Desiccation Stress Tolerance in Porphyra and Pyropia Species: A Latitudinal Analysis along the Chilean Coast
Abstract
:1. Introduction
2. Results
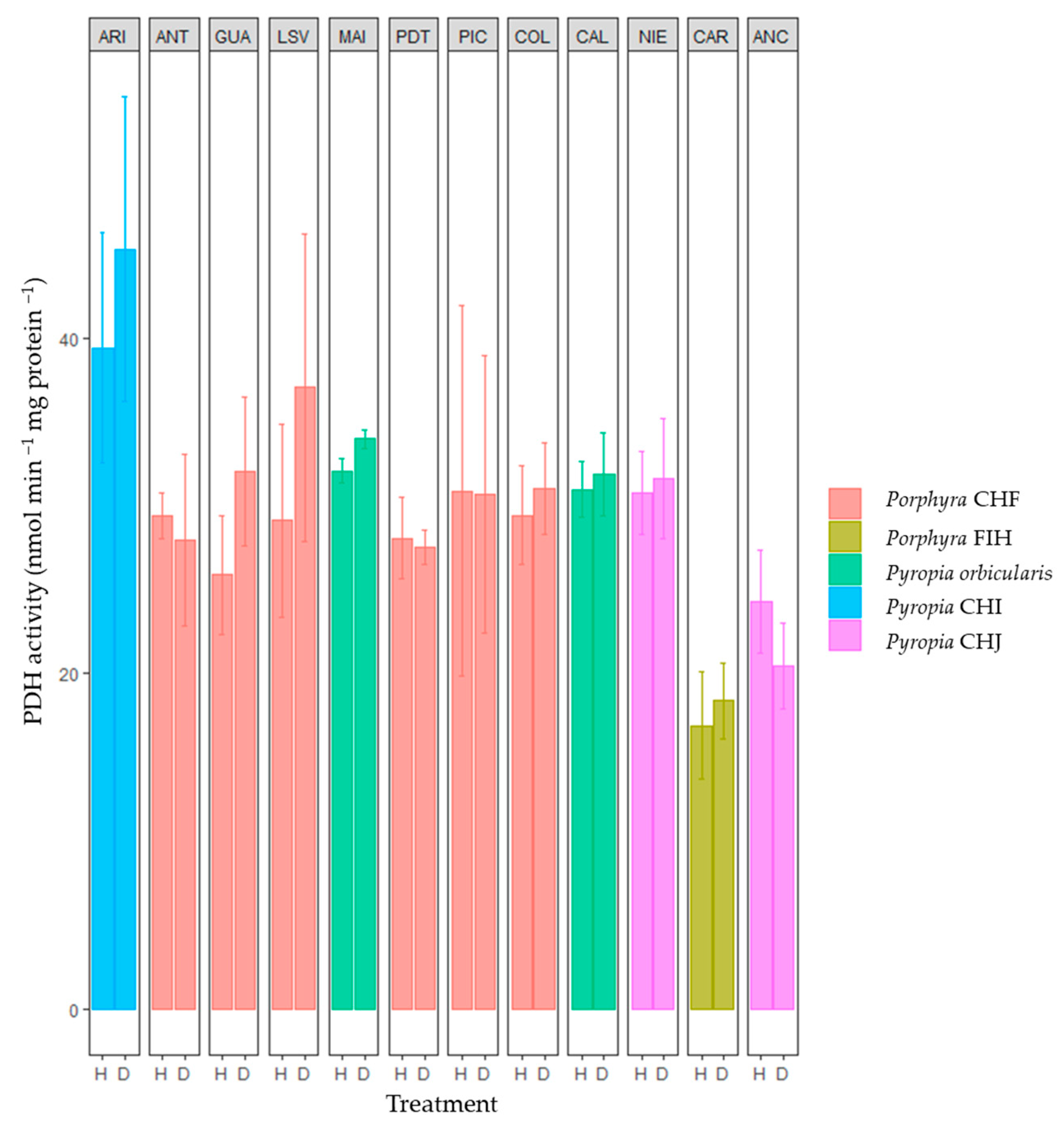
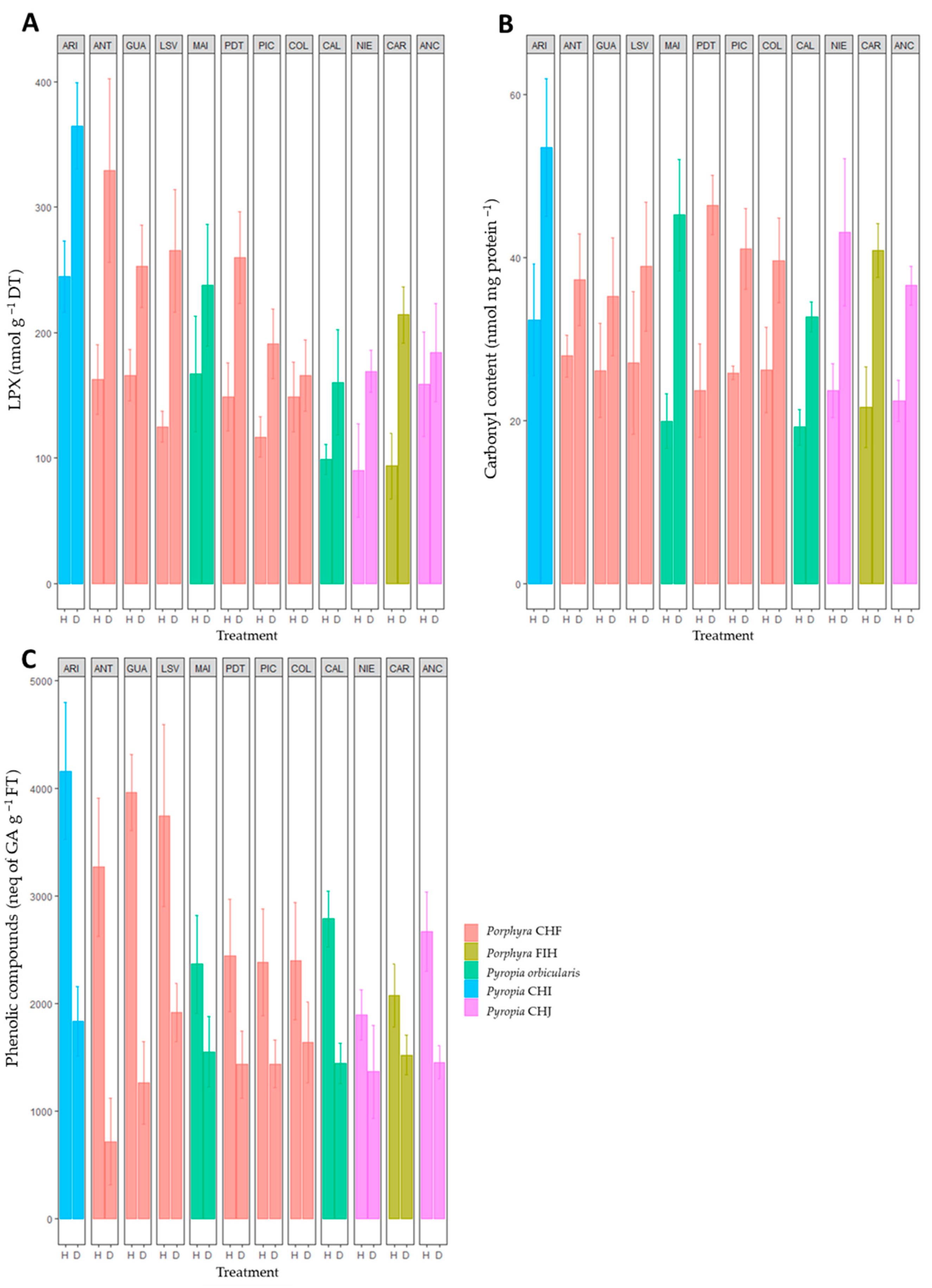
2.1. Specific Activity of Basal Metabolic and Antioxidant Enzymes and Metabolites along the Chilean Coast
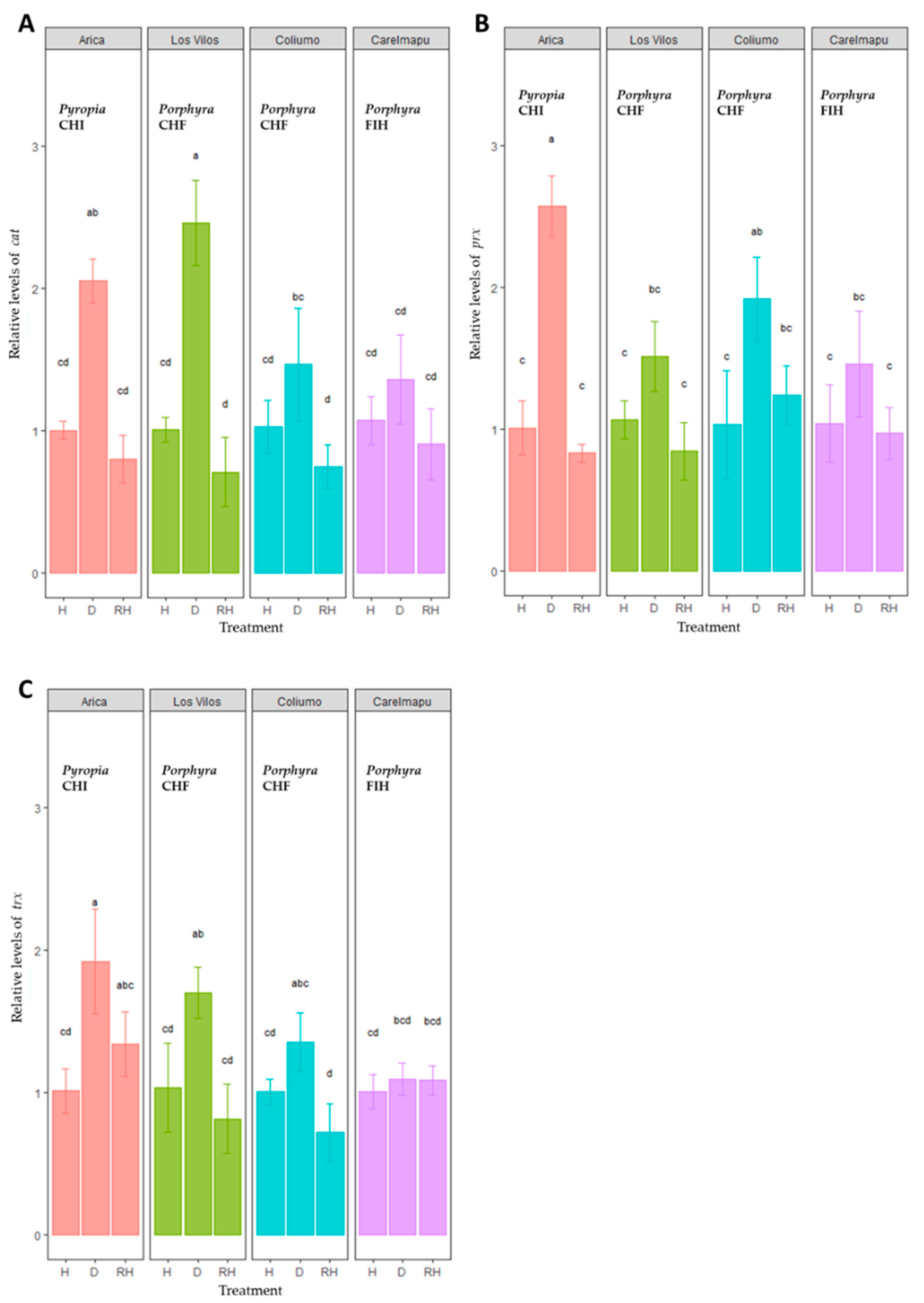
2.2. Differential Expression of Antioxidant Enzyme Transcripts
3. Discussion
4. Materials and Methods
4.1. Sampling, Study Sites, and Experimental Design
4.2. Extraction and Quantification of Protein Extracts
4.3. Biochemical Determinations
4.4. Differential Gene Expression
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Contreras-Porcia, L.; López-Cristoffanini, C.; Meynard, A.; Kumar, M. Tolerance Pathways to Desiccation Stress in Seaweeds. In Systems Biology of Marine Ecosystems; Kumar, M., Ralph, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 13–33. ISBN 978-3-319-62092-3. [Google Scholar] [CrossRef]
- Abdullah Al, M.; Akhtar, A.; Rahman, M.F.; Kamal, A.H.M.; Karim, N.U.; Hassan, M.L. Habitat structure and diversity patterns of seaweeds in the coastal waters of Saint Martin’s Island, Bay of Bengal, Bangladesh. Reg. Stud. Mar. Sci. 2020, 33, 100959. [Google Scholar] [CrossRef]
