Native Hyperaccumulator Plants with Differential Phytoremediation Potential in an Artisanal Gold Mine of the Ecuadorian Amazon
Abstract
:1. Introduction
2. Results
2.1. Metal Concentration in Soils
2.2. Metal Concentration in Plants
2.3. Bioaccumulation and Translocation Factors
2.4. Tolerance Index (TI) of Plant Yield
3. Discussion
4. Materials and Methods
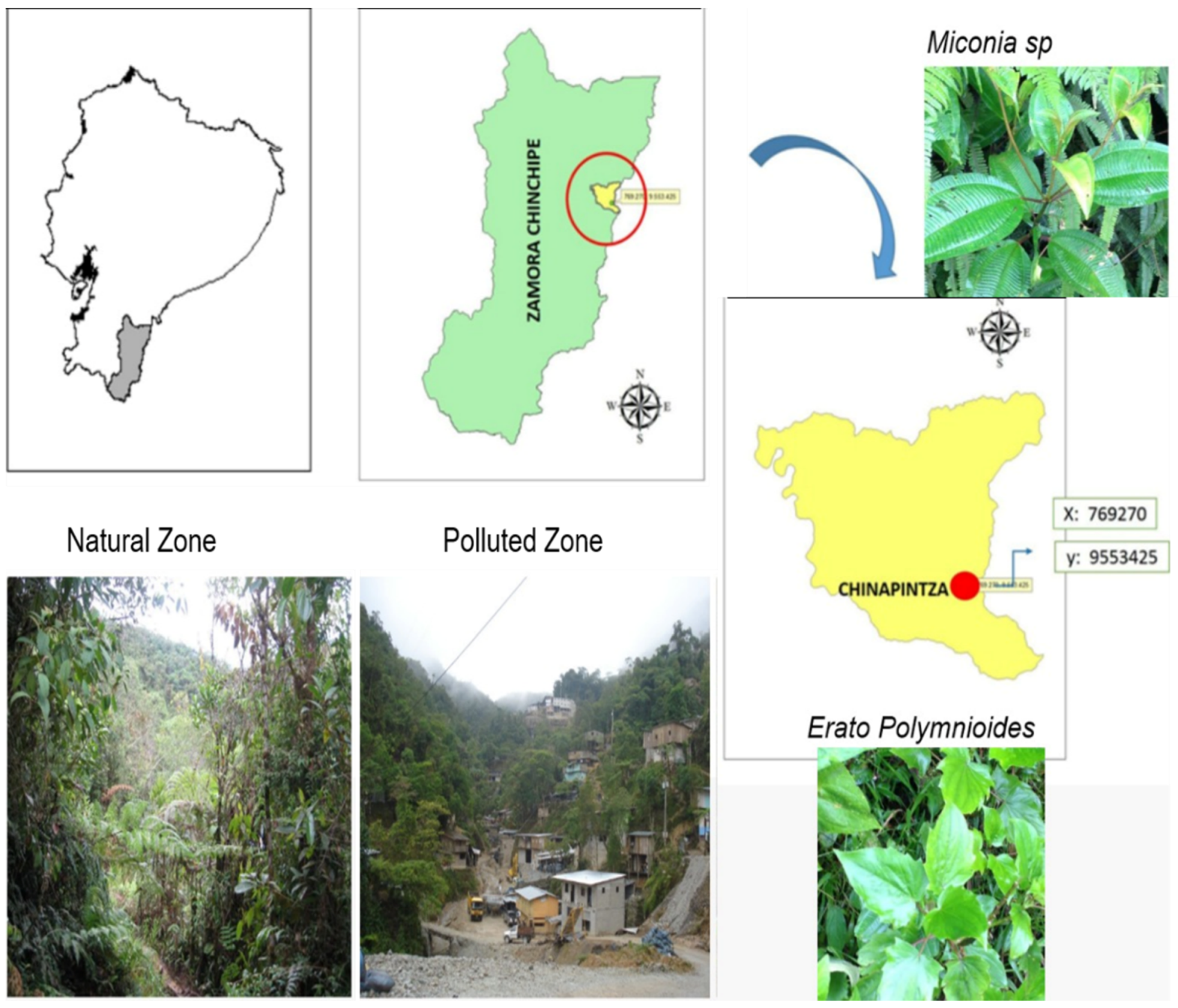
4.1. Study Area
4.2. Plant and Soil Sampling
4.3. Plant and Soil Analysis
4.4. Calculation of Bioaccumulation and Translocation Factors
4.5. Calculation of the Tolerance index (TI)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahugija, J.; Kasenya, Z.; Kilulya, K. Variations of Concentrations of Lead, Zinc, Iron, Copper and Cadmium in Urine of Primary School Pupils in Relation to Age, Sex and Academic Performance. Tanzan. J. Sci. 2020, 46, 190–204. [Google Scholar] [CrossRef]
- Mng’ong’o, M.; Munishi, L.; Ndakidemi, P.; Blake, W.; Comber, S.; Hutchinson, T. Toxic metals in East African agro-ecosystems: Key risks for sustainable food production. J. Environ. Manag. 2021, 294, 112973. [Google Scholar] [CrossRef]
- Emmanuel, A.; Jerry, C.; Dzigbodi, D. Review of Environmental and Health Impacts of Mining in Ghana. J. Health Pollut. 2018, 8, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obiri, S.; Yeboah, P.; Osae, S.; Adu-Kumi, S. Levels of arsenic, mercury, cadmium, copper, lead, zinc and manganese in serum and whole blood of resident adults from mining and non-mining communities in Ghana. Environ. Sci. Pollut. Res. 2016, 23, 16589–16597. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A. Responding to the Challenge of Artisanal and Small-Scale Mining Sustainable Markets; International Institute for Environment and Development (IIED): London, UK, 2013. [Google Scholar]
- Chamba, I.; Rosado, D.; Kalinhoff, C.; Thangaswamy, S.; Sánchez-Rodríguez, A.; Gazquez, M. Erato polymnioides—A novel Hg hyperaccumulator plant in ecuadorian rainforest acid soils with potential of microbe-associated phytoremediation. Chemosphere 2017, 188, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Berríos, N.; Aide, T. Global demand for gold is another threat for tropical forests. Environ. Res. Lett. 2015, 10, 014006. [Google Scholar] [CrossRef] [Green Version]
