Elimination of Eight Viruses and Two Viroids from Preclonal Candidates of Six Grapevine Varieties (Vitis vinifera L.) through In Vivo Thermotherapy and In Vitro Meristem Tip Micrografting
Abstract
1. Introduction
2. Results
2.1. Plant Regeneration
2.2. Virus and Viroid Elimination and Vine Acclimatization
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Virome Status of the Preclonal Candidates
4.3. Rootstock Source
4.4. In Vivo Thermotherapy and In Vitro Meristem Tip Micrografting
4.5. Verification of Virus and Viroid Elimination
4.6. Acclimatization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andret-Link, P.; Laporte, C.; Valat, L.; Ritzenthaler, C.; Demangeat, G.; Vigne, E.; Laval, V.; Pfeiffer, P.; Stussi-Garaud, C.; Fuchs, M. Grapevine fanleaf virus: Still a major threat to the grapevine industry. J. Plant. Pathol. 2004, 86, 183–195. [Google Scholar]
- Giampetruzzi, A.; Roumi, V.; Roberto, R.; Malossini, U.; Yoshikawa, N.; La Notte, P.; Terlizzi, F.; Credi, R.; Saldarelli, P. A new grapevine virus discovered by deep sequencing of virus- and viroid-derived small RNAs in Cv Pinot gris. Virus Res. 2012, 163, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Maree, H.J.; Almeida, R.P.P.; Bester, R.; Chooi, K.M.; Cohen, D.; Dolja, V.V.; Fuchs, M.F.; Golino, D.A.; Jooste, A.E.C.; Martelli, G.P.; et al. Grapevine leafroll-associated virus 3. Front. Microbiol. 2013, 4, 1–21. [Google Scholar] [CrossRef]
- Sudarshana, M.R.; Perry, K.L.; Fuchs, M.F. Grapevine red blotch-associated virus, an emerging threat to the grapevine industry. Phytopathology 2015, 105, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Křižan, B.; Ondrušiková, E.; Holleinová, V.; Moravcová, K.; Bláhová, L. Elimination of Grapevine fanleaf virus in Grapevine by in vivo and in vitro thermotherapy. Hortic. Sci. 2009, 36, 105–108. [Google Scholar] [CrossRef]
- Panattoni, A.; Triolo, E. Susceptibility of grapevine viruses to thermotherapy on in vitro collection of Kober 5BB. Sci. Hortic. 2010, 125, 63–67. [Google Scholar] [CrossRef]
- Duran-Vila, N.; Juárez, J.; Arregui, J.M. Production of Viroid-Free Grapevines by Shoot Tip Culture. Production 1988, 39, 217–220. [Google Scholar]
- Youssef, S.A.; Al-Dhaher, M.M.A.; Shalaby, A.A. Elimination of Grapevine fanleaf virus (GFLV) and Grapevine leaf roll-associated virus-1 (GLRaV-1) from infected grapevine plants using meristem tip culture. Int. J. Virol. 2009, 5, 89–99. [Google Scholar] [CrossRef]
- Kim, M.Y.; Cho, K.H.; Chun, J.A.; Park, S.J.; Kim, S.H.; Lee, H.C. Elimination of grapevine fleck virus from infected grapevines ‘Kyoho’ through meristem-tip culture of dormant buds. J. Plant. Biotechnol. 2017, 44, 401–408. [Google Scholar] [CrossRef]