- Fariña, J.M.; He, Q.; Silliman, B.R.; Bertness, M.D. Biogeography of salt marsh plant zonation on the Pacific coasts of South America. J. Biogeogr. 2018, 45, 238–247. [Google Scholar] [CrossRef]
- Holzinger, A.; Karsten, U. Desiccation stress and tolerance in green algae: Consequences for ultrastructure, physiological and molecular mechanisms. Front. Plant Sci. 2013, 4, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Shen, S.; Wang, G.; Niu, J.; Lin, A.; Pan, G. PSI-Driven Cyclic Electron Flow Allows Intertidal Macroalgae Ulva sp. (Chlorophyta) to Survive in Desiccated Conditions. Plant Cell. Physiol. 2011, 52, 885–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Xue, S.; Chen, J.; Shang, S.; Xiao, H.; Zang, Y.; Tang, X. Effects of different short-term UV-B radiation intensities on metabolic characteristics of Porphyra haitanensis. Int. J. Mol. Sci. 2021, 22, 2180. [Google Scholar] [CrossRef]
- Contreras-Porcia, L.; Thomas, D.; Flores, V.; Correa, J.A. Tolerance to oxidative stress induced by desiccation in Porphyra columbina (Bangiales, Rhodophyta). J. Exp. Bot. 2011, 62, 1815–1829. [Google Scholar] [CrossRef] [Green Version]
