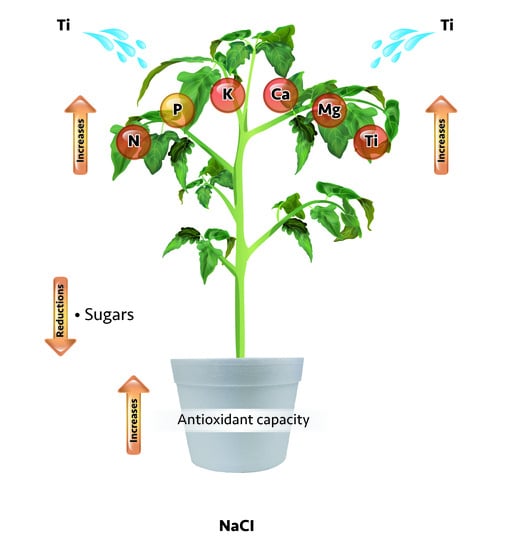
Titanium Increases the Antioxidant Activity and Macronutrient Concentration in Tomato Seedlings Exposed to Salinity in Hydroponics
Abstract
1. Introduction
2. Results
2.1. Seedling Height, Stem Diameter, and Leaf Area
2.2. SPAD Units and Photosynthetic Pigments
2.3. Total Sugars in Leaves, Stems, and Roots of Tomato Seedlings
2.4. Total Antioxidant Activity
2.5. Nutrient Concentration
3. Discussion
4. Materials and Methods
4.1. Plant Material, Saline and Leaf Treatments
4.2. Experimental Design
4.3. Growth Variables
4.4. SPAD Units and Concentration of Photosynthetic Pigments
4.5. Determination of Total Sugars
4.6. Antioxidant Activity
4.7. Nutrient Concentration
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Global Map of Salt Affected Soils. 2021. Available online: https://www.fao.org/3/cb7247en/cb7247en.pdf (accessed on 4 March 2022).
- Hussain, S.; Shaukat, M.; Ashraf, M.A.; Zhu, C.Q.; Jin, Q.; Zhang, J. Salinity stress in arid and semi-arid climates: Effects and management in field crops. In Climate Change and Agriculture, 1st ed.; Hussain, S., Ed.; InTech Publishers: Rijeka, Croatia, 2019; pp. 1–26. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. Biological Parts for Engineering Abiotic Stress Tolerance in Plants. BioDes. Res. 2022, 2022, 9819314. [Google Scholar] [CrossRef]
- Can-Chulim, A.; Cruz-Crespo, E.; Ortega-Escobar, H.M.; Sánchez-Bernal, E.I.; Madueño-Molina, A.; Bojorquez-Serrano, J.I.; Macilla-Villa, O.R. Phaseolus vulgaris response to salinity generated by NaCl, Na2SO4 and NaHCO3. Rev. Mex. Cienc. Agríc. 2017, 8, 1287–1300. [Google Scholar]
- Madueño-Molina, A.; García-Paredes, J.D.; Martínez-Hernández, J.; Bugarín-Montoya, R.; Bojorquez-Serrano, J.I. Salinidad inducida con NaCl y aplicación de fósforo sobre las propiedades bioquímicas de frijolillo (Rhynchosia minima L. (DC)). Univ. Y Cienc. 2011, 27, 43–51. [Google Scholar]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Mannino, G.; Beekwilder, J.; Contartese, V.; Karlova, R.; Bertea, C.M. The application of a biostimulant based on tannins affects root architecture and improves tolerance to salinity in tomato plants. Sci. Rep. 2021, 11, 354. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Vetrano, F. Use of microbial biostimulants to increase the salinity tolerance of vegetable transplants. Agronomy 2021, 11, 1143. [Google Scholar] [CrossRef]
- Saran, S.; Jayanth, T.A.S.; Anand, S.; Pandey, V.; Sumathi, N. Tomato Processing Industry Management. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2017, VI, 124–128. [Google Scholar]
- Laranjeira, T.; Costa, A.; Faria-Silva, C.; Ribeiro, D.; de Oliveira, J.M.P.F.; Simões, S.; Ascenso, A. Sustainable valorization of tomato by-products to obtain bioactive compounds: Their potential in inflammation and cancer management. Molecules 2022, 27, 1701. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Ciulca, S.I.; Poiana, M.A.; Moigradean, D.; Radulov, I.; Negrea, M.; Sumalan, R.L. The antioxidant profile evaluation of some tomato landraces with soil salinity tolerance correlated with high nutraceutical and functional value. Agronomy 2020, 10, 500. [Google Scholar] [CrossRef]
- Ladewig, P.; Trejo-Téllez, L.I.; Servín-Juárez, R.; Contreras-Oliva, A.; Gómez-Merino, F.C. Growth, yield and fruit quality of Mexican tomato landraces in response to salt stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12005. [Google Scholar] [CrossRef]
- Singh, J.; Sastry, E.V.; Singh, V. Effect of salinity on tomato (Lycopersicon esculentum Mill.) during seed germination stage. Physiol. Mol. Biol. Plants 2012, 18, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aversana, E.; Cirillo, V.; Van Oosten, M.J.; Di Stasio, E.; Saiano, K.; Woodrow, P.; Ciarmiello, L.F.; Maggio, A.; Carillo, P. Ascophyllum nodosum based extracts counteract salinity stress in tomato by remodeling leaf nitrogen metabolism. Plants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. FAOSTAT. Data. Production. 2022. Available online: https://www.fao.org/faostat/en/#data/QV (accessed on 12 March 2022).