- Turcsan, M.; Demian, E.; Varga, T.; Jaksa-Czotter, N.; Szegedi, E.; Olah, R.; Varallyay, E. Hts-based monitoring of the efficiency of somatic embryogenesis and meristem cultures used for virus elimination in grapevine. Plants 2020, 9, 1782. [Google Scholar] [CrossRef]
- Panattoni, A.; Luvisi, A.; Triolo, E. Selective chemotherapy on Grapevine leafroll-associated virus-1 and -3. Phytoparasitica 2011, 39, 503–508. [Google Scholar] [CrossRef]
- Skiada, F.G.; Maliogka, V.I.; Katis, N.I.; Eleftheriou, E.P. Elimination of Grapevine rupestris stem pitting-associated virus (GRSPaV) from two Vitis vinifera cultivars by in vitro chemotherapy. Eur. J. Plant. Pathol. 2013, 135, 407–414. [Google Scholar] [CrossRef]
- Komínek, P.; Komínková, M.; Jandová, B. Effect of repeated Ribavirin treatment on grapevine viruses. Acta Virol. 2016, 60, 400–403. [Google Scholar] [CrossRef]
- Guţa, I.C.; Buciumeanu, E.C.; Tataru, L.D.; Topala, C.M. Regeneration of grapevine virus-free plants by in vitro chemotherapy. Acta Hortic. 2017, 1188, 319–322. [Google Scholar] [CrossRef]
- Bi, W.L.; Hao, X.Y.; Cui, Z.H.; Pathirana, R.; Volk, G.M.; Wang, Q.C. Shoot tip cryotherapy for efficient eradication of grapevine leafroll-associated virus-3 from diseased grapevine in vitro plants. Ann. Appl. Biol. 2018, 173, 261–270. [Google Scholar] [CrossRef]
- Gambino, G.; Bondaz, J.; Gribaudo, I. Detection and elimination of viruses in callus, somatic embryos and regenerated plantlets of grapevine. Eur. J. Plant. Pathol. 2006, 114, 397–404. [Google Scholar] [CrossRef]
- Gambino, G.; di Matteo, D.; Gribaudo, I. Elimination of Grapevine fanleaf virus from three Vitis vinifera cultivars by somatic embryogenesis. Eur. J. Plant. Pathol. 2009, 123, 57–60. [Google Scholar] [CrossRef]
- Gambino, G.; Navarro, B.; Vallania, R.; Gribaudo, I.; Di Serio, F. Somatic embryogenesis efficiently eliminates viroid infections from grapevines. Eur. J. Plant. Pathol. 2011, 130, 511–519. [Google Scholar] [CrossRef]
- Bouamama-Gzara, B.; Selmi, I.; Chebil, S.; Melki, I.; Mliki, A.; Ghorbel, A.; Carra, A.; Carimi, F.; Mahfoudhi, N. Elimination of Grapevine leafroll associated virus-3, Grapevine rupestris stem pitting associated virus and Grapevine virus A from a Tunisian Cultivar by somatic embryogenesis and characterization of the somaclones using ampelographic descriptors. Plant. Pathol. J. 2017, 33, 561–571. [Google Scholar] [CrossRef]
- Guţa, I.C.; Buciumeanu, E.C.; Tataru, L.D.; Oprescu, B.; Topala, C.M. New approach of electrotherapy for grapevine virus elimination. Acta Hortic. 2019, 1242, 697–701. [Google Scholar] [CrossRef]
- Salami, S.A.; Ebadi, A.; Zamani, Z.; Habibi, M.K. Incidence of Grapevine fanleaf virus in Iran: A survey study and production of virus-free material using meristem culture and thermotherapy. Eur. J. Hortic. Sci. 2009, 74, 42–46. [Google Scholar]