- Flores-Molina, M.R.; Thomas, D.; Lovazzano, C.; Núñez, A.; Zapata, J.; Kumar, M.; Correa, J.A.; Contreras-Porcia, L. Desiccation stress in intertidal seaweeds: Effects on morphology, antioxidant responses and photosynthetic performance. Aquat. Bot. 2014, 113, 90–99. [Google Scholar] [CrossRef]
- Quan, W.; Ying, M.; Wang, Y.; Zhou, Q.; Xu, C. Chlorophyll Fluorescence Characteristics Responses of Two Strains of Porphyra haitanensis to Emersion and Submersion. Int. J. Agric. Biol. 2017, 19, 887–892. [Google Scholar] [CrossRef]
- Shi, J.; Wang, W.; Lin, Y.; Xu, K.; Xu, Y.; Ji, D.; Chen, C.; Xie, C. Insight into transketolase of Pyropia haitanensis under desiccation stress based on integrative analysis of omics and transformation. BMC Plant Biol. 2019, 19, 475. [Google Scholar] [CrossRef]
- Huang, L.-B.; Peng, L.-N.; Yan, X.-H. Multi-omics responses of red algae Pyropia haitanensis to intertidal desiccation during low tides. Algal Res. 2021, 58, 102376. [Google Scholar] [CrossRef]
- Ramírez, M.E.; Contreras-Porcia, L.; Guillemin, M.L.; Brodie, J.; Valdivia, C.; Flores-Molina, M.R.; Núñez, A.; Bulboa Contador, C.; Lovazzano, C. Pyropia orbicularis sp. nov. (Rhodophyta, Bangiaceae) based on a population previously known as Porphyra columbina from the central coast of Chile. Phytotaxa 2014, 158, 133–153. [Google Scholar] [CrossRef]
