.; Zheng, C.; Lu, Y.; Zhou, H.; Xu, H. Cytotoxic 7-methoxylated caged xanthones from the twigs of Garcinia oligantha. Chin. J. Chem. 2021, 39, 2898–2910. [Google Scholar] [CrossRef]
- Jiang, Y.; Xiao, L.; Fu, W.; Tang, Y.; Lertnimitphun, P.; Kim, N.; Zheng, C.; Tan, H.; Lu, Y.; Xu, H. Gaudichaudione H inhibits inflammatory responses in macrophages and dextran sodium sulfate-induced colitis in mice. Front. Pharmacol. 2020, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, W.; Deng, Z.W.; Lin, W.H. Assignment of the absolute stereochemistry of an unusual diterpenoid from the mangrove plant Excoecaria agallocha L. J. Chinese Pharm. Sci. 2010, 19, 387–392. [Google Scholar] [CrossRef]
- Esposito, F.; Sanna, C.; Del Vecchio, C.; Cannas, V.; Venditti, A.; Corona, A.; Bianco, A.; Serrilli, A.M.; Guarcini, L.; Parolin, C.; et al. Hypericum hircinum L. components as new single-molecule inhibitors of both HIV-1 reverse transcriptase-associated DNA polymerase and ribonuclease H activities. Pathog. Dis. 2013, 68, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.H.; Kwon, H.-J.; Kim, J.-H.; Ra, J.C.; Kim, J.A.; Kim, Y.H. An anti-influenza component of the bark of Alnus japonica. Arch. Pharmacal Res. 2010, 33, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, H.; Liu, J.; Liu, Y.; Wang, Y.; Xu, S.; Zhu, Z.; Xu, J. Synthesis, biological evaluation and mechanism studies of C-23 modified 23-hydroxybetulinic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2019, 182, 111659. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.R.; Li, X.B.; Luu, T.T.T.; Wang, M.Y. Liposoluble constituents of Urticae Rhizoma. Chin. Pharm. J. 2017, 52, 1217–1222. [Google Scholar]
- Zhang, Y.-B.; Li, W.; Zhang, Z.-M.; Chen, N.-H.; Zhang, X.-Q.; Jiang, J.-W.; Wang, G.-C.; Li, Y.-L. Two new triterpenoids from the roots of Rhodomyrtus tomentosa. Chem. Lett. 2016, 45, 368–370. [Google Scholar] [CrossRef]
- Higuchi, R.; Toshio, T. Pericarp saponins of Akebia quinata Decne. II. Arjunolic and norarjunolic acids, and their glycosides. Chem. Pharm. Bull. 1976, 24, 1314–1323. [Google Scholar] [CrossRef]
- Chaudhuri, P.K.; Singh, D. A new triterpenoid from the rhizomes of Nelumbo nucifera. Nat. Prod. Res. 2013, 27, 532–536. [Google Scholar] [CrossRef]
- Bisoli, E.; Freire, T.V.; Yoshida, N.C.; Garcez, W.S.; Queiroz, L.M.M.; Matos, M.F.C.; Perdomo, R.T.; Garcez, F.R. Cytotoxic phenanthrene, dihydrophenanthrene, and dihydrostilbene derivatives and other aromatic compounds from Combretum laxum. Molecules 2020, 25, 3154. [Google Scholar] [CrossRef] [PubMed]
- King, F.E.; King, T.J.; Ross, J.M. The chemistry of extractives from hardwoods. Part XVIII. The constitution of arjunolic acid, a triterpene from Terminalia arjuna. J. Chem. Soc. 1954, 29, 3995–4003. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kondo, Y.; Kimura, E.; Arihara, S. A lupane-triterpene and a 3(2→1) abeolupane glucoside from Hovenia trichocarea. Phytochemistry 1998, 49, 2057–2060. [Google Scholar] [CrossRef]
- Ge, Y. Studies on Chemical Constituents and Pharmacological Activity of the Leaves of Forsythia suspensa (Thunb.). Master’s Thesis, Hebei Medical University, Hebei Province, China, 2014. [Google Scholar]
- Forestrania, R.C.; Anaya-Eugenio, G.D.; Acuña, U.M.; Ren, Y.; Elya, B.; de Blanco, E.C. Secondary metabolites from Garcinia daedalanthera Pierre leaves (Clusiaceae). Nat. Prod. Res. 2020, 36, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gao, X.; Gao, G.; Wang, Y.B.; Cao, H.; Li, D.H.; Hua, H.M. Discovery of beta-carboline-(phenylsulfonyl)furoxan hybrids as potential anti-breast cancer agents. Bioorg. Med. Chem. Lett. 2021, 40, 127952–127964. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).