- Spilmont, A.-S.; Ruiz, A.; Grenan, S. Efficiency of micrografting of shoot apices as a sani- tation method against seven grapevine viruses (ArMV, GFLV, GLRaV-1, -2, -3, GFkV, GVA). In Proceedings of the Proceedings of the 17th Congress of the International Council for the Study of Virus and Virus-like Diseases of the Grapevine (ICVG), Davis, CA, USA, 7–14 October 2012; pp. 270–271. [Google Scholar]
- Hu, G.; Dong, Y.; Zhang, Z.; Fan, X.; Ren, F. Efficiency of chemotherapy combined with thermotherapy for eliminating grapevine leafroll-associated virus 3 (GLRaV-3). Sci. Hortic. 2020, 271, 109462. [Google Scholar] [CrossRef]
- Hu, G.J.; Dong, Y.F.; Zhang, Z.P.; Fan, X.D.; Fang, R.E.N. Elimination of grapevine fleck virus and grapevine rupestris stem pitting-associated virus from Vitis vinifera 87-1 by ribavirin combined with thermotherapy. J. Integr. Agric. 2021, 20, 2463–2470. [Google Scholar] [CrossRef]
- Panattoni, A.; Luvisi, A.; Triolo, E. Review. Elimination of viruses in plants: Twenty years of progress. Span. J. Agric. Res. 2013, 11, 173–188. [Google Scholar] [CrossRef]
- Cooper, V.C.; Walkey, D.G.A. Thermal inactivation of cherry leaf roll virus in tissue cultures of Nicotiana rustica raised from seeds and meristem-tips. Ann. Appl. Biol. 1978, 88, 273–278. [Google Scholar] [CrossRef]
- Wang, Q.; Cuellar, W.J.; Rajamäki, M.L.; Hirata, Y.; Valkonen, J.P.T. Combined thermotherapy and cryotherapy for efficient virus eradication: Relation of virus distribution, subcellular changes, cell survival and viral RNA degradation in shoot tips. Mol. Plant. Pathol. 2008, 9, 237–250. [Google Scholar] [CrossRef]
- Szittya, G.; Silhavy, D.; Molnár, A.; Havelda, Z.; Lovas, Á.; Lakatos, L.; Bánfalvi, Z.; Burgyán, J. Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J. 2003, 22, 633–640. [Google Scholar] [CrossRef]
- Chellappan, P.; Vanitharani, R.; Ogbe, F.; Fauquet, C.M. Effect of temperature on geminivirus-induced RNA silencing in plants. Plant. Physiol. 2005, 138, 1828–1841. [Google Scholar] [CrossRef]
- Qu, F.; Ye, X.; Hou, G.; Sato, S.; Clemente, T.E.; Morris, T.J. RDR6 Has a Broad-Spectrum but Temperature-Dependent Antiviral Defense Role in Nicotiana benthamiana. J. Virol. 2005, 79, 15209–15217. [Google Scholar] [CrossRef]