- Guillemin, M.L.; Contreras-Porcia, L.; Ramírez, M.E.; Macaya, E.C.; Bulboa Contador, C.; Woods, H.; Wyatt, C.; Brodie, J. The bladed Bangiales (Rhodophyta) of the South Eastern Pacific: Molecular species delimitation reveals extensive diversity. Mol. Phylogenet. Evol. 2016, 94, 814–826. [Google Scholar] [CrossRef]
- Meynard, A.; Zapata, J.; Salas, N.; Betancourtt, C.; Pérez-Lara, G.; Castañeda, F.; Ramírez, M.E.; Bulboa Contador, C.; Guillemin, M.L.; Contreras-Porcia, L. Genetic and morphological differentiation of Porphyra and Pyropia species (Bangiales, Rhodophyta) coexisting in a rocky intertidal in Central Chile. J. Phycol. 2019, 55, 297–313. [Google Scholar] [CrossRef]
- Thiel, M.; Macaya, E.C.; Acuña, E.; Arntz, W.E.; Bastias, H.; Brokordt, K.; Camus, P.A.; Castilla, J.C.; Castro, L.R.; Cortés, M.; et al. The Humboldt Current System of northern and central Chile. Oceanogr. Mar. Biol. 2007, 45, 195–344. [Google Scholar]
- Camus, P.A. Marine biogeography of continental Chile. Rev. Chil. Hist. Nat. 2001, 74, 587–617. [Google Scholar] [CrossRef] [Green Version]
- Tellier, F.; Meynard, A.P.; Correa, J.A.; Faugeron, S.; Valero, M. Phylogeographic analyses of the 30° S south-east Pacific biogeographic transition zone establish the occurrence of a sharp genetic discontinuity in the kelp Lessonia nigrescens: Vicariance or parapatry? Mol. Phylogenet. Evol. 2009, 53, 679–693. [Google Scholar] [CrossRef]
