Development of Microscopic Techniques for the Visualization of Plant–Root-Knot Nematode Interaction
Abstract
1. Introduction
2. Results
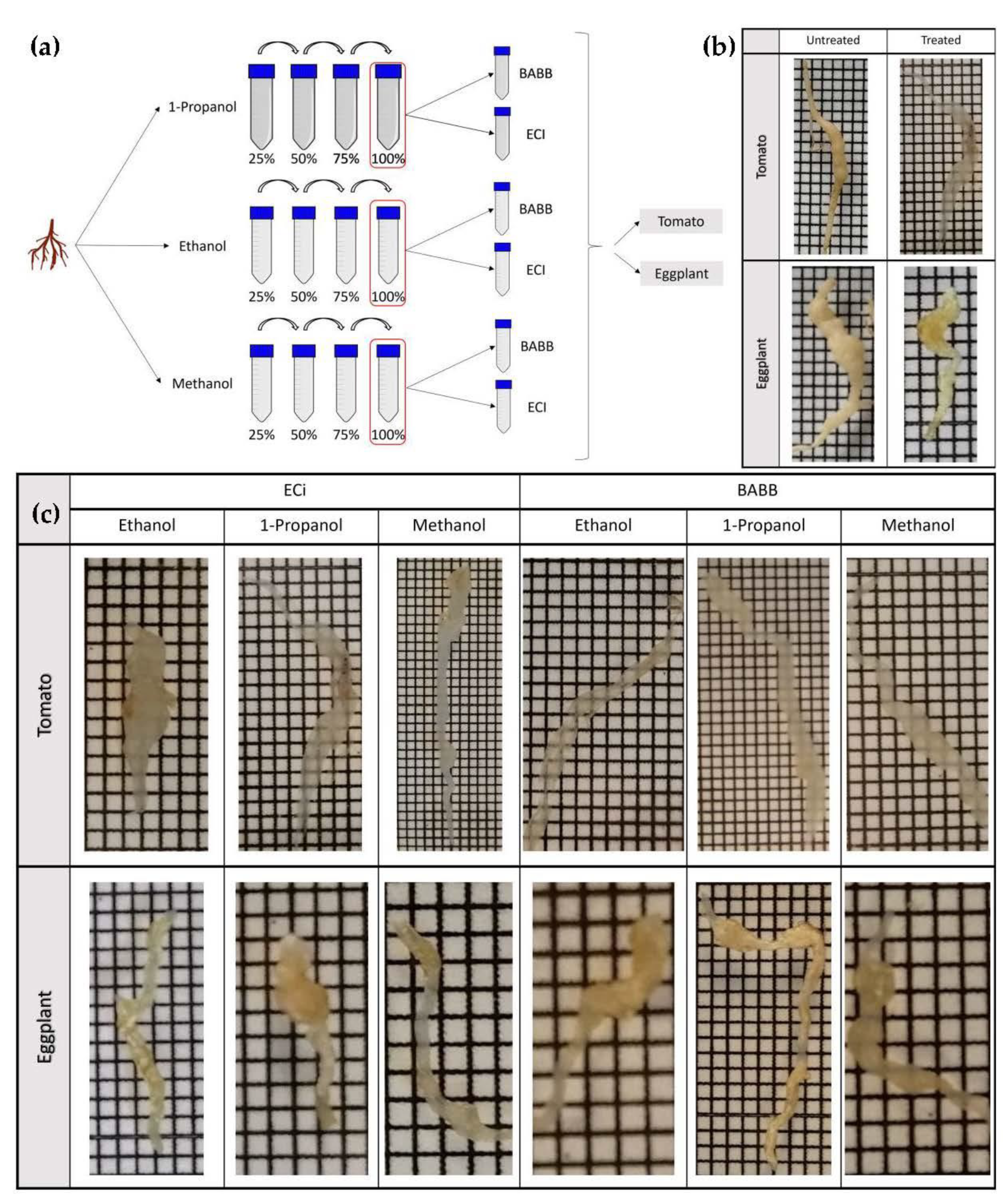
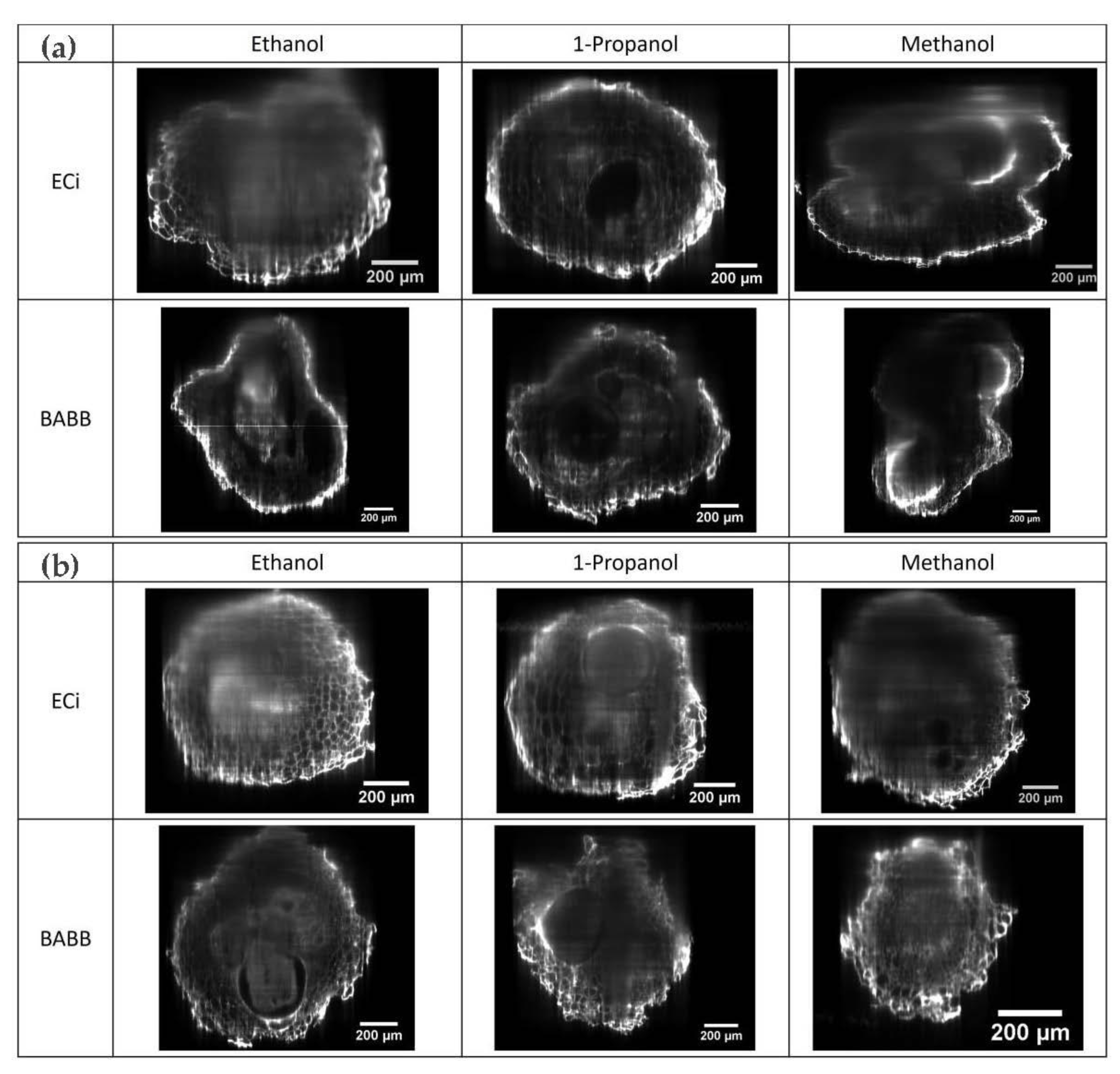
2.1. Dehydration and Clearing
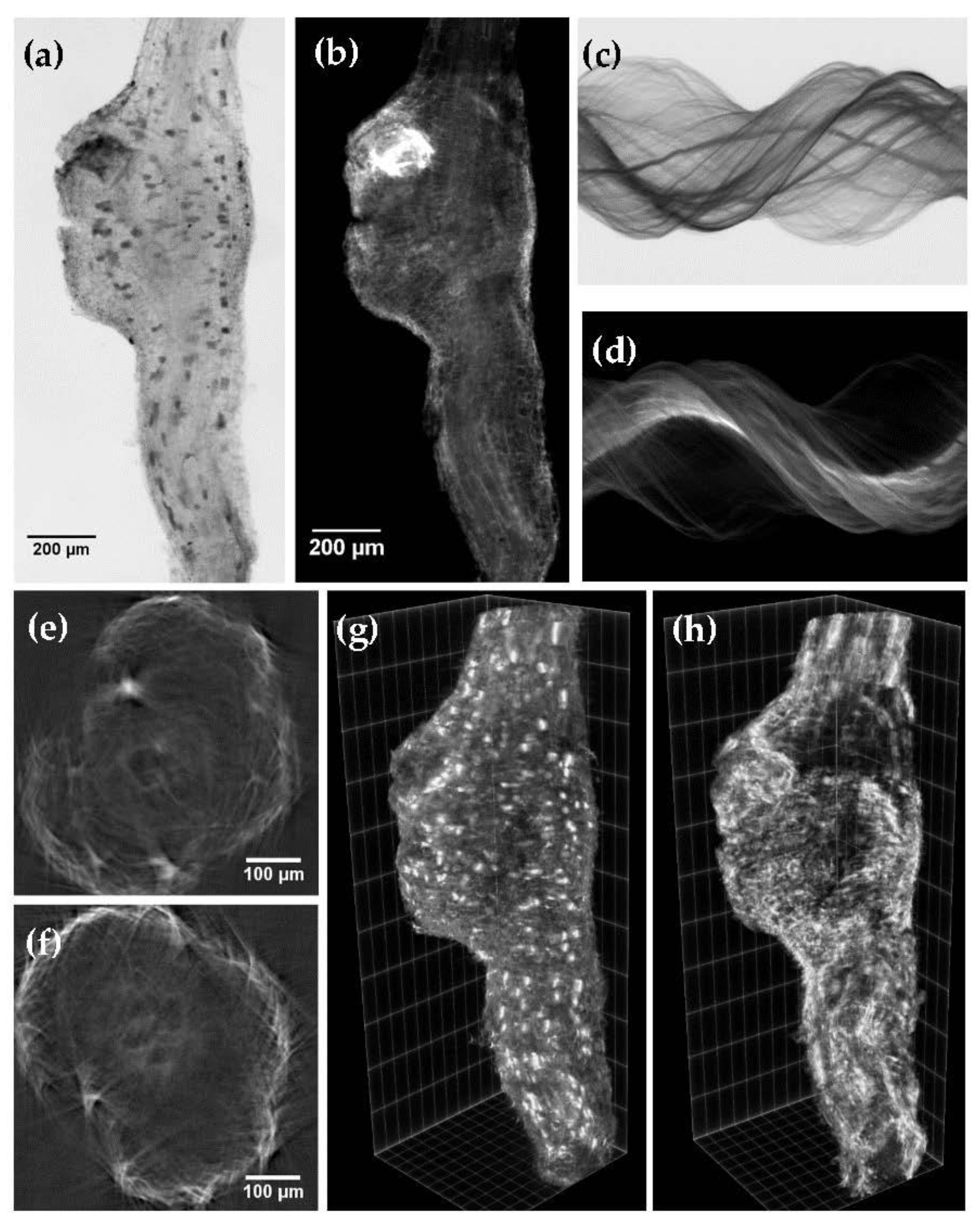
2.2. LSFM Imaging of Cleared Roots Galls
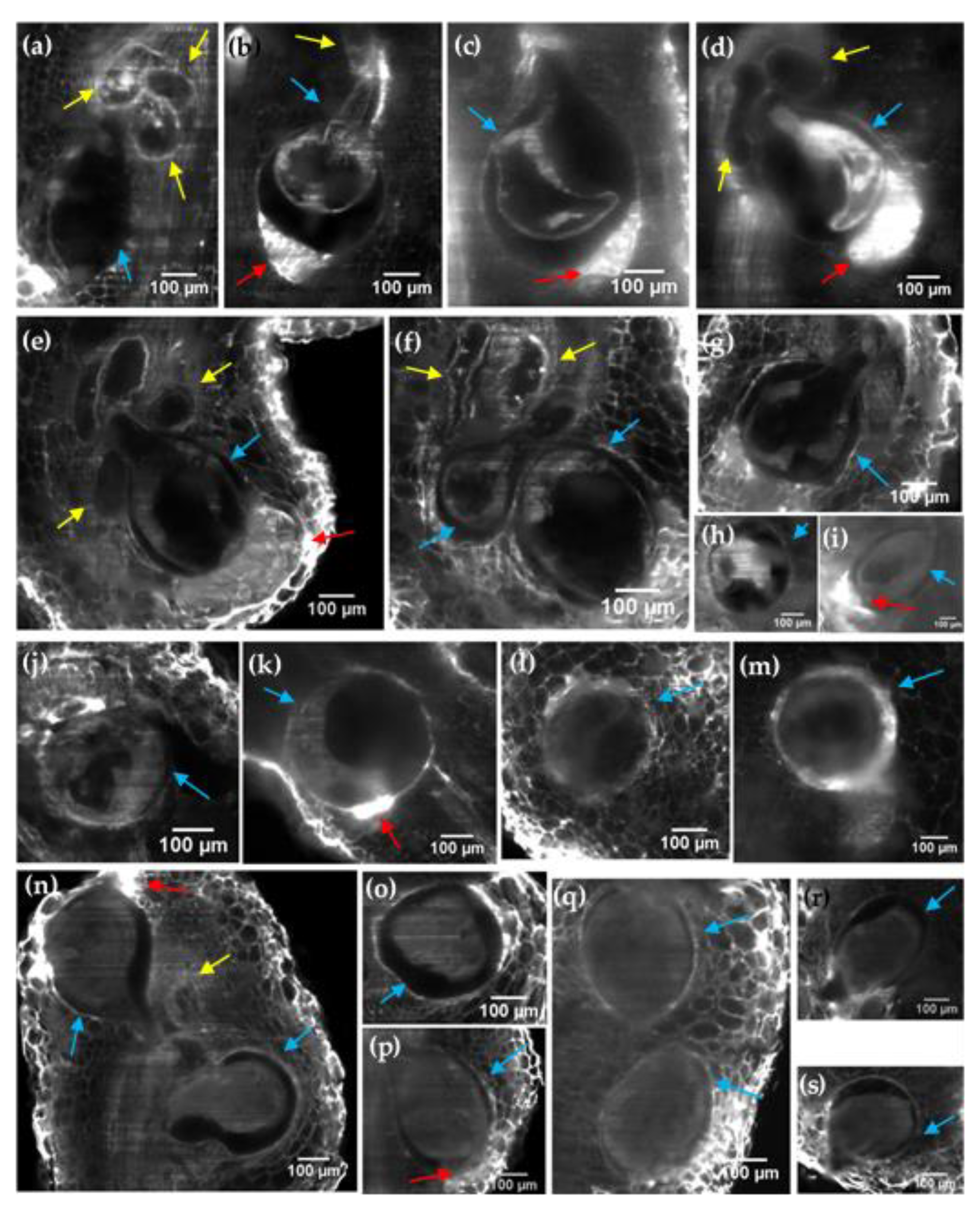
2.3. LSFM Imaging of Cleared Tomato and Eggplant Roots Galls
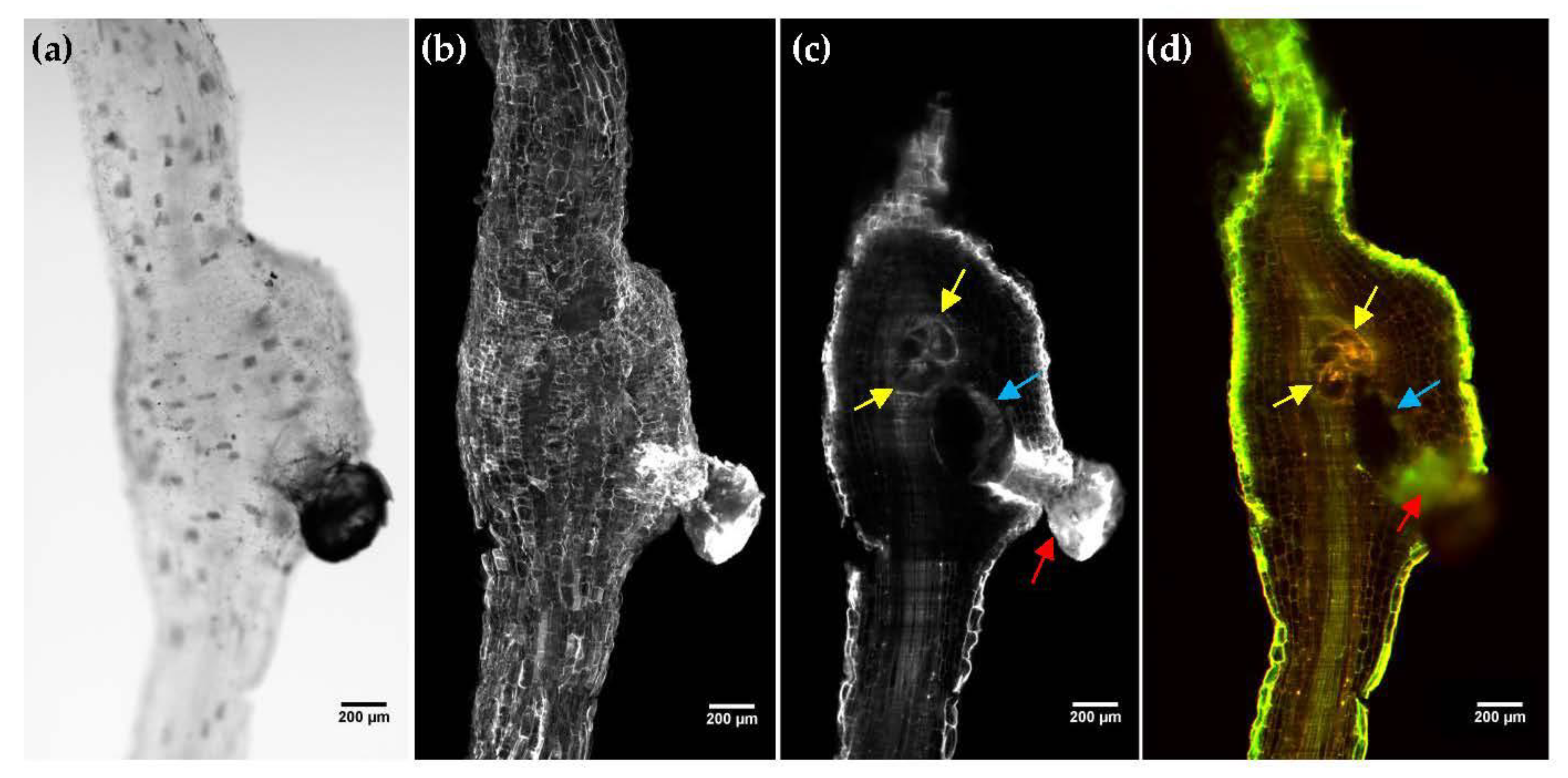
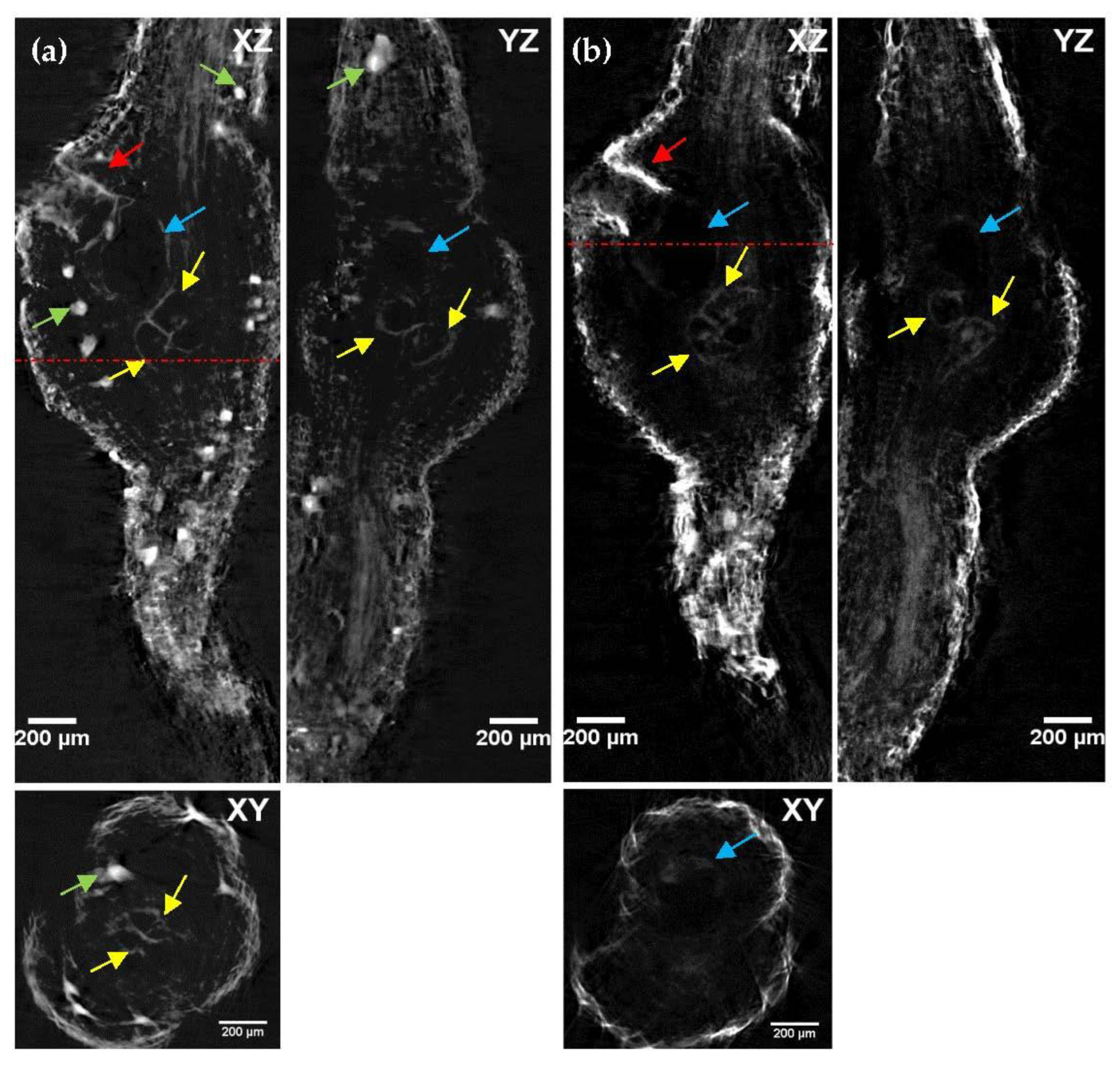
2.4. OPT Imaging of Cleared Tomato Root Galls
3. Discussion
4. Materials and Methods
4.1. Plant Material and Nematode Inoculums
4.2. Sample Preparation
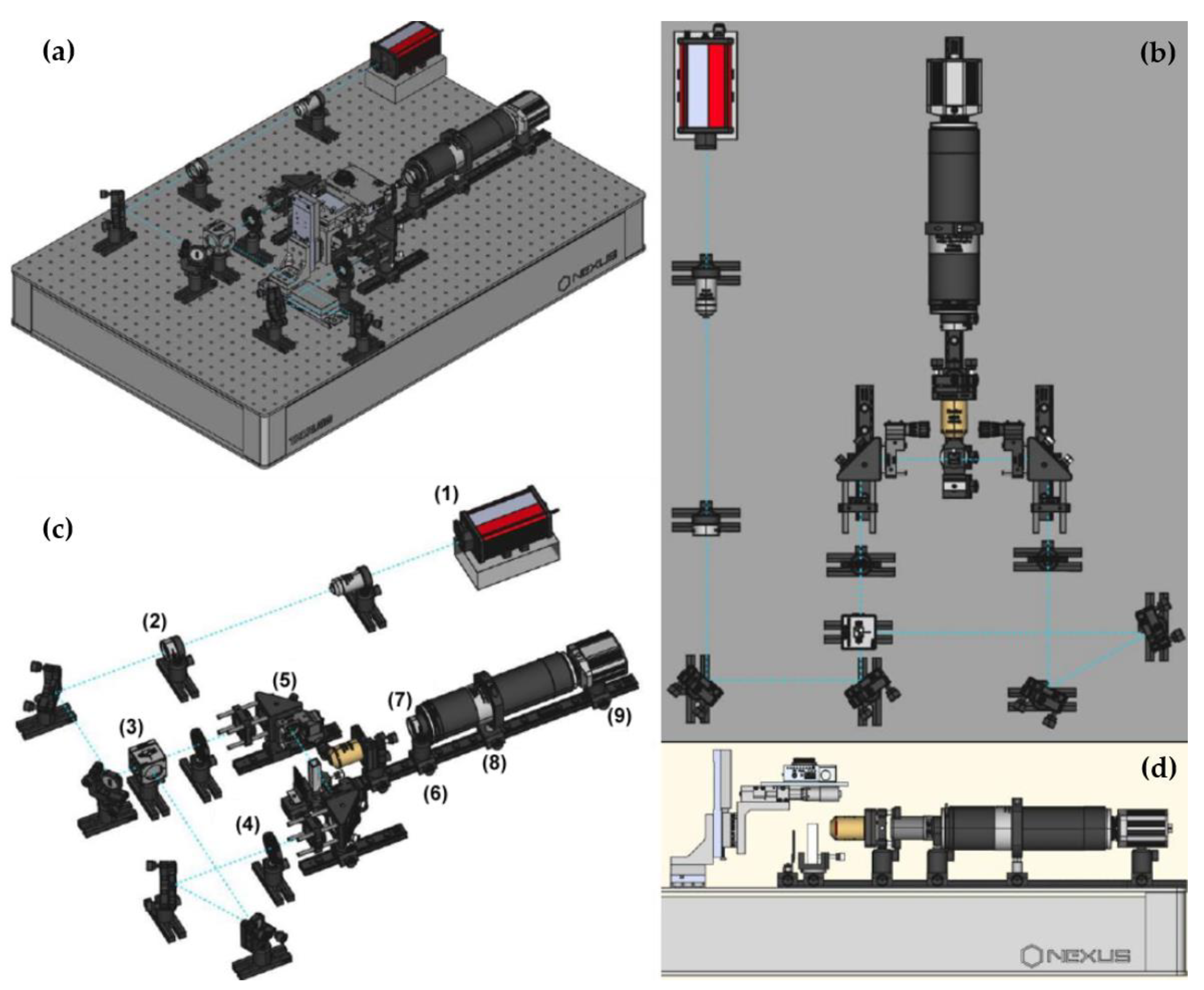
4.3. Experimental Setup
4.4. Image Post-Processing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Sorribas, F.J.; Djian-Caporalino, C.; Mateille, T. Nematodes. In Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M.L., Albajes, R., Nicot, P., Eds.; Springer: Cham, Switzerland, 2020; pp. 147–174. [Google Scholar]
- López-Gómez, M.; Giné, A.; Vela, M.D.; Ornat, C.; Sorribas, F.J.; Talavera, M.; Verdejo-Lucas, S. Damage functions and thermal requirements of Meloidogyne javanica and Meloidogyne incognita on watermelon. Ann. Appl. Biol. 2014, 165, 466–473. [Google Scholar] [CrossRef]