- Velázquez, K.; Renovell, A.; Comellas, M.; Serra, P.; García, M.L.; Pina, J.A.; Navarro, L.; Moreno, P.; Guerri, J. Effect of temperature on RNA silencing of a negative-stranded RNA plant virus: Citrus psorosis virus. Plant. Pathol. 2010, 59, 982–990. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.J.; Zhang, F.g.P.; Hong, N.; Wang, G.P.; Wang, A.; Wang, L.P. Identification and characterization of microRNAs from in vitro-grown pear shoots infected with Apple stem grooving virus in response to high temperature using small RNA sequencing. BMC Genom. 2015, 16, 945. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.J.; Yang, Y.K.; Hong, N.; Wang, G.P.; Wang, A.; Wang, L.P. Characterization of virus-derived small interfering RNAs in Apple stem grooving virus-infected in vitro-cultured Pyrus pyrifolia shoot tips in response to high temperature treatment. Virol. J. 2016, 13, 1–11. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.J.; Paek, K.H. Temperature-specific vsiRNA confers RNAi-mediated viral resistance at elevated temperature in Capsicum annuum. J. Exp. Bot. 2021, 72, 1432–1448. [Google Scholar] [CrossRef]
- Grout, B.W. Meristem-tip culture for propagation and virus elimination. Methods Mol. Biol. 1999, 111, 115–125. [Google Scholar]
- Jonard, R.; Hugard, J.; Macheix, J.J.; Martinez, J.; Mosella-Chancel, L.; Poessel, J.L.; Villemur, P. In vitro micrografting and its applications to fruit science. Sci. Hortic. 1983, 20, 147–159. [Google Scholar] [CrossRef]
- Hussain, G.; Wani, M.S.; Mir, M.A.; Rather, Z.A.; Bhat, K.M. Micrografting for fruit crop improvement. Afr. J. Biotechnol. 2014, 13, 2474–2483. [Google Scholar] [CrossRef][Green Version]
- Sharma, S.; Singh, B.; Rani, G.; Zaidi, A.A.; Hallan, V.K.; Nagpal, A.K.; Virk, G.S. In vitro production of Indian citrus ringspot virus (ICRSV) free Kinnow plants employing thermotherapy coupled with shoot tip grafting. Plant Cell. Tissue Organ Cult. 2008, 92, 85–92. [Google Scholar] [CrossRef]
- Chae, C.W.; Yun, S.H.; Park, J.H.; Hyun, J.W.; Koh, S.W.; Lee, D.H. Micrografting and Heat Treatment Combination for Eliminating Virus of CTV-infected Citrus. J. Life Sci. 2013, 23, 267–272. [Google Scholar] [CrossRef]
- Miljanić, V.; Jakše, J.; Kunej, U.; Rusjan, D.; Škvarč, A.; Štajner, N. Virome Status of Preclonal Candidates of Grapevine Varieties (Vitis vinifera L.) From the Slovenian Wine-Growing Region Primorska as Determined by High-Throughput Sequencing. Front. Microbiol. 2022, 13, 830866. [Google Scholar] [CrossRef]
- Maliogka, V.I.; Skiada, F.G.; Eleftheriou, E.P.; Katis, N.I. Elimination of a new ampelovirus (GLRaV-Pr) and Grapevine rupestris stem pitting associated virus (GRSPaV) from two Vitis vinifera cultivars combining in vitro thermotherapy with shoot tip culture. Sci. Hortic. 2009, 123, 280–282. [Google Scholar] [CrossRef]
- Gribaudo, I.; Gambino, G.; Cuozzo, D.; Mannini, F. Attempts to eliminate Grapevine rupestris stem pitting-associated virus from grapevine clones. J. Plant. Pathol. 2006, 88, 293–298. [Google Scholar] [CrossRef]