- González, A.V.; Beltrán, J.; Hiriart-Bertrand, L.; Flores, V.; de Reviers, B.; Correa, J.A.; Santelices, B. Identification of cryptic species in the Lessonia nigrescens complex (Phaeophyceae, Laminariales). J. Phycol. 2012, 48, 1153–1165. [Google Scholar] [CrossRef]
- López-Cristoffanini, C.; Tellier, F.; Otaíza, R.; Correa, J.A.; Contreras-Porcia, L. Tolerance to air exposure: A feature driving the latitudinal distribution of two sibling kelp species. Bot. Mar. 2013, 56, 431–440. [Google Scholar] [CrossRef]
- Contreras-Porcia, L.; Callejas, S.; Thomas, D.; Sordet, C.; Pohnert, G.; Contreras, A.; Lafuente, A.; Flores-Molina, M.R.; Correa, J.A. Seaweeds early development: Detrimental effects of desiccation and attenuation by algal extracts. Planta 2012, 235, 337–348. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). 2022a. Statical Query Panel for Global Aquaculture Production. Available online: https://www.fao.org/fishery/statistics-query/en/ (accessed on 1 July 2022).
- SERNAPESCA. Anuario estadístico de Pesca y Acuicultura. Servicio Nacional de Pesca, Valparaíso. 2021. Available online: http://www.sernapesca.cl/informacion-utilidad/anuarios-estadisticos-de-pesca-y-acuicultura (accessed on 10 September 2022).
