Bioaugmentation Improves Phytoprotection in Halimione portulacoides Exposed to Mild Salt Stress: Perspectives for Salinity Tolerance Improvement
Abstract
1. Introduction
2. Results
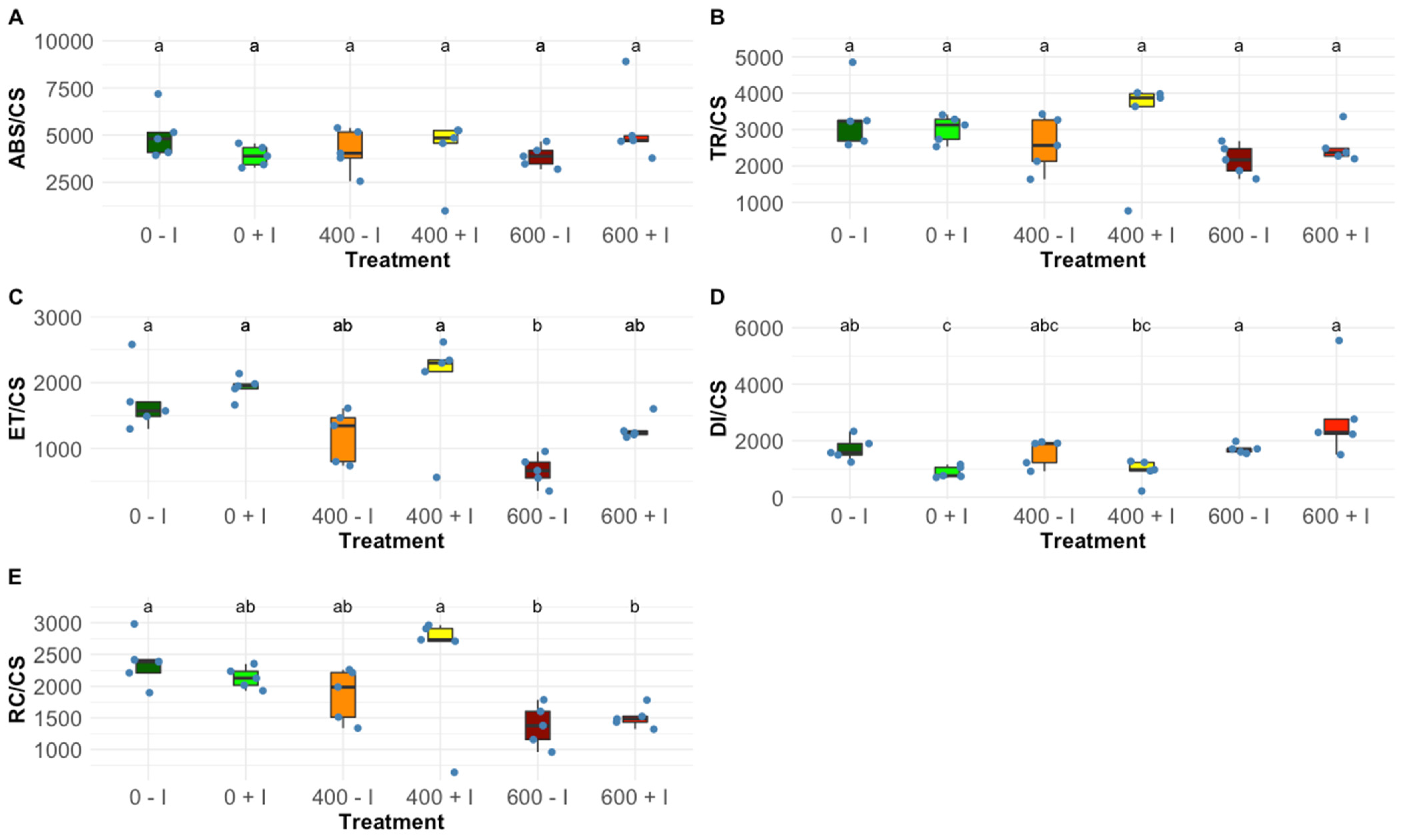
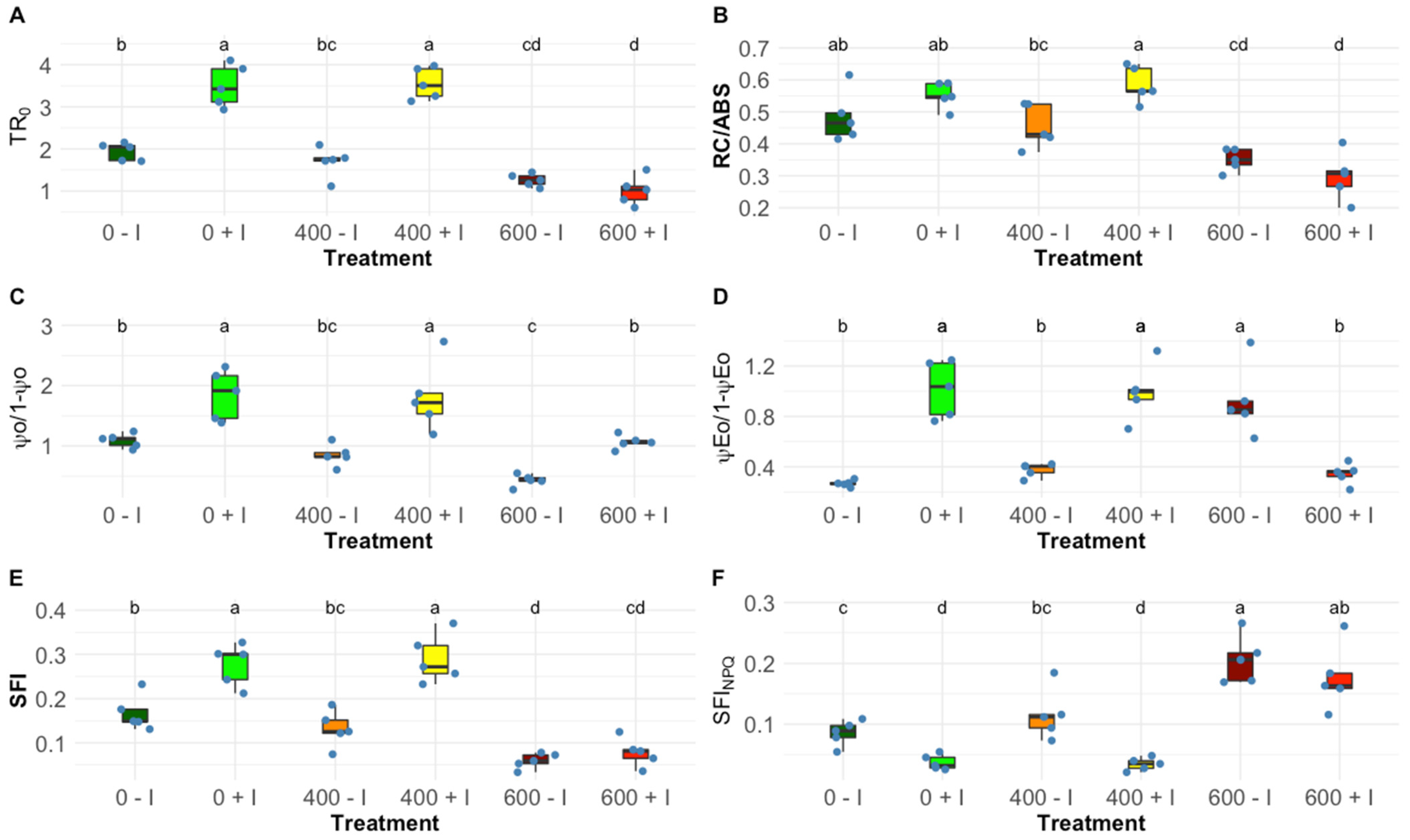
2.1. Photochemical Processes
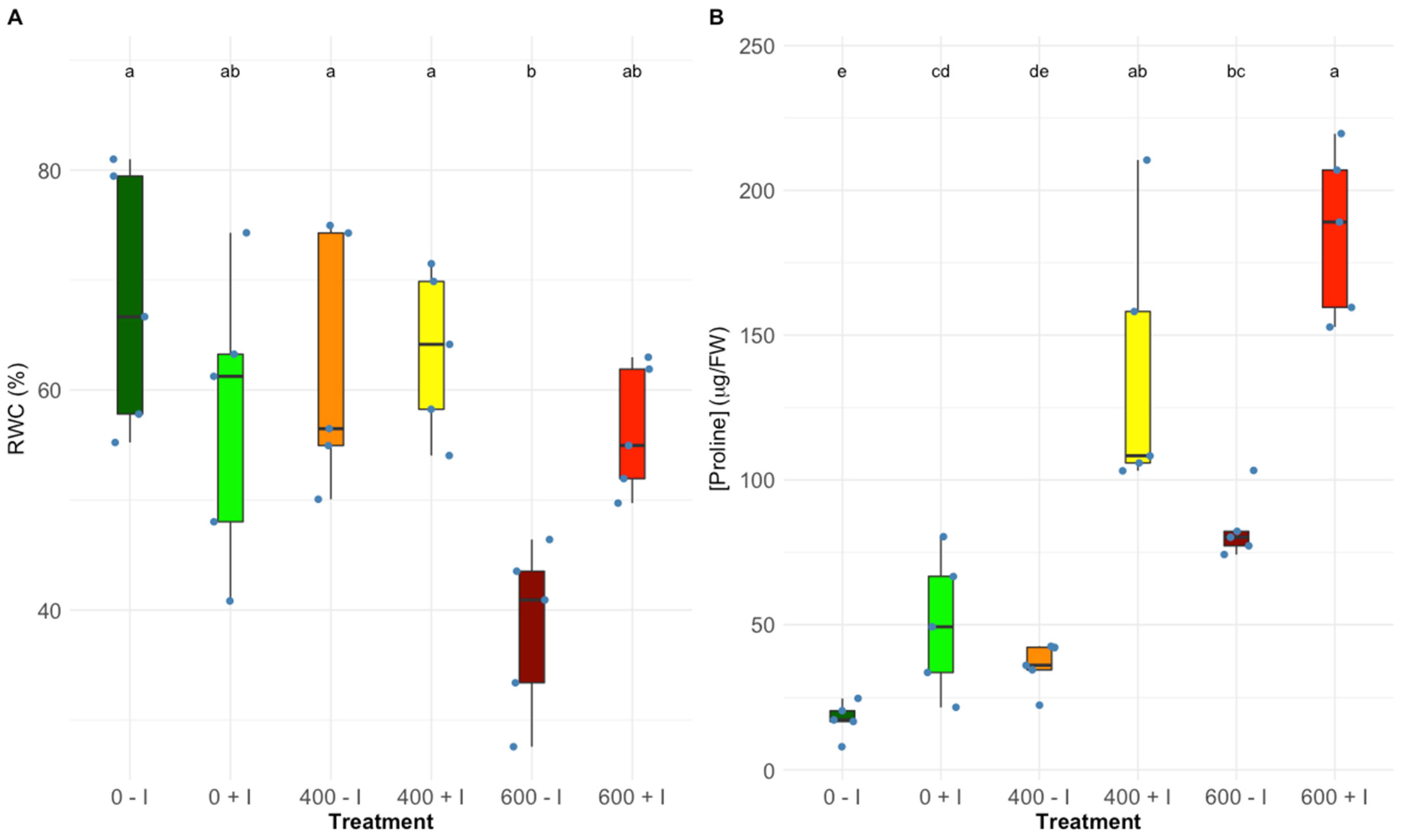
2.2. Na, K, Ca and Cl Accumulation in Leaf, Stem, and Root Tissues
2.3. Photosynthetic Pigments Profile
2.4. Antioxidant Enzymatic Activities
2.5. Proline Quantification
3. Discussion
4. Materials and Methods
4.1. Sampling Sites and Plant Material Collection
4.2. Rhizobacteria Used for Inoculation in This Study
4.3. Preparation of Bacterial Inoculants
4.4. Experimental Setup and Root Inoculation
4.5. Pulse Amplitude Modulated (PAM) Fluorometry
4.6. Ion Analysis
4.7. Pigment Profiling
4.8. Oxidative Stress Biomarkers
4.9. Proline Quantification
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, J.-Y.; Marotzke, J.; Nala, G.; Cao, L.; Corti, S.; Dunne, J.P.; Engelbrecht, F.; Fisher, E.; Fyfe, J.C.; Jones, C.; et al. Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; p. 3949. [Google Scholar]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Beattie, G.A. Mining Halophytes for Plant Growth-Promoting Halotolerant Bacteria to Enhance the Salinity Tolerance of Non-Halophytic Crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of Plant Growth by ACC Deaminase-Producing Soil Bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Valin, H.; Sands, R.D.; van der Mensbrugghe, D.; Nelson, G.C.; Ahammad, H.; Blanc, E.; Bodirsky, B.; Fujimori, S.; Hasegawa, T.; Havlik, P.; et al. The Future of Food Demand: Understanding Differences in Global Economic Models. Agric. Econ. 2014, 45, 51–67. [Google Scholar] [CrossRef]
- Akbarimoghaddam, H.; Galavi, M.; Ghanbari, A.; Panjehkeh, N. Salinity Effects on Seed Germination and Seedling Growth of Bread Wheat Cultivars. Trakia J. Sci. 2011, 9, 43–50. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Teixeira, A.; Duarte, B.; Caçador, I. Salt Marshes and Biodiversity. Sabkha Ecosyst. 2014, 47, 283–298. [Google Scholar] [CrossRef]
- Qadir, M.; Qureshi, R.H.; Ahmad, N. Reclamation of a Saline-Sodic Soil by Gypsum and Leptochloa Fusca. Geoderma 1996, 74, 207–217. [Google Scholar] [CrossRef]
- Indika Herath, Z.; Vithanage, M. Phytoremediation in Constructed Wetlands. In Phytoremediation: Management of Environmental Contaminants; Springer: Cham, Switzerland, 2015; Volume 2, pp. 243–263. [Google Scholar] [CrossRef]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Glassworts: From Wild Salt Marsh Species to Sustainable Edible Crops. Agriculture 2019, 9, 14. [Google Scholar] [CrossRef]