- Sabanadzovic, S.; Aboughanem-Sabanadzovic, N.; Martelli, G.P. Grapevine fleck and similar viruses. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Springer: Cham, Switzerland, 2017; pp. 331–349. ISBN 9783319577067. [Google Scholar]
- Bota, J.; Cretazzo, E.; Montero, R.; Rosselló, J.; Cifre, J. Grapevine fleck virus (GFkV) elimination in a selected clone of vitis vinifera l. CV Manto Negro and its effects on photosynthesis. J. Int. Sci. Vigne Vin 2014, 48, 11–19. [Google Scholar] [CrossRef]
- Eichmeier, A.; Kominkova, M.; Pecenka, J.; Kominek, P. High-throughput small RNA sequencing for evaluation of grapevine sanitation efficacy. J. Virol. Methods 2019, 267, 66–70. [Google Scholar] [CrossRef]
- Miljanić, V.; Jakše, J.; Kunej, U.; Rusjan, D.; Škvarč, A.; Štajner, N. First report of grapevine red globe virus, grapevine rupestris vein feathering virus and grapevine Syrah virus-1 infecting grapevine in Slovenia. Plant. Dis. 2022. [Google Scholar] [CrossRef]
- El Beaino, T.; Sabanadzovic, S.; Digiaro, M.; Abou Ghanem-Sabanadzovic, N.; Rowhani, A.; Kyriakopoulou, P.E.; Martelli, G.P. Molecular detection of Grapevine fleck virus-like viruses. Vitis 2001, 40, 65–68. [Google Scholar]
- Al Rwahnih, M.; Daubert, S.; Golino, D.; Rowhani, A. Deep sequencing analysis of RNAs from a grapevine showing Syrah decline symptoms reveals a multiple virus infection that includes a novel virus. Virology 2009, 387, 395–401. [Google Scholar] [CrossRef]
- Goussard, P.G.; Wiid, J. The Elimination of Fanleaf Virus from Grapevines Using in vitro Somatic Embryogenesis Combined with Heat Therapy. S. Afr. J. Enol. Vitic. 1992, 13, 81–83. [Google Scholar] [CrossRef][Green Version]
- Weiland, C.M.; Superior, E.P.; Cantos, M.; Troncoso, A.; Perez-Camacho, F. Regeneration of virus-free plants by in vitro chemotherapy of GFLV (grapevine fanleaf virus) infected explants of vitis vinifera cv zalema. Acta Hortic. 2004, 652, 463–466. [Google Scholar] [CrossRef]
- Mavrič Pleško, I.; Viršček Marn, M.; Seljak, G.; Žežlina, I. First report of grapevine Pinot gris virus infecting grapevine in Slovenia. Plant. Dis. 2014, 98, 1014. [Google Scholar] [CrossRef] [PubMed]
- Tarquini, G.; Ermacora, P.; Bianchi, G.L.; de Amicis, F.; Pagliari, L.; Martini, M.; Loschi, A.; Saldarelli, P.; Loi, N.; Musetti, R. Localization and subcellular association of Grapevine Pinot Gris Virus in grapevine leaf tissues. Protoplasma 2018, 255, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Gualandri, V.; Bianchedi, P.; Morelli, M.; Giampetruzzi, A.; Valenzano, P.; Giovanna, B.; Campanale, A.; Saldarelli, P. Production of Grapevine Pinot gris virus-free germplasm: Techniques and tools. In Proceedings of the 18th Congress of ICVG, Ankara, Turkey, 7–11 September 2015; pp. 10–12. [Google Scholar]