- Vela, M.D.; Giné, A.; López-Gómez, M.; Sorribas, F.J.; Ornat, C.; Verdejo-Lucas, S.; Talavera, M. Thermal time requirements of root-knot nematodes on zucchini-squash and population dynamics with associated yield losses on spring and autumn cropping cycles. Eur. J. Plant Pathol. 2014, 140, 481–490. [Google Scholar] [CrossRef]
- Expósito, A.; Pujolà, M.; Achaerandio, I.; Giné, A.; Escudero, N.; Fullana, A.M.; Cunquero, M.; Loza-Alvarez, P.; Sorribas, F.J. Tomato and melon meloidogyne resistant rootstocks improve crop yield but melon fruit quality is influenced by the cropping season. Front. Plant Sci. 2020, 11, 560024. [Google Scholar] [CrossRef]
- Sorribas, F.J.; Ornat, C.; Verdejo-Lucas, S.; Galeano, M.; Valero, J. Effectiveness and profitability of the Mi-resistant tomatoes to control root-knot nematodes. Eur. J. Plant Pathol. 2005, 111, 29–38. [Google Scholar] [CrossRef]
- Roberts, P.A. Concepts and consequences of resistance. In Plant Resistance to Parasitic Nematodes; Starr, J.L., Cook, R., Bridge, J., Eds.; CABI International: Wallingford, UK, 2002; pp. 23–41. [Google Scholar]
- Abad, P.; Castagnone-Sereno, P.; Rosso, M.N.; Engler, J.D.A.; Favery, B. Invasion, feeding and development. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; CABI international: Wallingford, UK, 2009; pp. 163–181. [Google Scholar]
- Seo, J.; Choe, M.; Kim, S.Y. Clearing and labeling techniques for large-scale biological tissues. Mol. Cells 2016, 39, 439–446. [Google Scholar] [CrossRef]
- Warner, C.A.; Biedrzycki, M.L.; Jacobs, S.S.; Wisser, R.J.; Caplan, J.L.; Janine Sherrier, D. An optical clearing technique for plant tissues allowing deep imaging and compatible with fluorescence microscopy. Plant Physiol. 2014, 166, 1684–1687. [Google Scholar] [CrossRef]
- Richardson, D.S.; Lichtman, J.W. Clarifying Tissue Clearing. Cell 2015, 162, 246–257. [Google Scholar] [CrossRef]
- Becker, K.; Jährling, N.; Saghafi, S.; Weiler, R.; Dodt, H.U. Chemical clearing and dehydration of GFP expressing mouse brains. PLoS ONE 2012, 7, e33916. [Google Scholar] [CrossRef]
- Klingberg, A.; Hasenberg, A.; Ludwig-Portugall, I.; Medyukhina, A.; Männ, L.; Brenzel, A.; Engel, D.R.; Figge, M.T.; Kurts, C.; Gunzer, M. Fully automated evaluation of total glomerular number and capillary tuft size in nephritic kidneys using lightsheet microscopy. J. Am. Soc. Nephrol. 2017, 28, 452–459. [Google Scholar] [CrossRef]
- Masselink, W.; Reumann, D.; Murawala, P.; Pasierbek, P.; Taniguchi, Y.; Bonnay, F.; Meixner, K.; Knoblich, J.A.; Tanaka, E.M. Broad applicability of a streamlined ethyl cinnamate-based clearing procedure. Development 2019, 146, dev166884. [Google Scholar] [CrossRef]