- Betancourtt, C.; Zapata, J.; Latorre, N.; Anguita, C.; Castañeda, F.; Meynard, A.; Fierro, C.; Espinoza, C.; Guajardo, E.; Núñez, A.; et al. Spatio-temporal variation in the composition of the macroalgae assemblage of the intertidal rocky zone from Maitencillo, Valparaíso, central coast of Chile. Rev. Biol. Mar. Oceanogr. 2018, 53, 105–117. [Google Scholar] [CrossRef]
- Zapata, J.; Meynard, A.; Anguita, C.; Espinoza, C.; Alvear, P.; Kumar, M.; Contreras-Porcia, L. Non-Random distribution and ecophysiological differentiation of Pyropia species (Bangiales, Rhodophyta) through environmental gradients. J. Phycol. 2019, 55, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Porcia, L.; López-Cristoffanini, C.; Lovazzano, C.; Flores-Molina, M.R.; Thomas, D.; Núñez, A.; Fierro, C.; Guajardo, E.; Correa, J.A.; Kube, M.; et al. Differential gene expression in Pyropia columbina (Bangiales, Rhodophyta) under natural hydration and desiccation conditions. Lat. Am. J. Aquat. Res. 2013, 41, 933–958. [Google Scholar] [CrossRef]
- López-Cristoffanini, C.; Zapata, J.; Gaillard, F.; Potin, P.; Correa, J.A.; Contreras-Porcia, L. Identification of proteins involved in desiccation tolerance in the red seaweed Pyropia orbicularis (Rhodophyta, Bangiales). Proteomics 2015, 15, 3954–3968. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, E.; Correa, J.A.; Contreras-Porcia, L. Role of abscisic acid (ABA) in activating antioxidant tolerance responses to desiccation stress in intertidal seaweed species. Planta 2016, 243, 767–781. [Google Scholar] [CrossRef]
- Fierro, C.; López-Cristoffanini, C.; Latorre, N.; Rivas, J.; Contreras-Porcia, L. Methylglyoxal metabolism in seaweeds during desiccation. Rev. Biol. Mar. Oceanogr. 2016, 51, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Fierro, C.; López-Cristoffanini, C.; Meynard, A.; Lovazzano, C.; Castañeda, F.; Guajardo, E.; Contreras-Porcia, L. Expression profile of desiccation tolerance factors in intertidal seaweed species during the tidal cycle. Planta 2017, 245, 1149–1164. [Google Scholar] [CrossRef]
- Lalegerie, F.; Gager, L.; Stiger-Pouvreau, V.; Connan, S. Chapter Eight—The Stressful life of Red and Brown Seaweeds on the Temperate Intertidal Zone: Effect of Abiotic and Biotic Parameters on the Physiology of Macroalgae and Content Variability of Particular Metabolites. In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 95, pp. 247–287. [Google Scholar] [CrossRef]
- Bellorín, A.; Bulboa, C.; Contreras-Porcia, L. Algas: Una Introducción a la Ficología, 1st ed.; RIL Editores: Santiago, Chile, 2022; ISBN 978-956-01-0899-9. [Google Scholar]
- Karsten, U. Seaweed Acclimation to Salinity and Desiccation Stress. In Seaweed Biology. Ecological Studies; Wiencke, C., Bischof, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 219. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, P.; Reddy, C.R.K.; Jha, B. Chapter Four—Salinity and Desiccation Induced Oxidative Stress Acclimation in Seaweeds. In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71. [Google Scholar] [CrossRef]
- Karsten, U. Seasonal variation in heteroside concentrations of field-collected Porphyra species (Rhodophyta) from different biogeographic regions. New. Phytol. 1999, 143, 561–571. [Google Scholar] [CrossRef]
- Weng, Z.; Zhao, J.; Wang, Z.; Chen, J.; Luo, Q.; Yang, R.; Chen, H.; Zhang, P.; Wang, T. Responses of isomeric floridosides under stress in two heteromorphic generations of Neoporphyra haitanensis. Algal Res. 2022, 65, 102724. [Google Scholar] [CrossRef]