- Meng, B.; Rowhani, A. Grapevine rupestris stem pitting-associated virus. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Springer: Cham, Switzerland, 2017; pp. 257–287. ISBN 9783319577067. [Google Scholar]
- Hu, G.; Dong, Y.; Zhang, Z.; Fan, X.; Ren, F.; Li, Z.; Zhang, S. Elimination of Grapevine rupestris stem pitting-associated virus from Vitis vinifera ‘Kyoho’ by an antiviral agent combined with shoot tip culture. Sci. Hortic. 2018, 229, 99–106. [Google Scholar] [CrossRef]
- Mavrič, I.; Marn, M.V.; Koron, D.; Žežlina, I. First Report of Raspberry bushy dwarf virus on Red Raspberry and Grapevine in Slovenia. Plant. Dis. 2003, 87, 1148. [Google Scholar] [CrossRef]
- Jevremovic, D.; Paunovic, S. Raspberry bushy dwarf virus: A grapevine pathogen in Serbia. Pestic. Fitomed. 2011, 26, 55–60. [Google Scholar] [CrossRef]
- Pleško, I.M.; Marn, M.V.; Nyerges, K.; Lázár, J. First Report of Raspberry bushy dwarf virus Infecting Grapevine in Hungary. Plant. Dis. 2012, 96, 1582. [Google Scholar] [CrossRef]
- Czotter, N.; Molnar, J.; Szabó, E.; Demian, E.; Kontra, L.; Baksa, I.; Szittya, G.; Kocsis, L.; Deak, T.; Bisztray, G.; et al. NGS of virus-derived small RNAs as a diagnostic method used to determine viromes of Hungarian Vineyards. Front. Microbiol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Navrotskaya, E.; Porotikova, E.; Yurchenko, E.; Galbacs, Z.N.; Varallyay, E.; Vinogradova, S. High-throughput sequencing of small rnas for diagnostics of grapevine viruses and viroids in Russia. Viruses 2021, 13, 2432. [Google Scholar] [CrossRef]
- Theiler-Hedtrich, R.; Baumann, G. Elimination of Apple Mosaic Virus and Raspberry Bushy Dwarf Virus from Infected Red Raspberry (Rubus idaeus L.) by Tissue Culture. J. Phytopathol. 1989, 127, 193–199. [Google Scholar] [CrossRef]
- Weber, C.A. Eliminating Raspberry bushy dwarf virus (RBDV) from infected raspberry tissue cultures with ribavirin. Acta Hortic. 2016, 1133, 473–477. [Google Scholar] [CrossRef]
- Mathew, L.; Tiffin, H.; Erridge, Z.; McLachlan, A.; Hunter, D.; Pathirana, R. Efficiency of eradication of Raspberry bushy dwarf virus from infected raspberry (Rubus idaeus) by in vitro chemotherapy, thermotherapy and cryotherapy and their combinations. Plant. Cell. Tissue Organ. Cult. 2021, 144, 133–141. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]

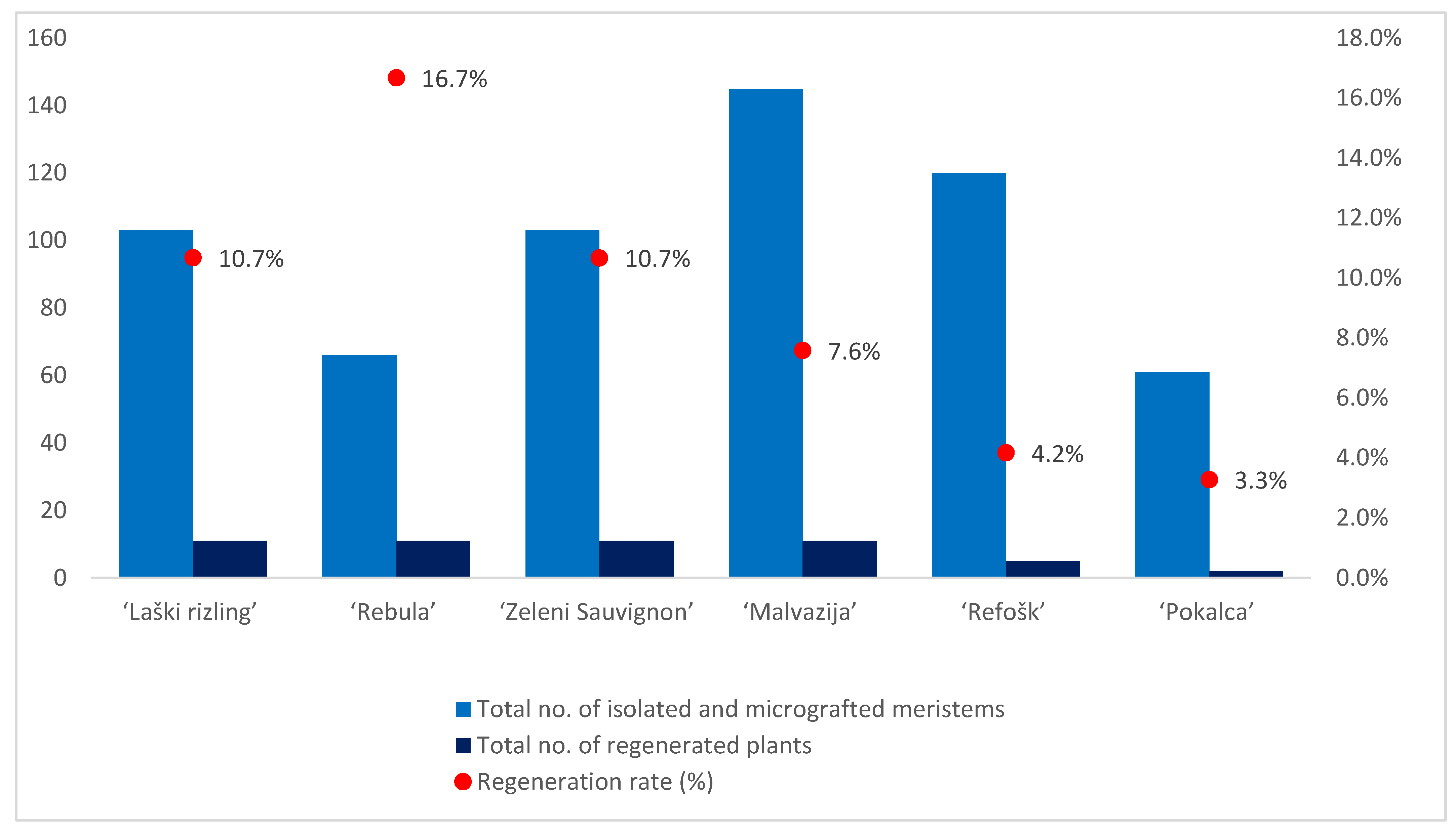




| Sample Name | No. of Isolated and Micrografted Meristems | No. of Regenerated Plants | Regeneration Rate (%) |
|---|---|---|---|
| Laški rizling 3/34B | 26 | 2 | 7.7 |
| Laški rizling 3/45B | 28 | 0 | - |
| Laški rizling 3/64B | 27 | 4 | 14.8 |
| Laški rizling 3/56B | 22 | 5 | 22.7 |