- Stoffersen, B.; Appel, P.; Na-Oy, L.; Sekamane, A.; Ruiz, I.; Kóster-Rasmussen, R. Introduction of Mercury-Free Gold Extraction to Small-Scale Miners in the Cabo Delgado Province in Mozambique. J. Health Pollut. 2018, 8, 180909. [Google Scholar] [CrossRef]
- Yeleliere, E.; Cobbina, S.; Duwiejuah, A. Review of Ghana’s water resources: The quality and management with particular focus on freshwater resources. Appl. Water Sci. 2018, 8, 93. [Google Scholar] [CrossRef]
- López-Blanco, C.; Collahuazo, L.; Torres, S. Mercury Pollution in Soils from the Yacuambi River (Ecuadorian Amazon) as a Result of Gold Placer Mining. Bull. Environ. Contam. Toxicol. 2015, 95, 311–316. [Google Scholar] [CrossRef]
- Wieczorek, J.; Baran, A.; Urbański, K.; Mazurek, R.; Klimowicz-Pawlas, A. Assessment of the pollution and ecological risk of lead and cadmium in soils. Environ. Geochem. Health 2018, 40, 2325–2342. [Google Scholar] [CrossRef]
- Rama, K.; Nishita, O.; Sadiqa, A.; Sonal, B. A review on heavy metal contamination at mining sites and remedial techniques. IOP Conf. Ser. Earth Environ. Sci. 2021, 796, 012013. [Google Scholar]
- Xin, J.; Ma, S.; Li, Y.; Zhao, C.; Tian, R. Pontederia cordata, an ornamental aquatic macrophyte with great potential in phytoremediation of heavy-metal-contaminated wetlands. Ecotoxicol. Environ. Saf. 2020, 203, 111024. [Google Scholar] [CrossRef] [PubMed]
- Salas-Moreno, M.; Marrugo-Negrete, J. Phytoremediation potential of Cd and Pb-contaminated soils by Paspalum fasciculatum Willd. ex Flüggé. Int. J. Phytoremediation 2020, 22, 87–97. [Google Scholar] [CrossRef]
- Gupta, R.; Mohapatra, H. Microbial biomass: An economical alternative for removal of heavy metals from waste water. Indian J. Exp. Biol. 2003, 41, 945–966. [Google Scholar] [PubMed]
- Sardar, U.; Bhargavi, E.; Devi, I.; Bhunia, B.; Tiwari, O. Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: A critical review. Carbohydr. Polym. 2018, 199, 353–364. [Google Scholar]
- Ashraf, S.; Ali, Q.; Zahir, Z.; Asghar, H. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Emenike, C.; Jayanthi, B.; Agamuthu, P.; Fauziah, S. Biotransformation and removal of heavy metals: A review of phytoremediation and microbial remediation assessment on contaminated soil. Environ. Rev. 2018, 26, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhao, B.; Jin, M.; Hu, L.; Zhong, H.; He, Z. A comprehensive survey on the horizontal and vertical distribution of heavy metals and microorganisms in soils of a Pb/Zn smelter. J. Hazard. Mater. 2020, 400, 123255. [Google Scholar] [CrossRef]