- Zhan, W.; Wang, Y. Mexican Tomatoes Are Winning the American Market. University of California at Davis. 2021. Available online: https://asmith.ucdavis.edu/news/mexican-tomatoes-are-winning-american-domestic-markets (accessed on 28 February 2022).
- SADER, ASERCA y CIMA. Reporte del Mercado de Tomate Rojo. 2019. Secretaría de Agricultura y Desarrollo Rural. Available online: https://www.cima.aserca.gob.mx/work/models/cima/pdf/cadena/2019/Reporte_mercado_jitomate_130319.pdf (accessed on 2 January 2022).
- Bacilieri, F.S.; Pereira de Vasconcelos, A.C.; Quintao Lana, R.M.; Mageste, J.G.; Torres, J.L.R. Titanium (Ti) In Plant Nutrition-A Review. Aust. J. Crop Sci. 2017, 11, 382–386. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a beneficial element for crop production. Front. Plant Sci. 2017, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Bedinger, G.M. Titanium: 2016 Mineral Yearbook. United State Geological Survey. 2018. Available online: https://www.usgs.gov/centers/national-minerals-information-center/titanium-statistics-and-information (accessed on 15 January 2022).
- Gao, L.; Rao, B.; Dai, H.; Xie, H.; Wang, P.; Ma, F. Kinetics of sulphuric acid leaching of titanium from refractory anatase under atmospheric pressure. Physicochem. Probl. Miner. Process. 2019, 55, 467–478. [Google Scholar] [CrossRef]
- Cervantes-Avilés, P.; Souza-Brito, E.; Bernal-Martínez, A.; Reyes-Aguilera, J.A.; de la Rosa, G.; Cuevas-Rodríguez, G. Impacto de los nanocontaminantes en biorreactores aerobios para tratamiento de aguas residuales. Rev. Mex. Ing. Quím. 2017, 16, 247–261. [Google Scholar]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 Nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef]
- Rezaei, F.; Moaveni, P.; Mozafari, H. Effect of different concentrations and time of nano TiO2 spraying on quantitative and qualitative yield of soybean (Glycine max L.) at Shahr-e-Qods, Iran. Biol. Forum 2015, 7, 957–964. [Google Scholar]
- Abdel-Latef, A.A.H.; Srivastava, A.K.; El-sadek, M.S.A.; Kordrostami, M.; Tran, L.S.P. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad. Dev. 2018, 29, 1065–1073. [Google Scholar] [CrossRef]
- Servin, A.D.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Diaz, B.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron Micro-XRF and Micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2012, 46, 7637–7643. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A Review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- López-Vargas, E.R.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Juárez-Maldonado, A. Seed treatment with carbon nanomaterials impacts growth and nutrient absorption in tomato under saline stress. Agronomy 2021, 8, 1–21. [Google Scholar] [CrossRef]
- Saldaña, T.M.; Bejarano, C.A.; Guaqueta, S. Efecto de la salinidad en el crecimiento de plantas de tomate tipo chonto. Rev. Colomb. Cienc. Hortíc. 2017, 11, 329–342. [Google Scholar] [CrossRef]
- Goykovic, V.; Saavedra, G.R. Some effects of salinity on the tomato cultivars and agronomic practices in its managing. Idesia 2007, 25, 47–58. [Google Scholar] [CrossRef][Green Version]
- Coca, A.; Carranza, C.; Miranda, D.; Rodríguez, M. NaCl Effects on growth, yield and quality parameters in the onion (Allium cepa L.) under controlled conditions. Rev. Colomb. Cienc. Hortíc. 2012, 6, 196–212. [Google Scholar] [CrossRef]
- Casierra, F.; Arias, J.; