Mercury Phytotoxicity and Tolerance in Three Wild Plants during Germination and Seedling Development
Abstract
1. Introduction
2. Results
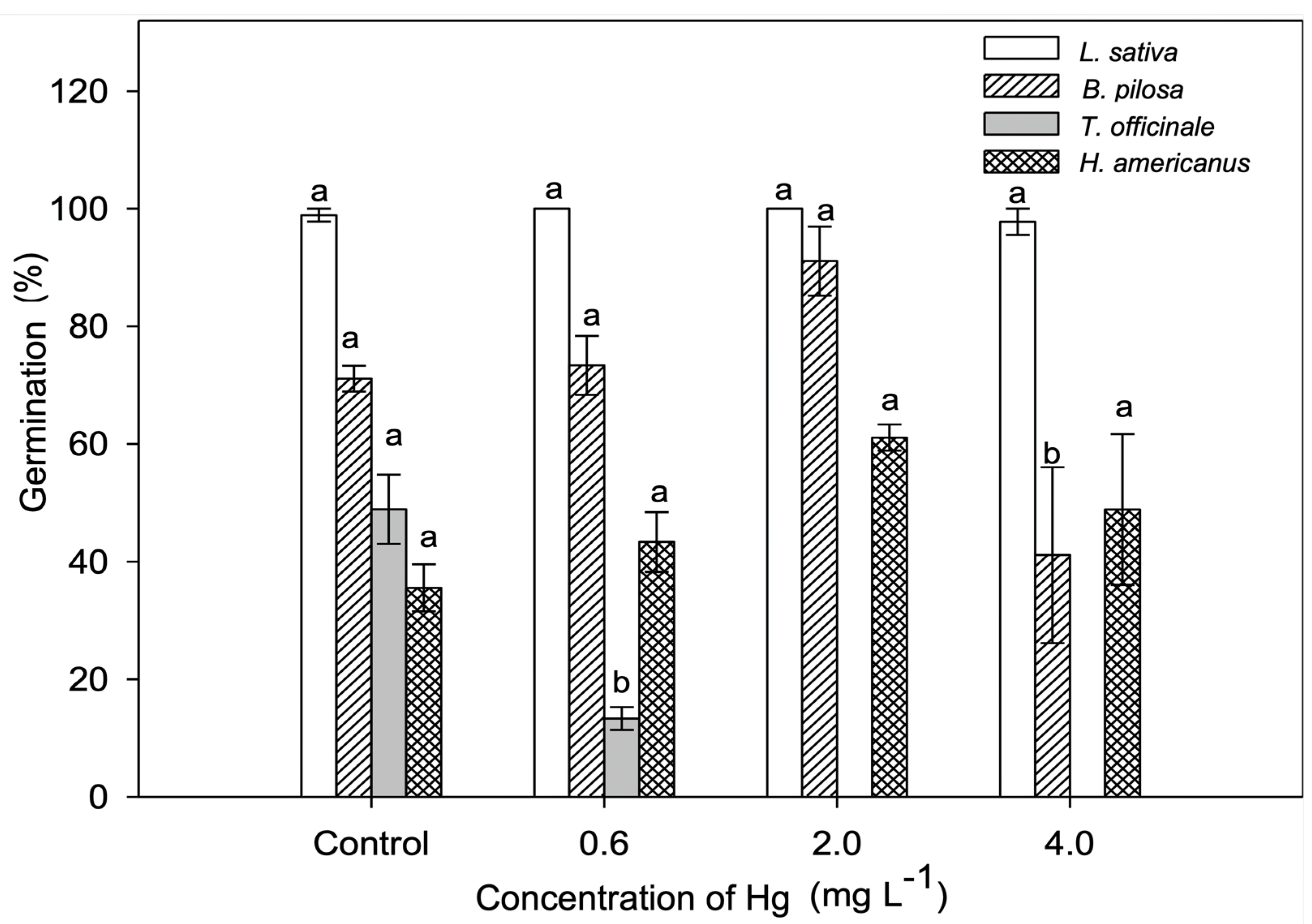
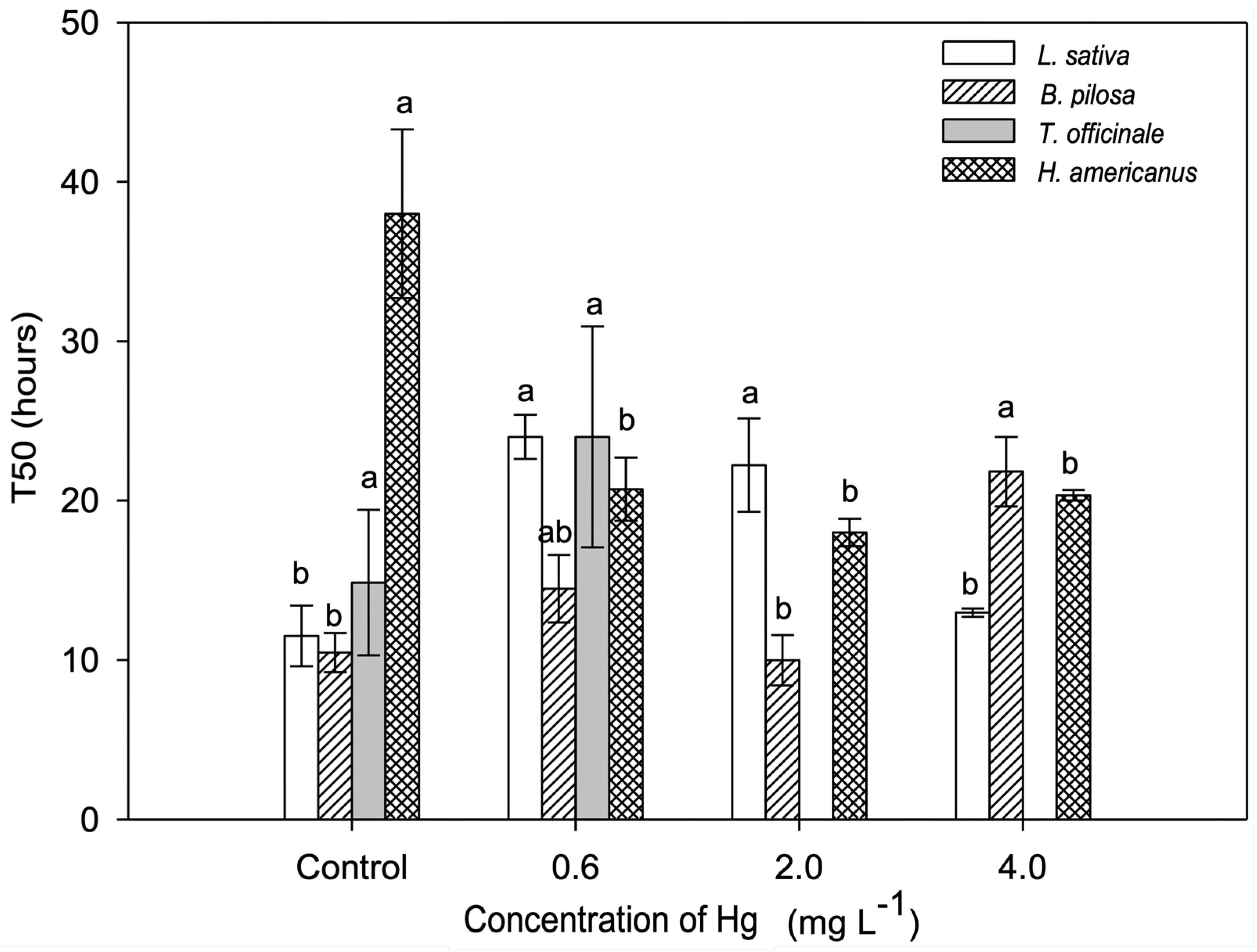
2.1. In Vitro Test
2.2. Seedling Growth
3. Discussion
4. Materials and Methods
4.1. Collection, Sterilization, and Storage of Seeds
4.2. In Vitro Test
4.3. Germination Response Variables
4.4. Growth Response Variables
4.5. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Treatment | Germination (%) |
|---|---|---|
| L. sativa | Control | 95 (1.73) a |
| 0.6 | 93 (1.71) a | |
| 2.0 | 98(1.76) a | |
| B. pilosa | Control | 70 (1.48) a |
| 0.6 | 88 (1.67) a | |
| 2.0 | 82(1.60) a | |
| T. officinale | Control | 45 (1.19) a |
| 0.6 | 15(0.68) b | |
| 2.0 | 2(0.22) c | |
| H. americanus | Control | 43 (1.16) b |
| 0.6 | 32(1.00) b | |
| 2.0 | 58(1.31) a |
References
- Chamba, I.; Rosado, D.; Kalinhoff, C.; Thangaswamy, S.; Sánchez-Rodríguez, A.; Gazquez, M.J. Erato Polymnioides—A Novel Hg Hyperaccumulator Plant in Ecuadorian Rainforest Acid Soils with Potential of Microbe-Associated Phytoremediation. Chemosphere 2017, 188, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Ralph, O.; Gilles, N.; Fon, N.; Luma, H.; Greg, N. Impact of Artisanal Gold Mining on Human Health and the Environment in the Batouri Gold District, East Cameroon. Acad. J. Interdiscip. Stud. 2018, 7, 25–44. [Google Scholar] [CrossRef]
- Veiga, M.M.; Angeloci, G.; Hitch, M.; Colon Velasquez-Lopez, P. Processing Centres in Artisanal Gold Mining. J. Clean. Prod. 2014, 64, 535–544. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, X.; Liu, B.; Nan, Y. A Qualitative Study of the Critical Conditions for the Initiation of Mine Waste Debris Flows. Water 2020, 12, 1536. [Google Scholar] [CrossRef]
- Gallo Corredor, J.A.; Humberto Pérez, E.; Figueroa, R.; Figueroa Casas, A. Water Quality of Streams Associated with Artisanal Gold Mining; Suárez, Department of Cauca, Colombia. Heliyon 2021, 7, e07047. [Google Scholar] [CrossRef] [PubMed]
- Yevugah, L.L.; Darko, G.; Bak, J. Does Mercury Emission from Small-Scale Gold Mining Cause Widespread Soil Pollution in Ghana? Environ. Pollut. 2021, 284, 116945. [Google Scholar] [CrossRef] [PubMed]