- Caçador, I.; Neto, J.M.; Duarte, B.; Barroso, D.V.; Pinto, M.; Marques, J.C. Development of an Angiosperm Quality Assessment Index (AQuA-Index) for Ecological Quality Evaluation of Portuguese Water Bodies—A Multi-Metric Approach. Ecol. Indic. 2013, 25, 141–148. [Google Scholar] [CrossRef]
- Duarte, B.; Baeta, A.; Rousseau-Gueutin, M.; Ainouche, M.; Marques, J.C.; Caçador, I. A Tale of Two Spartinas: Climatic, Photobiological and Isotopic Insights on the Fitness of Non-Indigenous versus Native Species. Estuar. Coast. Shelf Sci. 2015, 167, 178–190. [Google Scholar] [CrossRef]
- Duarte, B.; Feijão, E.; Pinto, M.V.; Matos, A.R.; Silva, A.; Figueiredo, A.; Fonseca, V.F.; Reis-Santos, P.; Caçador, I. Nutritional Valuation and Food Safety of Endemic Mediterranean Halophytes Species Cultivated in Abandoned Salt Pans under a Natural Irrigation Scheme. Estuar. Coast. Shelf Sci. 2021, 265, 107733. [Google Scholar] [CrossRef]
- Glick, B.R. The Enhancement of Plant Growth by Free-Living Bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Jha, B.; Gontia, I.; Hartmann, A. The Roots of the Halophyte Salicornia Brachiata Are a Source of New Halotolerant Diazotrophic Bacteria with Plant Growth-Promoting Potential. Plant Soil 2012, 356, 265–277. [Google Scholar] [CrossRef]
- Mesa-Marín, J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Bernabeu-Meana, M.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Redondo-Gómez, S. Effect of Plant Growth-Promoting Rhizobacteria on Salicornia Ramosissima Seed Germination under Salinity, CO2 and Temperature Stress. Agronomy 2019, 9, 655. [Google Scholar] [CrossRef]
- Glick, B.R.; Bashan, Y. Genetic Manipulation of Plant Growth-Promoting Bacteria to Enhance Biocontrol of Phytopathogens. Biotechnol. Adv. 1997, 15, 353–378. [Google Scholar] [CrossRef]
- Inbaraj, M.P. Plant-Microbe Interactions in Alleviating Abiotic Stress—A Mini Review. Front. Agron. 2021, 3, 28. [Google Scholar] [CrossRef]
- John, P.; Arshad, M.; Frankenberger, W.T., Jr. Ethylene: Agricultural Sources and Applications. Ann. Bot. 2002, 90, 424. [Google Scholar] [CrossRef][Green Version]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Giongo, A.; Ambrosini, A.; Vargas, L.K.; Freire, J.R.J.; Bodanese-Zanettini, M.H.; Passaglia, L.M.P. Evaluation of Genetic Diversity of Bradyrhizobia Strains Nodulating Soybean [Glycine Max (L.) Merrill] Isolated from South Brazilian Fields. Appl. Soil Ecol. 2008, 38, 261–269. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Cunha, A.; Figueiredo, S.; Faustino, P.; Patinha, C.; Silva, H.; Sierra-Garcia, I.N. The Root Microbiome of Salicornia Ramosissima as a Seedbank for Plant-Growth Promoting Halotolerant Bacteria. Appl. Sci. 2021, 11, 2233. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Romano-Rodríguez, E.; Mesa-Marín, J.; Sola-Elías, C.; Mateos-Naranjo, E. Consortia of Plant-Growth-Promoting Rhizobacteria Isolated from Halophytes Improve the Response of Swiss Chard to Soil Salinization. Agronomy 2022, 12, 468. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, D.P.; Saikia, R. Genetic Diversity of Plant Growth Promoting Rhizobacteria Isolated from Rhizospheric Soil of Wheat under Saline Condition. Curr. Microbiol. 2009, 59, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Figueira, C.; Ferreira, M.J.; Silva, H.; Cunha, A. Improved Germination Efficiency of Salicornia Ramosissima Seeds Inoculated with Bacillus Aryabhattai SP1016-20. Ann. Appl. Biol. 2020, 174, 319–328. [Google Scholar] [CrossRef]
- Schmidt, C.S.; Alavi, M.; Cardinale, M.; Müller, H.; Berg, G. Stenotrophomonas Rhizophila DSM14405 T Promotes Plant Growth Probably by Altering Fungal Communities in the Rhizosphere. Biol. Fertil. Soils 2012, 48, 947–960. [Google Scholar] [CrossRef]
- Fidalgo, C.; Proença, D.N.; Morais, P.V.; Henriques, I.; Alves, A. The Endosphere of the Salt Marsh Plant Halimione Portulacoides Is a Diversity Hotspot for the Genus Salinicola: Description of Five Novel Species Salinicola Halimionae Sp. Nov., Salinicola Aestuarinus Sp. Nov., Salinicola Endophyticus Sp. Nov., Salinicola Halophyticus Sp. Nov. and Salinicola Lusitanus Sp. Nov. Int. J. Syst. Evol. Microbiol. 2019, 69, 46–62. [Google Scholar] [CrossRef]
- IPCC. IPCC Future Global Climate: Scenario-Based Projections and Near-Term Information. Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021; p. 195. [Google Scholar]
- Morales-García, Y.E.; Baez, A.; Quintero-Hernández, V.; Molina-Romero, D.; Rivera-Urbalejo, A.P.; Pazos-Rojas, L.A.; Muñoz-Rojas, J. Bacterial Mixtures, the Future Generation of Inoculants for Sustainable Crop Production. In Field Crops: Sustainable Management by PGPR; Springer: Cham, Switzerland, 2019; pp. 11–44. [Google Scholar] [CrossRef]
- Esitken, A.; Yildiz, H.E.; Ercisli, S.; Figen Donmez, M.; Turan, M.; Gunes, A. Effects of Plant Growth Promoting Bacteria (PGPB) on Yield, Growth and Nutrient Contents of Organically Grown Strawberry. Sci. Hortic. 2010, 124, 62–66. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhowmik, P.C.; Hossain, M.A.; Rahman, M.M.; Prasad, M.N.V.; Ozturk, M.; Fujita, M. Potential Use of Halophytes to Remediate Saline Soils. BioMed Res. Int. 2014, 2014, 589341. [Google Scholar] [CrossRef]
- Mateos-Naranjo, E.; Jurado, J.L.; Redondo-Gómez, S.; Pérez-Romero, J.A.; Glick, B.R.; Rodríguez-Llorente, I.D.; Pajuelo, E.; Echegoyan, A.; Mesa-Marín, J. Uncovering PGPB Vibrio Spartinae Inoculation-Triggered Physiological Mechanisms Involved in the Tolerance of Halimione Portulacoides to NaCl Excess. Plant Physiol. Biochem. 2020, 154, 151–159. [Google Scholar] [CrossRef]