- Dodt, H.U.; Leischner, U.; Schierloh, A.; Jährling, N.; Mauch, C.P.; Deininger, K.; Deussing, J.M.; Eder, M.; Zieglgänsberger, W.; Becker, K. Ultramicroscopy: Three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods 2007, 4, 331–336. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.H.; Zhang, J.Y.; Chen, N.; Zhi, G.Y. High-yield synthesis of bioactive ethyl cinnamate by enzymatic esterification of cinnamic acid. Food Chem. 2016, 190, 629–633. [Google Scholar] [CrossRef]
- Antonino de Souza Junior, J.D.; Pierre, O.; Coelho, R.R.; Grossi-de-Sa, M.F.; Engler, G.; de Almeida Engler, J. Application of Nuclear Volume Measurements to Comprehend the Cell Cycle in Root-Knot Nematode-Induced Giant Cells. Front. Plant Sci. 2017, 8, 961. [Google Scholar] [CrossRef]
- Kurihara, D.; Mizuta, Y.; Sato, Y.; Higashiyama, T. ClearSee: A rapid optical clearing reagent for whole-plant fluorescence imaging. Development 2015, 142, 4168–4179. [Google Scholar] [CrossRef]
- Hasegawa, J.; Sakamoto, Y.; Nakagami, S.; Aida, M.; Sawa, S.; Matsunaga, S. Three-Dimensional Imaging of Plant Organs Using a Simple and Rapid Transparency Technique. Plant. Cell Physiol. 2016, 57, 462–472. [Google Scholar] [CrossRef]
- Minsky, M. Memoir on inventing the confocal scanning microscope. Scanning 1988, 10, 128–138. [Google Scholar] [CrossRef]
- Huisken, J.; Swoger, J.; Del Bene, F.; Wittbrodt, J.; Stelzer, E.H.K. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 2004, 305, 1007–1009. [Google Scholar] [CrossRef]