- Lara-Rodríguez, J.S. All That Glitters Is Not Gold or Platinum: Institutions and the Use of Mercury in Mining in Chocó, Colombia. Extr. Ind. Soc. 2018, 5, 308–318. [Google Scholar] [CrossRef]
- Esdaile, L.J.; Chalker, J.M. The Mercury Problem in Artisanal and Small-Scale Gold Mining. Chem. Eur. J. 2018, 24, 6905–6916. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, J.M. Recent Advances in Mercury Research. Sci. Total Environ. 2020, 738, 139955. [Google Scholar] [CrossRef] [PubMed]
- Taux, K.; Kraus, T.; Kaifie, A. Mercury Exposure and Its Health Effects in Workers in the Artisanal and Small-Scale Gold Mining (ASGM) Sector—A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2081. [Google Scholar] [CrossRef]
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the Terrestrial Environment: A Review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing Phytoremediation of Soils Polluted with Heavy Metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Durango-Hernández, J.; Pinedo-Hernández, J.; Olivero-Verbel, J.; Díez, S. Phytoremediation of Mercury-Contaminated Soils by Jatropha Curcas. Chemosphere 2015, 127, 58–63. [Google Scholar] [CrossRef]
- Xun, Y.; Feng, L.; Li, Y.; Dong, H. Mercury Accumulation Plant Cyrtomium Macrophyllum and Its Potential for Phytoremediation of Mercury Polluted Sites. Chemosphere 2017, 189, 161–170. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, B.; Wang, L.; Urbanovich, O.; Nagorskaya, L.; Li, X.; Tang, L. A Review on Phytoremediation of Mercury Contaminated Soils. J. Hazard. Mater. 2020, 400, 123138. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.; Rodriguez, E. Phytotoxicity of Mercury in Plants: A Review. J. Bot. 2012, 2012, e848614. [Google Scholar] [CrossRef]
- Bae, J.; Mercier, G.; Watson, A.K.; Benoit, D.L. Seed Germination Test for Heavy Metal Phytotoxicity Assessment. Can. J. Plant Sci. 2014, 94, 1519–1521. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants. Biomolecules 2022, 12, 43. [Google Scholar] [CrossRef]
- Mukhraiya, D.; Bhat, J. Assessment of Mercury Toxicity on Seed Germination, Growth and Antioxidant Enzyme Expression of Sorghum Vulgare Var. SG-1000 Seedlings. Int. J. Theor. Appl. Sci. 2017, 9, 45–50. [Google Scholar]