- Duarte, B.; Santos, D.; Marques, J.C.; Caçador, I. Ecophysiological Adaptations of Two Halophytes to Salt Stress: Photosynthesis, PS II Photochemistry and Anti-Oxidant Feedback—Implications for Resilience in Climate Change. Plant Physiol. Biochem. 2013, 67, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Diner, B.A.; Wollman, F.-A. Biosynthesis of Photosystem II Reaction Centers, Antenna and Plastoquinone Pool in Greening Cells of Cyanidium Caldarium Mutant III-C. Plant Physiol. 1979, 63, 26. [Google Scholar] [CrossRef]
- Allen, J.F.; Bennett, J.; Steinback, K.E.; Arntzen, C.J. Chloroplast Protein Phosphorylation Couples Plastoquinone Redox State to Distribution of Excitation Energy between Photosystems. Nature 1981, 291, 25–29. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Govindjee; Bosa, K.; Kościelniak, J.; Zuk-Gołaszewska, K. Effects of Salt Stress on Photosystem II Efficiency and CO2 Assimilation of Two Syrian Barley Landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Duarte, B.; Cabrita, M.T.; Gameiro, C.; Matos, A.R.; Godinho, R.; Marques, J.C.; Caçador, I. Disentangling the Photochemical Salinity Tolerance in Aster Tripolium L.: Connecting Biophysical Traits with Changes in Fatty Acid Composition. Plant Biol. 2017, 19, 239–248. [Google Scholar] [CrossRef]
- Duarte, B.; Santos, D.; Marques, J.C.; Caçador, I. Biophysical Probing of Spartina Maritima Photo-System II Changes during Prolonged Tidal Submersion Periods. Plant Physiol. Biochem. 2014, 77, 122–132. [Google Scholar] [CrossRef]
- Beer, S.; Björk, M. Measuring Rates of Photosynthesis of Two Tropical Seagrasses by Pulse Amplitude Modulated (PAM) Fluorometry. Aquat. Bot. 2000, 66, 69–76. [Google Scholar] [CrossRef]
- Demetriou, G.; Neonaki, C.; Navakoudis, E.; Kotzabasis, K. Salt Stress Impact on the Molecular Structure and Function of the Photosynthetic Apparatus—The Protective Role of Polyamines. Biochim. Biophys. Acta (BBA)—Bioenerg. 2007, 1767, 272–280. [Google Scholar] [CrossRef]
- Duarte, B.; Sleimi, N.; Cagador, I. Biophysical and Biochemical Constraints Imposed by Salt Stress: Learning from Halophytes. Front. Plant Sci. 2014, 5, 746. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Saegusa, D.; Fujita, M.; Phan Tran, L.S. Hydrogen Sulfide Regulates Salt Tolerance in Rice by Maintaining Na+/K+ Balance, Mineral Homeostasis and Oxidative Metabolism under Excessive Salt Stress. Front. Plant Sci. 2015, 6, 1055. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Roles and Transport of Sodium and Potassium in Plants. Met. Ions Life Sci. 2016, 16, 291–324. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Davy, A.J.; Fernández-Muñoz, F.; Castellanos, E.M.; Luque, T.; Figueroa, M.E. Growth and Photosynthetic Responses to Salinity of the Salt-Marsh Shrub Atriplex Portulacoides. Ann. Bot. 2007, 100, 555. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.; Rai, S.; Ilyas, N.; Sayyed, R.Z.; Al-Turki, A.I.; El Enshasy, H.A.; Simarmata, T. Halotolerant Rhizobacteria for Salinity-Stress Mitigation: Diversity, Mechanisms and Molecular Approaches. Sustainability 2022, 14, 490. [Google Scholar] [CrossRef]