- Sharpe, J.; Ahlgren, U.; Perry, P.; Hill, B.; Ross, A.; Hecksher-Sorensen, J.; Baldock, R.; Davidson, D. Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science 2002, 296, 541–545. [Google Scholar] [CrossRef]
- Olarte, O.E.; Andilla, J.; Gualda, E.J.; Loza-Alvarez, P. Light-sheet microscopy: A tutorial. Adv. Opt. Photon. 2018, 10, 111–179. [Google Scholar] [CrossRef]
- Quintana, L.; Sharpe, J. Optical projection tomography of vertebrate embryo development. Cold Spring Harb. Protoc. 2011, 6, 586–594. [Google Scholar] [CrossRef][Green Version]
- Berthet, B.; Maizel, A. Light sheet microscopy and live imaging of plants. J. Microsc. 2016, 263, 158–164. [Google Scholar] [CrossRef]
- Gualda, E.; Moreno, N.; Tomancak, P.; Martins, G.G. Going “open” with mesoscopy: A new dimension on multi-view imaging. Protoplasma 2014, 251, 363–372. [Google Scholar] [CrossRef]
- Lee, K.J.I.; Calder, G.M.; Hindle, C.R.; Newman, J.L.; Robinson, S.N.; Avondo, J.J.H.Y.; Coen, E.S. Macro optical projection tomography for large scale 3D imaging of plant structures and gene activity. J. Exp. Bot. 2017, 68, 527–538. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Gualda, E.J.; Vale, T.; Almada, P.; Feijo, J.; Martins, G.G.; Moreno, N. OpenSpinMicroscopy: An open-source integrated microscopy platform. Nat. Methods 2013, 10, 599–600. [Google Scholar] [CrossRef]