- Bae, J.; Benoit, D.L.; Watson, A.K. Effect of Heavy Metals on Seed Germination and Seedling Growth of Common Ragweed and Roadside Ground Cover Legumes. Environ. Pollut. 2016, 213, 112–118. [Google Scholar] [CrossRef]
- González-Valdez, E.; Alarcón, A.; Ferrera-Cerrato, R.; Vega-Carrillo, H.R.; Maldonado-Vega, M.; Salas-Luévano, M.Á. Seed Germination and Seedling Growth of Five Plant Species for Assessing Potential Strategies to Stabilizing or Recovering Metals from Mine Tailings. Water Air Soil Pollut. 2016, 227, 24. [Google Scholar] [CrossRef]
- Mahbub, K.; Kader, M.; Krishnan, K.; Labbate, M.; Naidu, R.; Mallavarapu, M. Toxicity of Inorganic Mercury to Native Australian Grass Grown in Three Different Soils. Bull. Environ. Contam. Toxicol. 2017, 98, 850–855. [Google Scholar] [CrossRef]
- Rodríguez-Alonso, J.; Sierra, M.J.; Lominchar, M.A.; Millán, R. Mercury Tolerance Study in Holm Oak Populations from the Almadén Mining District (Spain). Environ. Exp. Bot. 2017, 133, 98–107. [Google Scholar] [CrossRef]
- Nikolić, M.; Stevović, S. Family Asteraceae as a Sustainable Planning Tool in Phytoremediation and Its Relevance in Urban Areas. Urban For. Urban Green. 2015, 14, 782–789. [Google Scholar] [CrossRef]
- Vishnoi, N.; Singh, D.P. Efficiency of an Industrially Important Crop Hibiscus Cannabinus for Phytoremediation and Bioenergy Production. In Phytoremediation Potential of Bioenergy Plants; Bauddh, K., Singh, B., Korstad, J., Eds.; Springer: Singapore, 2017; pp. 255–268. [Google Scholar]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. Global Database for Plants That Hyperaccumulate Metal and Metalloid Trace Elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef]
- Graziani, N.S.; Salazar, M.J.; Pignata, M.L.; Rodriguez, J.H. Assessment of the Root System of Brassica juncea (L.) Czern. and Bidens pilosa L. Exposed to Lead Polluted Soils Using Rhizobox Systems. Int. J. Phytoremediat. 2016, 18, 235–244. [Google Scholar] [CrossRef]
- Sharma, R.; Bhardwaj, R.; Gautam, V.; Bali, S.; Kaur, R.; Kaur, P.; Sharma, M.; Kumar, V.; Sharma, A.; Sonia; et al. Phytoremediation in Waste Management: Hyperaccumulation Diversity and Techniques. In Plants Under Metal and Metalloid Stress: Responses, Tolerance and Remediation; Hasanuzzaman, M., Nahar, K., Fujita, M., Eds.; Springer: Singapore, 2018; pp. 277–302. [Google Scholar]