- Hasan, M.; Uddin, M.; Ara-Sharmeen, I.; Alharby, H.; Alzahrani, Y.; Hakeem, K.; Zhang, L. Assisting Phytoremediation of Heavy Metals Using Chemical Amendments. Plants 2019, 8, 295. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.; Wang, Y.; Tan, S.; Mohd-Yusof, M.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Favas, P.; Pratas, J.; Varun, M.; Souza, R.; Paul, M. Phytoremediation of Soils Contaminated with Metals and Metalloids at Mining Areas: Potential of Native Flora. In Environmental Risk Assessment of Soil Contaminatio; IntechOpen: London, UK, 2014; pp. 485–517. [Google Scholar]
- Wei, Z.; Van, L.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.; Sonne, C. A review on phytoremediation of contaminants in air, water and soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef] [PubMed]
- Baker, A. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M. Phytoremediation of heavy metals--concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Kachenga, L.; Chabwela, H.; Mwauluka, K. Phytoremediation Potential of Indigenous Plants Growing at Nchanga Mine in Chingola, Zambia. Open J. Ecol. 2020, 10, 45–61. [Google Scholar] [CrossRef] [Green Version]
- Nkansah, F.; Belford, E. Heavy Metals Accumulation by Indigenous Plants Growing in Contaminated Soil in a Gold Mining Area in Ghana. J. Nat. Sci. Res. 2017, 7, 41–47. [Google Scholar]
- Tariq, F.; Samsuri, A.; Karam, D.; Aris, A. Phytoremediation of Gold Mine Tailings Amended with Iron-Coated and Uncoated Rice Husk Ash by Vetiver Grass (Vetiveria zizanioides (Linn.) Nash). Appl. Environ. Soil Sci. 2016, 12, 4151898. [Google Scholar] [CrossRef] [Green Version]
- Surbhi, S. Difference between Standard Deviation and Standard Error (with Comparison Chart)—Key Differences. 2018. Available online: https://keydifferences.com/difference-between-standard-deviation-and-standard-error.html (accessed on 17 February 2022).
- Norma de Calidad Ambiental del Recurso Suelo y Criterios de Remediación Para Suelos Contaminados. Anexo 2 Libro VI 1. Available online: https://www.dspace.espol.edu.ec/bitstream/123456789/6078/39/LIBRO%20VI%20Anexo%202%20Remediacion%20de%20suelos.pdf (accessed on 10 February 2022).