- Mayer, J.; Robert-Moreno, A.; Danuser, R.; Stein, J.V.; Sharpe, J.; Swoger, J. OPTiSPIM: Integrating optical projection tomography in light sheet microscopy extends specimen characterization to nonfluorescent contrasts. Opt. Lett. 2014, 39, 1053–1056. [Google Scholar] [CrossRef]
- Bassi, A.; Schmid, B.; Huisken, J. Optical tomography complements light sheet microscopy for in toto imaging of zebrafish development. Development 2015, 142, 1016–1020. [Google Scholar] [CrossRef]
- Fester, T.; Berg, R.H.; Taylor, C.G. An easy method using glutaraldehyde-introduced fluorescence for the microscopic analysis of plant biotrophic interactions. J. Microsc. 2008, 231, 342–348. [Google Scholar] [CrossRef]
- Cabrera, J.; Olmo, R.; Ruiz-Ferrer, V.; Abreu, I.; Hermans, C.; Martinez-Argudo, I.; Fenoll, C.; Escobar, C. A Phenotyping Method of Giant Cells from Root-Knot Nematode Feeding Sites by Confocal Microscopy Highlights a Role for CHITINASE-LIKE 1 in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 429. [Google Scholar] [CrossRef]
- Pitrone, P.; Schindelin, J.; Stuyvenberg, L.; Preibisch, S.; Weber, M.; Eliceiri, K.; Huisken, J.; Tomancak, P. OpenSPIM: An open-access light sheet microscopy platform. Nat. Methods 2013, 10, 598–599. [Google Scholar] [CrossRef]
- LEGOLish: Light Sheet Imaging for Everybody. Available online: http://legolish.org (accessed on 16 February 2022).