- El-Akhal, M.R.; Rincón, A.; Coba de la Peña, T.; Lucas, M.M.; El Mourabit, N.; Barrijal, S.; Pueyo, J.J. Effects of Salt Stress and Rhizobial Inoculation on Growth and Nitrogen Fixation of Three Peanut Cultivars. Plant Biol. 2013, 15, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Dodd, I.C.; Hontzeas, N.; Theobald, J.C.; Safronova, V.I.; Davies, W.J. Rhizosphere Bacteria Containing 1-Aminocyclopropane-1-Carboxylate Deaminase Increase Yield of Plants Grown in Drying Soil via Both Local and Systemic Hormone Signalling. New Phytol. 2009, 181, 413–423. [Google Scholar] [CrossRef]
- Munns, R. Why Measure Osmotic Adjustment? Funct. Plant Biol. 1988, 15, 717–726. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A.; Al-Qurainy, F.; Foolad, M.R. Drought Tolerance: Roles of Organic Osmolytes, Growth Regulators, and Mineral Nutrients. Adv. Agron. 2011, 111, 249–296. [Google Scholar] [CrossRef]
- Nicola, S.; Grigoriadou, K.; Schunko, C.; Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Stewart, G.R.; Lee, J.A. The Role of Proline Accumulation in Halophytes. Planta 1974, 120, 279–289. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline Accumulation in Plants: A Review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.B.; Boyer, K.E. Nitrogen Further Promotes a Dominant Salt Marsh Plant in an Increasingly Saline Environment. J. Plant Ecol. 2012, 5, 429–441. [Google Scholar] [CrossRef]
- Caçador, I.; Tibério, S.; Cabral, H.N. Species Zonation in Corroios Salt Marsh in the Tagus Estuary (Portugal) and Its Dynamics in the Past Fifty Years. Hydrobiologia 2007, 587, 205–211. [Google Scholar] [CrossRef]
- Carreiras, J.; Alberto Pérez-Romero, J.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Rita Matos, A.; Caçador, I.; Duarte, B. The Effect of Heavy Metal Contamination Pre-Conditioning in the Heat Stress Tolerance of Native and Invasive Mediterranean Halophytes. Ecol. Indic. 2020, 111, 106045. [Google Scholar] [CrossRef]