- Ramírez, M.; Ramosa, J.; Angélica, R.; Brabob, E. Assessment of Hg-contamination in soils and stream sediments in the mineral district of Nambija, Ecuadorian Amazon (example of an impacted area affected by artisanal gold mining). Appl. Geochem. 2002, 18, 371–381. [Google Scholar] [CrossRef]
- Anda-Aguirre, A. Zamora de Quito y el oro de nambija; Casa de la Cultura Ecuatoriana “Benjamín Carrión”, Núcleo de Loja: Loja, Ecuador, 1986. [Google Scholar]
- Chamba, I.; Gazquez, M.J.; Selvaraj, T.; Calva, J.; Toledo, J.J.; Armijos, C. Selection of a suitable plant for phytoremediation in mining artisanal zones. Int. J. Phytoremediation 2016, 18, 853–860. [Google Scholar] [CrossRef]
- Nurcholis, M.; Yudiantoro, D.F.; Haryanto, D.; Mirzam, A. Heavy metals distribution in the Artisanal gold mining area in Wonogiri. Indones. J. Geogr. 2017, 49, 133–144. [Google Scholar] [CrossRef]
- Faz, A.; Zornoza, R.; Muñoz, M.; Acosta, J. Metals and metalloids in primary gold mining districts of Western Bolivia: Anthropogenic and natural sources. Environ. Earth Sci. 2013, 71, 5027–5036. [Google Scholar] [CrossRef]
- Hasnaoui, S.E.; Fahr, M.; Keller, C.; Levard, C.; Angeletti, B.; Chaurand, P.; Triqui, Z.E.A.; Guedira, A.; Rhazi, L.; Colin, F.; et al. Screening of native plants growing on a Pb/Zn mining area in eastern Morocco: Perspectives for phytoremediation. Plants 2020, 9, 1458. [Google Scholar] [CrossRef]
- Dan-Badjo, T.; Ibrahim, O.; Guéro, Y.; Morel, J.; Feidt, C.; Echevarria, G. Impacts of artisanal gold mining on soil, water and plant contamination by trace elements at Komabangou, Western Niger. J. Geochem. Explor. 2019, 205, 106328. [Google Scholar] [CrossRef]
- Fernández, S.; Poschenrieder, C.; Marcenò, C.; Gallego, J.; Jiménez-Gámez, D.; Bueno, A.; Afif, E. Phytoremediation capability of native plant species living on Pb-Zn and Hg-As mining wastes in the Cantabrian range, north of Spain. J. Geochem. Explor. 2017, 174, 10–20. [Google Scholar] [CrossRef]
- Basri, U.; Sakakibara, M.; Sera, K. Mercury in soil and forage plants from artisanal and small-scale gold mining in the bombana area, Indonesia. Toxics 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Peng, H.; Sheng, M.; Luo, H.; Wang, X.; Zhang, R.; Xu, F.; Xu, H. Evaluation of phytoremediation potential of native dominant plants and spatial distribution of heavy metals in abandoned mining area in Southwest China. Ecotoxicol. Environ. Saf. 2021, 220, 112368. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, G.; Rodella, A.; Aparecida, C.; Coscione, A. Vegetable species for phytoextraction of boron, copper, lead, manganese and zinc from contaminated soil. Sci. Agric. 2010, 67, 713–719. [Google Scholar]
- Liu, Z.; Chen, B.; Wang, L.; Urbanovich, O.; Nagorskaya, L.; Li, X.; Tang, L. A review on phytoremediation of mercury contaminated soils. J. Hazard. Mater. 2020, 400, 123138. [Google Scholar] [CrossRef]
- Susarla, S.; Medina, V.F.; McCutcheon, S.C. Phytoremediation: An ecological solution to organic chemical contamination. Ecol. Eng. 2002, 18, 647–658. [Google Scholar] [CrossRef]
- Kobina, A.; Marschner, B.; Antoniadis, V.; Stemn, E.; Shaheen, S.; Rinklebe, J. Human health risk via soil ingestion of potentially toxic elements and remediation potential of native plants near an abandoned mine spoil in Ghana. Sci. Total Environ. 2021, 798, 149272. [Google Scholar] [CrossRef]