- Diederich, B.; Lachmann, R.; Carlstedt, S.; Marsikova, B.; Wang, H.; Uwurukundo, X.; Mosig, A.S.; Heintzmann, R. A versatile and customizable low-cost 3D-printed open standard for microscopic imaging. Nat. Commun. 2020, 11, 5979. [Google Scholar] [CrossRef]
- Palomares-Rius, J.E.; Escobar, C.; Cabrera, J.; Vovlas, A.; Castillo, P. Anatomical alterations in plant tissues induced by plant-parasitic nematodes. Front. Plant Sci. 2017, 8, 1987. [Google Scholar] [CrossRef]
- Giné, A.; Sorribas, F.J. Quantitative approach for the early detection of selection for virulence of Meloidogyne incognita on resistant tomato in plastic greenhouses. Plant Pathol. 2017, 66, 1338–1344. [Google Scholar] [CrossRef]
- García-Mendívil, H.A.; Escudero, N.; Sorribas, F.J. Host suitability of Solanum torvum cultivars to Meloidogyne incognita and M. javanica and population dynamics. Plant Pathol. 2019, 68, 1215–1224. [Google Scholar] [CrossRef]
- Hussey, R.S.; Barker, K.R. A comparison of methods of collecting inoculate of Meloidogyne spp. including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Whitehead, A.G.; Hemming, J.R. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Vallejo Ramirez, P.P.; Zammit, J.; Vanderpoorten, O.; Riche, F.; Blé, F.X.; Zhou, X.H.; Spiridon, B.; Valentine, C.; Spasov, S.E.; Oluwasanya, P.W.; et al. OptiJ: Open-source optical projection tomography of large organ samples. Sci. Rep. 2019, 9, 15693. [Google Scholar] [CrossRef]