- Tcherkez, G.; Mahé, A.; Gauthier, P.; Mauve, C.; Gout, E.; Bligny, R.; Cornic, G.; Hodges, M. In Folio respiratory fluxomics revealed by 13C-isotopic labeling and H/D isotope effects highlight the noncyclic nature of the tricarboxylic acid “cycle” in illuminated leaves. Plant Physiol. 2009, 151, 620–630. [Google Scholar] [CrossRef] [Green Version]
- Araújo, W.L.; Nunes-Nesi, A.; Nikoloski, Z.; Sweetlove, L.J.; Fernie, A.R. Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ. 2012, 35, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Sampath-Wiley, P.; Neefus, C.D.; Jahnke, L.S. Seasonal effects of sun exposure and emersion on intertidal seaweed physiology: Fluctuations in antioxidant contents, photosynthetic pigments and photosynthetic efficiency in the red alga Porphyra umbilicalis Kützing (Rhodophyta, Bangiales). J. Exp. Mar. Biol. Ecol. 2008, 361, 83–91. [Google Scholar] [CrossRef]
- Lin, R.; Stekoll, M.S. Phycobilin content of the conchocelis phase of Alaskan Porphyra (Bangiales, Rhodophyta) species: Responses to environmental variables. J. Phycol. 2011, 47, 208–214. [Google Scholar] [CrossRef]
- Im, S.; Choi, S.; Hwang, M.S.; Park, E.-J.; Jeong, W.-J.; Choi, D.-W. De novo assembly of transcriptome from the gametophyte of the marine red algae Pyropia seriata and identification of abiotic stress response genes. J. Appl. Phycol. 2015, 27, 1343–1353. [Google Scholar] [CrossRef]
- Wang, W.; Lin, Y.; Teng, F.; Ji, D.; Xu, Y.; Chen, C.; Xie, C. Comparative transcriptome analysis between heat-tolerant and sensitive Pyropia haitanensis strains in response to high temperature stress. Algal Res. 2018, 29, 104–112. [Google Scholar] [CrossRef]
- Cao, M.; Wang, D.; Kong, F.; Wang, J.; Xu, K.; Mao, Y. A genome-wide identification of osmotic stress-responsive microRNAs in Pyropia haitanensis (Bangiales, Rhodophyta). Front. Mar. Sci. 2019, 6, 766. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Mo, Z.; Tang, X.; Gao, T.; Mao, Y. Genome-wide analysis of HSP70 gene superfamily in Pyropia yezoensis (Bangiales, Rhodophyta): Identification, characterization and expression profiles in response to dehydration stress. BMC Plant Biol. 2021, 21, 435. [Google Scholar] [CrossRef]
- Zhong, Z.-H.; Wang, W.-J.; Sun, X.-T.; Liu, F.-L.; Liang, Z.-R.; Wang, F.-J.; Chen, W.-Z. Developmental and physiological properties of Pyropia dentata (Bangiales, Rhodophyta) conchocelis in culture. J. Appl. Phycol. 2016, 28, 3435–3445. [Google Scholar] [CrossRef]
- Knoop, J.; Griffin, J.N.; Barrento, S. Cultivation of early life history stages of Porphyra dioica from the British Isles. J. Appl. Phycol. 2020, 32, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Brawley, S.H.; Blouin, N.A.; Ficko-Blean, E.; Wheeler, G.L.; Lohr, M.; Goodson, H.V.; Jenkins, J.W.; Blaby-Haas, C.E.; Helliwell, K.E.; Chan, C.X.; et al. Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta). Proc. Natl. Acad. Sci. USA 2017, 114, E6361–E6370. [Google Scholar] [CrossRef] [Green Version]
- Cho, T.J.; Rhee, M.S. Health Functionality and Quality Control of Laver (Porphyra, Pyropia): Current Issues and Future Perspectives as an Edible Seaweed. Mar. Drugs 2020, 18, 14. [Google Scholar] [CrossRef] [Green Version]
- FAO (Food and Agriculture Organization of the United Nations). 2022b. Thinking about the Future of Food Safety—A Foresight Report. Available online: https://www.fao.org/documents/card/en/c/cb8667en (accessed on 1 July 2022).