- Tanhan, P.; Kruatrachue, M.; Pokethitiyook, P.; Chaiyarat, R. Uptake and Accumulation of Cadmium, Lead and Zinc by Siam Weed [Chromolaena Odorata (L.) King & Robinson]. Chemosphere 2007, 68, 323–329. [Google Scholar] [CrossRef]
- Maleci, L.; Buffa, G.; Wahsha, M.; Bini, C. Morphological Changes Induced by Heavy Metals in Dandelion (Taraxacum Officinale Web.) Growing on Mine Soils. J. Soils Sediments 2014, 14, 731–743. [Google Scholar] [CrossRef]
- Rehmus, A.; Bigalke, M.; Valarezo, C.; Castillo, J.M.; Wilcke, W. Aluminum Toxicity to Tropical Montane Forest Tree Seedlings in Southern Ecuador: Response of Biomass and Plant Morphology to Elevated Al Concentrations. Plant Soil 2014, 382, 301–315. [Google Scholar] [CrossRef]
- Benalcázar, H.H.; Gagnon, D.; Davidson, R. Crecimiento y producción inicial de 15 especies de árboles tropicales de la Amazonía ecuatoriana de estados sucesionales diferentes. Siembra 2015, 2, 69–75. [Google Scholar] [CrossRef]
- Kimáková, T.; Bernasovská, K. The Mercury Concentration in Particular Parts of Taraxacum officinale (Dandelion) in different Areas of Slovakia. Planta Med. 2007, 73, 268. [Google Scholar] [CrossRef]
- Bijelić, L.; Puntarić, D.; Gvozdić, V.; Vidosavljević, D.; Lončarić, Z.; Puntarić, A.; Puntarić, E.; Puntarić, I.; Šijanović, S.; Vidosavljević, M. Dandelion (Taraxacum officinale) as a possible indicator of war contamination in Eastern Croatia. Acta Medica Croat. Časopis Akad. Med. Znan. Hrvat. 2017, 71, 25–31. [Google Scholar]
- Vargas Aguirre, C.F.; Rivera Páez, F.A.; Escobar Vargas, S. Effect of Arbuscular Mycorrhizae and Mercury on Lactuca Sativa (Asteraceae) Seedling Morpho—Histology. Environ. Exp. Bot. 2018, 156, 197–202. [Google Scholar] [CrossRef]
- Salazar, M.J.; Wannaz, E.D.; Blanco, A.; Miranda Pazcel, E.M.; Pignata, M.L. Pb Tolerance and Accumulation Capabilities of Bidens pilosa L. Growing in Polluted Soils Depend on the History of Exposure. Chemosphere 2021, 269, 128732. [Google Scholar] [CrossRef]