- Royer, L.; Weigert, M.; Günther, U.; Maghelli, N.; Jug, F.; Sbalzarini, I.F.; Myers, E.W. ClearVolume: Open-source live 3D visualization for light-sheet microscopy. Nat. Methods 2015, 12, 480–481. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernet, H.; Fullana, A.M.; Sorribas, F.J.; Gualda, E.J. Development of Microscopic Techniques for the Visualization of Plant–Root-Knot Nematode Interaction. Plants 2022, 11, 1165. https://doi.org/10.3390/plants11091165
Vernet H, Fullana AM, Sorribas FJ, Gualda EJ. Development of Microscopic Techniques for the Visualization of Plant–Root-Knot Nematode Interaction. Plants. 2022; 11(9):1165. https://doi.org/10.3390/plants11091165
Chicago/Turabian StyleVernet, Helena, Aïda Magdalena Fullana, Francisco Javier Sorribas, and Emilio J. Gualda. 2022. "Development of Microscopic Techniques for the Visualization of Plant–Root-Knot Nematode Interaction" Plants 11, no. 9: 1165. https://doi.org/10.3390/plants11091165
APA StyleVernet, H., Fullana, A. M., Sorribas, F. J., & Gualda, E. J. (2022). Development of Microscopic Techniques for the Visualization of Plant–Root-Knot Nematode Interaction. Plants, 11(9), 1165. https://doi.org/10.3390/plants11091165