- Contreras, L.; Moenne, A.; Correa, J.A. Antioxidant responses in Scytosiphon lomentaria (Phaeophycae) inhabiting copper-enriched coastal environments. J. Phycol. 2005, 41, 1184–1195. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Lovazzano, C.; Serrano, C.; Correa, J.A.; Contreras-Porcia, L. Comparative analysis of peroxiredoxin activation in the brown macroalgae Scytosiphon gracilis and Lessonia nigrescens (Phaeophyceae) under copper stress. Physiol. Plant. 2013, 149, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef] [PubMed]



| Site Name | Geographic Coordinates | Species |
|---|---|---|
| Arica, Playa Corazones (ARI) | 18°32′55″ S, 70°19′52″ W | Pyropia CHI |
| Antofagasta, Playa El Lenguado (ANT) | 23°46′30″ S, 70°28′33″ W | Porphyra CHF |
| Guanaquerillos (GUA) | 30°11′41″ S, 71°25′18″ W | Porphyra CHF |
| Los Vilos (LSV) | 31°55′10″ S, 71°31′08″ W | Porphyra CHF |
| Maitencillo, Playa Marbella (MAI) | 32°39′13″ S, 71°26′37″ W | Pyropia orbicularis |
| Punta de Tralca, Playa las Conchitas de Isla Negra (PDT) | 33°26′22″ S, 71°41′18″ W | Porphyra CHF |
| Pichilemu, Playa Principal (PIC) | 34°22′50″ S, 72°00′58″ W | Porphyra CHF |
| Coliumo, Playa Las Rocas (COL) | 36°31′36″ S, 72°57′23″ W | Porphyra CHF |
| Calfuco (CAL) | 39°46′48″ S, 73°23′34″ W | Pyropia orbicularis |
| Niebla, Playa De Los Enamorados (NIE) | 39°51′36″ S, 73°23′38″ W | Pyropia CHJ |
| Carelmapu (CAR) | 41°44′32″ S, 73°44′36″ W | Porphyra FIH |
| Ancud, Playa Mar Brava (ANC) | 41°51′55″ S, 74°00′45″ W | Pyropia CHJ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Porcia, L.; Meynard, A.; Piña, F.; Kumar, M.; Lovazzano, C.; Núñez, A.; Flores-Molina, M.R. Desiccation Stress Tolerance in Porphyra and Pyropia Species: A Latitudinal Analysis along the Chilean Coast. Plants 2023, 12, 12. https://doi.org/10.3390/plants12010012
Contreras-Porcia L, Meynard A, Piña F, Kumar M, Lovazzano C, Núñez A, Flores-Molina MR. Desiccation Stress Tolerance in Porphyra and Pyropia Species: A Latitudinal Analysis along the Chilean Coast. Plants. 2023; 12(1):12. https://doi.org/10.3390/plants12010012
Chicago/Turabian StyleContreras-Porcia, Loretto, Andrés Meynard, Florentina Piña, Manoj Kumar, Carlos Lovazzano, Alejandra Núñez, and María Rosa Flores-Molina. 2023. "Desiccation Stress Tolerance in Porphyra and Pyropia Species: A Latitudinal Analysis along the Chilean Coast" Plants 12, no. 1: 12. https://doi.org/10.3390/plants12010012
APA StyleContreras-Porcia, L., Meynard, A., Piña, F., Kumar, M., Lovazzano, C., Núñez, A., & Flores-Molina, M. R. (2023). Desiccation Stress Tolerance in Porphyra and Pyropia Species: A Latitudinal Analysis along the Chilean Coast. Plants, 12(1), 12. https://doi.org/10.3390/plants12010012