- Samreen, S.; Khan, A.A.; Khan, M.R.; Ansari, S.A.; Khan, A. Assessment of Phytoremediation Potential of Seven Weed Plants Growing in Chromium- and Nickel-Contaminated Soil. Water Air Soil Pollut. 2021, 232, 209. [Google Scholar] [CrossRef]
- Manori, S.; Shah, V.; Soni, V.; Dutta, K.; Daverey, A. Phytoremediation of Cadmium-Contaminated Soil by Bidens pilosa L.: Impact of Pine Needle Biochar Amendment. Environ. Sci. Pollut. Res. 2021, 28, 58872–58884. [Google Scholar] [CrossRef]
- Dai, H.; Wei, S.; Skuza, L.; Zhang, Q. Phytoremediation of Two Ecotypes Cadmium Hyperaccumulator Bidens pilosa L. Sourced from Clean Soils. Chemosphere 2021, 273, 129652. [Google Scholar] [CrossRef]
- Escobar-Vargas, S.; Aguirre, C.F.V.; Páez, F.A.R. Arbuscular Mycorrhizal Fungi Prevent Mercury Toxicity in Lactuca sativa (L.) Seed Germination. Pollution 2022, 8, 1014–1025. [Google Scholar] [CrossRef]
- Castillo, G.C.; Vila, I.C.; Neild, E. Ecotoxicity Assessment of Metals and Wastewater Using Multitrophic Assays. Environ. Toxicol. 2000, 15, 370–375. [Google Scholar] [CrossRef]
- Casado, M.P.; Macken, A.; Byrne, H.J. Ecotoxicological Assessment of Silica and Polystyrene Nanoparticles Assessed by a Multitrophic Test Battery. Environ. Int. 2013, 51, 97–105. [Google Scholar] [CrossRef]
- Mei, L.; Zhu, Y.; Zhang, X.; Zhou, X.; Zhong, Z.; Li, H.; Li, Y.; Li, X.; Daud, M.K.; Chen, J.; et al. Mercury-Induced Phytotoxicity and Responses in Upland Cotton (Gossypium hirsutum L.) Seedlings. Plants 2021, 10, 1494. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alonso, J.; Sierra, M.J.; Lominchar, M.Á.; Millán, R. Effects of Mercury on the Germination and Growth of Quercus ilex L. Seedlings. Environ. Sci. Pollut. Res. 2019, 26, 30930–30940. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.Á.; e Borges, E.E.L.; de Souza, G.A.; da Silva, C.J.; Pires, R.M.O.; Dias, D.C.F.S. Seed Imbibition and Germination of Plothymenia Reticulata Benth. (Fabaceae) Affected by Mercury: Possible Role of Aquaporins. Acta Bot. Bras. 2015, 29, 285–291. [Google Scholar] [CrossRef]
- Ranal, M.A.; de Santana, D.G. How and Why to Measure the Germination Process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Jeavons, R.A.; Jarvis, B.C. The Breaking of Dormancy in Hazel Seed by Pretreatment with Ethanol and Mercuric Chloride. New Phytol. 1984, 96, 551–554. [Google Scholar] [CrossRef]