- Carreiras, J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Matos, A.R.; Caçador, I.; Duarte, B. Heavy Metal Pre-Conditioning History Modulates Spartina Patens Physiological Tolerance along a Salinity Gradient. Plants 2021, 10, 2072. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The Role of the Xanthophyll Cycle and of Lutein in Photoprotection of Photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection and Other Responses of Plants to High Light Stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Carotenoids and Photoprotection in Plants: A Role for the Xanthophyll Zeaxanthin. Biochim. Biophys. Acta (BBA)—Bioenerg. 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Havaux, M.; Dall’Osto, L.; Bassi, R. Zeaxanthin Has Enhanced Antioxidant Capacity with Respect to All Other Xanthophylls in Arabidopsis Leaves and Functions Independent of Binding to PSII Antennae. Plant Physiol. 2007, 145, 1506–1520. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A.V.; Walters, R.G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 655–684. [Google Scholar] [CrossRef]
- Siefermann-Harms, D. Carotenoids in Photosynthesis. I. Location in Photosynthetic Membranes and Light-Harvesting Function. Biochim. Biophys. Acta (BBA)—Rev. Bioenerg. 1985, 811, 325–355. [Google Scholar] [CrossRef]
- Duarte, B.; Santos, D.; Marques, J.C.; Caçador, I. Ecophysiological Constraints of Two Invasive Plant Species under a Saline Gradient: Halophytes versus Glycophytes. Estuar. Coast. Shelf Sci. 2015, 167, 154–165. [Google Scholar] [CrossRef]
- Ruban, A.V.; Phillip, D.; Young, A.J.; Horton, P. Excited-State Energy Level Does Not Determine the Differential Effect of Violaxanthin and Zeaxanthin on Chlorophyll Fluorescence Quenching in the Isolated Light-Harvesting Complex of Photosystem II. Photochem. Photobiol. 1998, 68, 829–834. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The Role of Endogenous Nitric Oxide in Salicylic Acid-Induced up-Regulation of Ascorbate-Glutathione Cycle Involved in Salinity Tolerance of Pepper (Capsicum Annuum L.) Plants. Plant Physiol. Biochem. PPB 2020, 147, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of Inoculation with Plant Growth-Promoting Bacteria (PGPB) on Amelioration of Saline Stress in Maize (Zea Mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Duarte, B.; Santos, D.; Caçador, I. Halophyte Anti-Oxidant Feedback Seasonality in Two Salt Marshes with Different Degrees of Metal Contamination: Search for an Efficient Biomarker. Funct. Plant Biol. FPB 2013, 40, 922–930. [Google Scholar] [CrossRef]
- Zhou, H.; Lin, H.; Chen, S.; Becker, K.; Yang, Y.; Zhao, J.; Kudla, J.; Schumaker, K.S.; Guo, Y. Inhibition of the Arabidopsis Salt Overly Sensitive Pathway by 14-3-3 Proteins. Plant Cell 2014, 26, 1166–1182. [Google Scholar] [CrossRef]