- Mariwy, A.; Manuhutu, J.B.; Frans, D. Bioaccumulated Mercury by Several Types of Plants in Ex-Traditional Gold Processing Area, Gogorea Village, Buru Island. Indones. J. Chem. Res. 2021, 9, 105–110. [Google Scholar] [CrossRef]
- Xun, Y.; Feng, L.; Li, Y.; Dong, H. Mercury accumulation plant Cyrtomium macrophyllum and its potential for phytoremediation of mercury polluted sites. Chemosphere 2017, 189, 161–170. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kowalewska, A.; Mikołajczak, S.; Kołodziej, B.; Bryk, M.; Spychaj-Fabisiak, E.; Babula, J. Phytoextraction of heavy metals after application of bottom ash and municipal sewage sludge considering the risk of environmental pollution. J. Environ. Manag. 2022, 306, 114517. [Google Scholar] [CrossRef]
- Nikolić, M.; Stevović, S. Family Asteraceae as a sustainable planning tool in phytoremediation and its relevance in urban areas. Urban For. Urban Green. 2015, 14, 782–789. [Google Scholar] [CrossRef]
- Sacher, W. Revisión Crítica Parcial del Estudio de Impacto Ambiental para la Fase de Beneficio del Proyecto Minero de Cobre Mirador de la Empresa Ecuacorriente; de la Empresa Ecuacorriente: Quito, Ecuador, 2011. [Google Scholar]
- Raj, D.; Chowdhury, A.; Maiti, S. Ecological risk assessment of mercury and other heavy metals in soils of coal mining area: A case study from the eastern part of a Jharia coal field, India. Hum. Ecol. Risk Assess. 2017, 23, 767–787. [Google Scholar] [CrossRef]
- Santoro, A.; Held, A.; Linsinger, T.; Perez, A.; Ricci, M. Comparison of total and aqua regia extractability of heavy metals in sewage sludge: The case study of a certified reference material. Trends Anal. Chem. 2017, 89, 34. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Tapia, W.; Villamarín-Ortiz, A. Verificación del método analítico de espectroscopía de absorción atómica con horno de grafito para la cuantificación de cadmio en almendra de cacao (Theobroma cacao). La Granja 2020, 31, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Welna, M.; Pohl, P. Potential of the hydride generation technique coupled to inductively coupled plasma optical emission spectrometry for non-chromatographic As speciation. J. Anal. At. Spectrom. 2017, 32, 1766–1779. [Google Scholar] [CrossRef] [Green Version]
- Usman, K.; Al-Ghouti, M.; Abu-Dieyeh, M. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci. Rep. 2019, 9, 5658. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, A.F.R.; Hamzah, H.A. Heavy metals accumulation in suburban roadside plants of a tropical area (Jengka, Malaysia). Ecol. Processes 2018, 7, 28. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Chen, G.; Chen, Y.; Wang, L.; Shan, X.Q. Seedling growth and metal accumulation of selected woody species in copper and lead/zinc mine tailings. J. Environ. Sci. 2011, 23, 266–274. [Google Scholar] [CrossRef]
- Zuur, A.; Leno, E.; Walker, N.; Saveliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology with R; Springer Nature: New York, NY, USA, 2009. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, J.; Bates, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-157. 2022. Available online: https://CRAN.R-project.org/package=nlme (accessed on 18 February 2022).
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.7.2. 2022. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 18 February 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 18 February 2022).