- Ishtiyaq, S.; Kumar, H.; Varun, M.; Kumar, B.; Paul, M.S. Heavy Metal Toxicity and Antioxidative Response in Plants: An Overview. In Plants Under Metal and Metalloid Stress: Responses, Tolerance and Remediation; Hasanuzzaman, M., Nahar, K., Fujita, M., Eds.; Springer: Singapore, 2018; pp. 77–106. [Google Scholar]
- Muszyńska, E.; Labudda, M. Dual Role of Metallic Trace Elements in Stress Biology—From Negative to Beneficial Impact on Plants. Int. J. Mol. Sci. 2019, 20, 3117. [Google Scholar] [CrossRef]
- Li, W.; Khan, M.A.; Yamaguchi, S.; Kamiya, Y. Effects of Heavy Metals on Seed Germination and Early Seedling Growth of Arabidopsis Thaliana. Plant Growth Regul. 2005, 46, 45–50. [Google Scholar] [CrossRef]
- Mondal, N.K.; Das, C.; Datta, J.K. Effect of Mercury on Seedling Growth, Nodulation and Ultrastructural Deformation of Vigna radiata (L) Wilczek. Environ. Monit. Assess. 2015, 187, 241. [Google Scholar] [CrossRef]
- Kranner, I.; Colville, L. Metals and Seeds: Biochemical and Molecular Implications and Their Significance for Seed Germination. Environ. Exp. Bot. 2011, 72, 93–105. [Google Scholar] [CrossRef]
- Algreen, M.; Trapp, S.; Rein, A. Phytoscreening and Phytoextraction of Heavy Metals at Danish Polluted Sites Using Willow and Poplar Trees. Environ. Sci. Pollut. Res. 2014, 21, 8992–9001. [Google Scholar] [CrossRef]
- Urgiles, N.; Loján, P.; Aguirre, N.; Blaschke, H.; Günter, S.; Stimm, B.; Kottke, I. Application of Mycorrhizal Roots Improves Growth of Tropical Tree Seedlings in the Nursery: A Step towards Reforestation with Native Species in the Andes of Ecuador. New For. 2009, 38, 229–239. [Google Scholar] [CrossRef]
- Doğaroğlu, Z.G. KADMİYUM, KURŞUN VE ÇİNKO METALLERİNİN MARUL (Lactuca sativa) TOHUMLARININ ÇİMLENME ÖZELLİKLERİ ÜZERİNE ETKİSİ. Uludağ Üniv. Mühendis. Fakültesi Derg. 2018, 23, 299–308. [Google Scholar] [CrossRef][Green Version]
- Farooq, M.; Basra, S.M.A.; Ahmad, N.; Hafeez, K. Thermal Hardening: A New Seed Vigor Enhancement Tool in Rice. J. Integr. Plant Biol. 2005, 47, 187–193. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/7469942 (accessed on 5 July 2022).