- Jithesh, M.N.; Prashanth, S.R.; Sivaprakash, K.R.; Parida, A.K. Antioxidative Response Mechanisms in Halophytes: Their Role in Stress Defence. J. Genet. 2006, 85, 237–254. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the Molecular Mechanisms Mediating Plant Salt-Stress Responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Khan, I.; Raza, M.A.; Awan, S.A.; Shah, G.A.; Rizwan, M.; Ali, B.; Tariq, R.; Hassan, M.J.; Alyemeni, M.N.; Brestic, M.; et al. Amelioration of Salt Induced Toxicity in Pearl Millet by Seed Priming with Silver Nanoparticles (AgNPs): The Oxidative Damage, Antioxidant Enzymes and Ions Uptake Are Major Determinants of Salt Tolerant Capacity. Plant Physiol. Biochem. PPB 2020, 156, 221–232. [Google Scholar] [CrossRef]
- Feijão, E.; Cruz de Carvalho, R.; Duarte, I.A.; Matos, A.R.; Cabrita, M.T.; Novais, S.C.; Lemos, M.F.L.; Caçador, I.; Marques, J.C.; Reis-Santos, P.; et al. Fluoxetine Arrests Growth of the Model Diatom Phaeodactylum Tricornutum by Increasing Oxidative Stress and Altering Energetic and Lipid Metabolism. Front. Microbiol. 2020, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, S.M.; de Oliveira, C.A.; Andrade, D.L.; de Carvalho, C.G.; Ribeiro, V.P.; Pastina, M.M.; Marriel, I.E.; de Paula Lana, U.G.; Gomes, E.A. Tropical Bacillus Strains Inoculation Enhances Maize Root Surface Area, Dry Weight, Nutrient Uptake and Grain Yield. J. Plant Growth Regul. 2021, 40, 867–877. [Google Scholar] [CrossRef]
- Zhu, X.G.; Govindjee; Baker, N.R.; DeSturler, E.; Ort, D.R.; Long, S.P. Chlorophyll a Fluorescence Induction Kinetics in Leaves Predicted from a Model Describing Each Discrete Step of Excitation Energy and Electron Transfer Associated with Photosystem II. Planta 2005, 223, 114–133. [Google Scholar] [CrossRef]
- Towett, E.K.; Shepherd, K.D.; Cadisch, G. Quantification of Total Element Concentrations in Soils Using Total X-Ray Fluorescence Spectroscopy (TXRF). Sci. Total Environ. 2013, 463–464, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Küpper, H.; Seibert, S.; Parameswaran, A. Fast, Sensitive, and Inexpensive Alternative to Analytical Pigment HPLC: Quantification of Chlorophylls and Carotenoids in Crude Extracts by Fitting with Gauss Peak Spectra. Anal. Chem. 2007, 79, 7611–7627. [Google Scholar] [CrossRef] [PubMed]
- Tiryakioglu, M.; Eker, S.; Ozkutlu, F.; Husted, S.; Cakmak, I. Antioxidant Defense System and Cadmium Uptake in Barley Genotypes Differing in Cadmium Tolerance. J. Trace Elem. Med. Biol. 2006, 20, 181–189. [Google Scholar] [CrossRef]
- Teranishi, Y.; Tanaka, A.; Osumi, M.; Fukui, S. Catalase Activities of Hydrocarbon-Utilizing Candida Yeasts. Agric. Biol. Chem. 1974, 38, 1213–1220. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Gawehn, K.; Grassl, M. Lactatedehydrogenase, UV-Assay with Pyruvate and NADH. In Methods of Enzymatic Analysis; Academic Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Bradford, M.M. Determinación de Proteínas: Método de Bradford. Anal. Biochem. 1976, 254, 248–254. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Mendiburu, F.; Simon, R.; De Mendiburu, F. Agricolae—Ten Years of an Open Source Statistical Tool for Experiments in Breeding, Agriculture and Biology. PeerJ 2015, 3, e1404v1. [Google Scholar] [CrossRef]