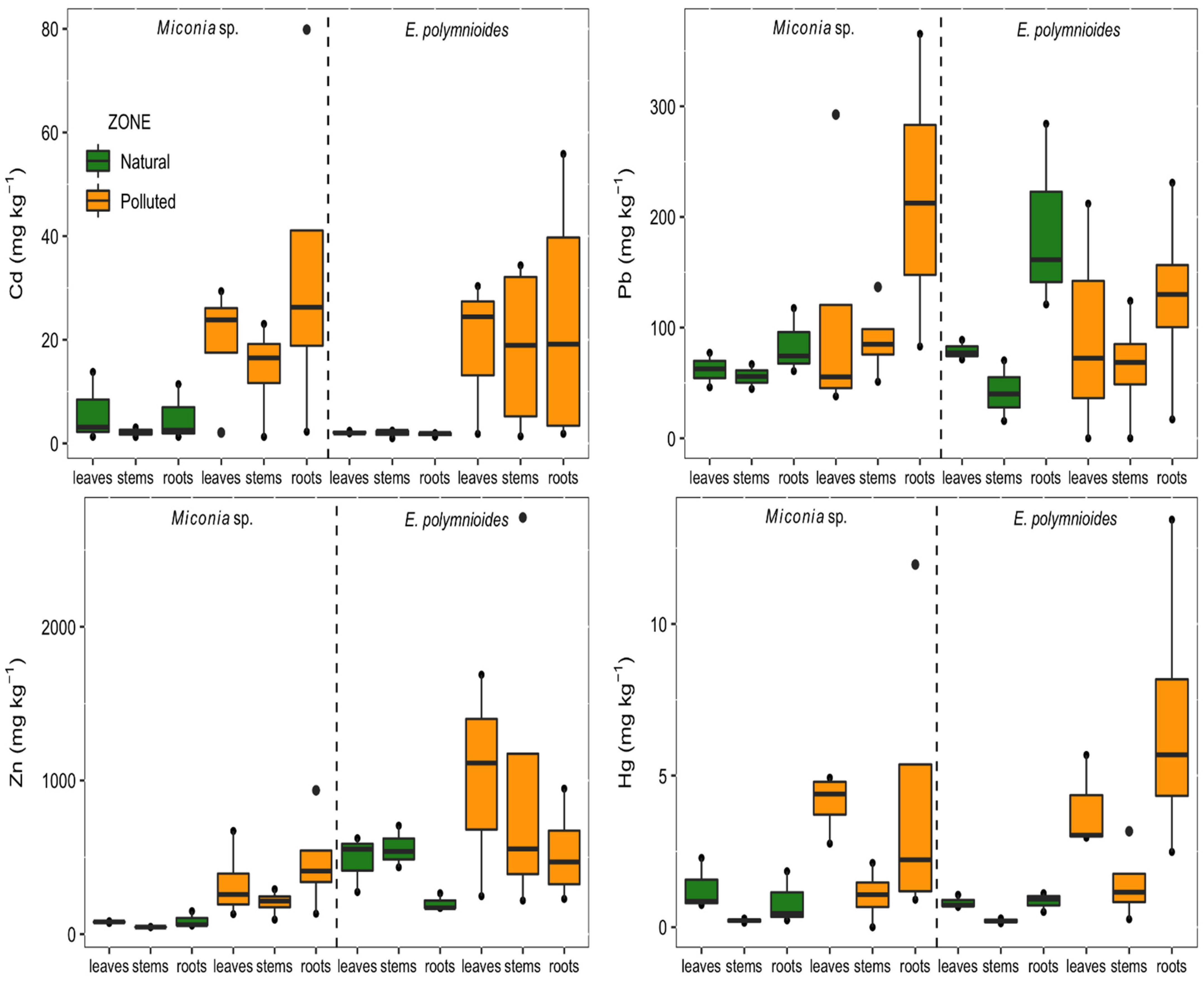
| Cd | Pb | Zn | Hg | ||
|---|---|---|---|---|---|
| Miconia sp. | NZ | 1.62 ± 0.59 37% | 510 ± 150 29% | 85 ± 16 19% | 1.62 ± 0.54 33% |
| PZ | 4.44 ± 1.68 38% | 523 ± 63 12% | 390 ± 140 36% | 6.1 ± 3.6 60% | |
| E. polymnioides | NZ | 0.42 ± 0.24 57% | 326 ± 75 23% | 169 ± 93 55% | 1.20 ± 0.22 18% |
| PZ | 5.15 ± 2.23 43% | 430 ± 250 58% | 589 ± 305 52% | 10.2 ± 6.0 59% |
| Total Plant Weight (g) | Plant Part Weight as Percentage of Total (%) | ||||
|---|---|---|---|---|---|
| Leaves | Stems | Roots | |||
| Miconia sp. | NZ | 10.7 ± 3.6 | 39.96 ± 0.92 | 39.7 ± 4.7 | 20.3 ± 5.6 |
| PZ | 1.96 ± 0.27 | 60.0 ± 1.3 | 23.5 ± 1.9 | 16.5 ± 2.1 | |
| E. polymnioides | NZ | 52 ± 11 | 17.1 ± 2.3 | 75.7 ± 1.8 | 7.23 ± 0.74 |
| PZ | 18.6 ± 2.9 | 25.5 ± 5.6 | 56 ± 12 | 18.4 ± 7.1 | |
| Cd | Pb | Zn | Hg | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NZ | PZ | NZ | PZ | NZ | PZ | NZ | PZ | ||
| Bioaccumulation factor (BCF) | |||||||||
| Miconia sp. | Leaves | 3.47 | 3.82 | 0.14 | 0.23 | 1.11 | 0.99 | 2.05 | 1.66 |
| Stems | 3.08 | 2.91 | 0.12 | 0.20 | 0.87 | 0.78 | 1.03 | 0.57 | |
| Roots | 3.09 | 6.50 | 0.20 | 0.49 | 1.48 | 2.34 | 1.10 | 1.05 | |
| E. polymnioides | Leaves | 2.88 | 6.19 | 0.25 | 0.53 | 4.48 | 3.28 | 0.73 | 1.19 |
| Stems | 2.85 | 4.62 | 0.12 | 0.38 | 4.53 | 2.08 | 0.17 | 0.31 | |
| Roots | 2.42 | 5.64 | 0.55 | 0.80 | 1.71 | 1.52 | 0.76 | 1.12 | |
| Translocation factor (TF) | |||||||||