| Rebula 15/3B | 13 | 3 | 23.1 |
| Rebula 16/1B | 22 | 1 | 4.5 |
| Rebula 19/2B | 12 | 3 | 25.0 |
| Rebula 22/3B | 19 | 4 | 21.1 |
| Zeleni Sauvignon 14/2P | 18 | 1 | 5.6 |
| Zeleni Sauvignon 14/5P | 17 | 3 | 17.6 |
| Zeleni Sauvignon 14/7P | 25 | 2 | 8.0 |
| Zeleni Sauvignon 15/2P | 17 | 1 | 5.9 |
| Zeleni Sauvignon 15/3P | 26 | 4 | 15.4 |
| Malvazija 32/1B | 27 | 3 | 11.1 |
| Malvazija 32/2B | 12 | 1 | 8.3 |
| Malvazija 32/3B | 25 | 1 | 4.0 |
| Malvazija 20/47P | 13 | 1 | 7.7 |
| Malvazija 21/8P | 24 | 1 | 4.2 |
| Malvazija 23/2P | 25 | 1 | 4.0 |
| Malvazija 23/3P | 19 | 3 | 15.8 |
| Refošk 11/4P | 28 | 2 | 7.1 |
| Refošk 12/3P | 18 | 0 | - |
| Refošk 12/6P | 27 | 0 | - |
| Refošk 12/18P | 25 | 2 | 8.0 |
| Refošk 12/19P | 22 | 1 | 4.5 |
| Pokalca 3/4P | 20 | 1 | 5.0 |
| Pokalca 3/6P | 22 | 1 | 4.5 |
| Pokalca 9/2G | 19 | 0 | - |
| Virus/Viroid | No. of Infected Preclonal Candidates before the Sanitation Process | No. of Tested Vines after the Sanitation Process | No. of Virus/Viroid-Free Vines | Elimination Rate (%) |
|---|---|---|---|---|
| GRSPaV | 26 | 49 | 49 | 100 |
| GPGV | 26 | 49 | 49 | 100 |
| GFLV | 3 | 2 | 2 | 100 |
| GLRaV-3 | 1 | 2 | 2 | 100 |
| GFkV | 13 | 26 | 26 | 100 |
| GRVFV | 19 | 33 | 33 | 100 |
| GSyV-1 | 3 | 2 | 2 | 100 |
| RBDV | 4 | 11 | 11 | 100 |
| HSVd | 28 | 51 | 20 | 39.2 |
| GYSVd-1 | 27 | 47 | 20 | 42.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miljanić, V.; Rusjan, D.; Škvarč, A.; Chatelet, P.; Štajner, N. Elimination of Eight Viruses and Two Viroids from Preclonal Candidates of Six Grapevine Varieties (Vitis vinifera L.) through In Vivo Thermotherapy and In Vitro Meristem Tip Micrografting. Plants 2022, 11, 1064. https://doi.org/10.3390/plants11081064
Miljanić V, Rusjan D, Škvarč A, Chatelet P, Štajner N. Elimination of Eight Viruses and Two Viroids from Preclonal Candidates of Six Grapevine Varieties (Vitis vinifera L.) through In Vivo Thermotherapy and In Vitro Meristem Tip Micrografting. Plants. 2022; 11(8):1064. https://doi.org/10.3390/plants11081064
Chicago/Turabian StyleMiljanić, Vanja, Denis Rusjan, Andreja Škvarč, Philippe Chatelet, and Nataša Štajner. 2022. "Elimination of Eight Viruses and Two Viroids from Preclonal Candidates of Six Grapevine Varieties (Vitis vinifera L.) through In Vivo Thermotherapy and In Vitro Meristem Tip Micrografting" Plants 11, no. 8: 1064. https://doi.org/10.3390/plants11081064
APA StyleMiljanić, V., Rusjan, D., Škvarč, A., Chatelet, P., & Štajner, N. (2022). Elimination of Eight Viruses and Two Viroids from Preclonal Candidates of Six Grapevine Varieties (Vitis vinifera L.) through In Vivo Thermotherapy and In Vitro Meristem Tip Micrografting. Plants, 11(8), 1064. https://doi.org/10.3390/plants11081064