| Plant-Growth Promoting Traits | ||||||||
|---|---|---|---|---|---|---|---|---|
| Bacterial Strains | Sampling Site | Limit Salt Tolerance (mM) | P-Solubilization | Siderophore | ACC Deaminase (nm mg−1 h−1) | IAA (µg mL−1) | N-Fixation | EPS (OD540) |
| Bacillus aryabhattai SP20 | Horta dos Peixinhos, Portugal | 856 | + | + | − | − | − | 0.80 ± 0.01 |
| Stenotrophomonas rhizophila EH7 | Horta dos Peixinhos, Portugal | 856 | + | + | - | 15.02 ± 0.31 | + | − |
| Pseudomonas oryzihabitans RL18 | Tagus Estuary, Portugal | 1711 | + | + | +* (26.9 ± 14.13) | 39.55 ± 1.01 | − | 0.40 ± 0.01 |
| Salinicola endophyticus EL13 | Tagus Estuary, Portugal | 1711 | + | + | +* | 71.35 ± 6.86 | − | 0.54 ± 0.01 |
| Ψ0/(1 − Ψ0) | Contribution of the dark reactions from quinone A to plastoquinone |
| ΨEo/(1 − ΨEo) | The equilibrium constant for the redox reactions between PS II and PS I |
| RC/ABS | Reaction centre II density within the antenna chlorophyll bed of PS II |
| TR0/DI0 | Contribution or partial performance due to the light reactions for primary photochemistry |
| SFI | Structure functional index for photosynthesis |
| SFI (NO) | Non-photosynthetic or dissipation structure functional index |
| ABS/CS | Absorbed energy flux per cross-section |
| TR/CS | Trapped energy flux per cross-section |
| ET/CS | Electron transport energy flux per cross-section |
| DI/CS | Dissipated energy flux per cross-section |
| RC/CS | The number of available reaction centres per cross-section |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreiras, J.; Caçador, I.; Duarte, B. Bioaugmentation Improves Phytoprotection in Halimione portulacoides Exposed to Mild Salt Stress: Perspectives for Salinity Tolerance Improvement. Plants 2022, 11, 1055. https://doi.org/10.3390/plants11081055
Carreiras J, Caçador I, Duarte B. Bioaugmentation Improves Phytoprotection in Halimione portulacoides Exposed to Mild Salt Stress: Perspectives for Salinity Tolerance Improvement. Plants. 2022; 11(8):1055. https://doi.org/10.3390/plants11081055
Chicago/Turabian StyleCarreiras, João, Isabel Caçador, and Bernardo Duarte. 2022. "Bioaugmentation Improves Phytoprotection in Halimione portulacoides Exposed to Mild Salt Stress: Perspectives for Salinity Tolerance Improvement" Plants 11, no. 8: 1055. https://doi.org/10.3390/plants11081055
APA StyleCarreiras, J., Caçador, I., & Duarte, B. (2022). Bioaugmentation Improves Phytoprotection in Halimione portulacoides Exposed to Mild Salt Stress: Perspectives for Salinity Tolerance Improvement. Plants, 11(8), 1055. https://doi.org/10.3390/plants11081055