| Miconia sp. | Leaves/roots | 1.14 | 0.84 | 0.75 | 0.65 | 1.00 | 0.73 | 2.53 | 1.72 |
| Stems/roots | 1.00 | 0.59 | 0.65 | 0.50 | 0.70 | 0.44 | 0.87 | 0.36 | |
| E. polymnioides | Leaves/roots | 1.27 | 1.55 | 0.46 | 0.53 | 2.40 | 2.21 | 1.04 | 1.27 |
| Stems/roots | 1.08 | 1.07 | 0.23 | 0.43 | 2.79 | 1.45 | 0.25 | 0.41 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamba-Eras, I.; Griffith, D.M.; Kalinhoff, C.; Ramírez, J.; Gázquez, M.J. Native Hyperaccumulator Plants with Differential Phytoremediation Potential in an Artisanal Gold Mine of the Ecuadorian Amazon. Plants 2022, 11, 1186. https://doi.org/10.3390/plants11091186
Chamba-Eras I, Griffith DM, Kalinhoff C, Ramírez J, Gázquez MJ. Native Hyperaccumulator Plants with Differential Phytoremediation Potential in an Artisanal Gold Mine of the Ecuadorian Amazon. Plants. 2022; 11(9):1186. https://doi.org/10.3390/plants11091186
Chicago/Turabian StyleChamba-Eras, Irene, Daniel M. Griffith, Carolina Kalinhoff, Jorge Ramírez, and Manuel Jesús Gázquez. 2022. "Native Hyperaccumulator Plants with Differential Phytoremediation Potential in an Artisanal Gold Mine of the Ecuadorian Amazon" Plants 11, no. 9: 1186. https://doi.org/10.3390/plants11091186
APA StyleChamba-Eras, I., Griffith, D. M., Kalinhoff, C., Ramírez, J., & Gázquez, M. J. (2022). Native Hyperaccumulator Plants with Differential Phytoremediation Potential in an Artisanal Gold Mine of the Ecuadorian Amazon. Plants, 11(9), 1186. https://doi.org/10.3390/plants11091186