| Species | Treatment | Hypocotyl Length (mm) | Radicle Length (mm) |
|---|---|---|---|
| L. sativa | Control | 38.9 (3.60) a | 28.0 (5.35) a |
| 0.6 | 31.0 (2.01) b | 13.2 (2.55) b | |
| 2.0 | 16.6 (2.07) c | 7.2 (2.33) c | |
| 4.0 | 16.5 (2.18) c | 3.7 (1.33) c | |
| B. pilosa | Control | 36.4 (11.04) a | 15.0 (7.35) a |
| 0.6 | 37.7 (3.73) a | 17.3 (3.01) a | |
| 2.0 | 31.8 (3.28) a | 10.9 (3.73) a | |
| 4.0 | 26.8 (6.74) b | 6.7 (4.82) b | |
| T. officinale | Control | 18.4 (2.08) a | 5.4 (1.23) a |
| 0.6 | 14.2 (3.17) b | 5.4 (1.27) a | |
| 2.0 | - | - | |
| 4.0 | - | - | |
| H. americanus | Control | 26.6 (2.95) a | 9.2 (1.76) a |
| 0.6 | 26.6 (2.39) a | 8.4 (1.25) a | |
| 2.0 | 19.5 (4.04) b | 6.2 (1.05) b | |
| 4.0 | 19.0 (3.21) b | 6.3 (0.94) b |
| Species | Treatment | Hypocotyl Diameter (mm) | Radicle Diameter (mm) |
|---|---|---|---|
| L. sativa | Control | 0.21 (0.03) a | 0.09 (0.02) a |
| 0.6 | 0.17 (0.02) b | 0.09 (0.01) a | |
| 2.0 | 0.20 (0.02) a | 0.09 (0.01) a | |
| 4.0 | 0.22 (0.02) a | 0.10 (0.02) a | |
| B. pilosa | Control | 0.16 (0.05) a | 0.04 (0.02) a |
| 0.6 | 0.19 (0.04) a | 0.04 (0.02) a | |
| 2.0 | 0.18 (0.03) a | 0.06 (0.01) a | |
| 4.0 | 2.43 (3.84) b | 1.07 (1.69) b | |
| T. officinale | Control | 0.18 (0.03) a | 0.08 (0.02) a |
| 0.6 | 0.17 (0.03) a | 0.06 (0.02) a | |
| 2.0 | - | - | |
| 4.0 | - | - | |
| H. americanus | Control | 0.20 (0.03) a | 0.07 (0.02) a |
| 0.6 | 0.17 (0.03) b | 0.06 (0.01) a | |
| 2.0 | 0.20 (0.02) a | 0.07 (0.01) a | |
| 4.0 | 0.16 (0.03) c | 0.06 (0.01) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinhoff, C.; Calderón, N.-T. Mercury Phytotoxicity and Tolerance in Three Wild Plants during Germination and Seedling Development. Plants 2022, 11, 2046. https://doi.org/10.3390/plants11152046
Kalinhoff C, Calderón N-T. Mercury Phytotoxicity and Tolerance in Three Wild Plants during Germination and Seedling Development. Plants. 2022; 11(15):2046. https://doi.org/10.3390/plants11152046
Chicago/Turabian StyleKalinhoff, Carolina, and Norma-Thalia Calderón. 2022. "Mercury Phytotoxicity and Tolerance in Three Wild Plants during Germination and Seedling Development" Plants 11, no. 15: 2046. https://doi.org/10.3390/plants11152046
APA StyleKalinhoff, C., & Calderón, N.-T. (2022). Mercury Phytotoxicity and Tolerance in Three Wild Plants during Germination and Seedling Development. Plants, 11(15), 2046. https://doi.org/10.3390/plants11152046






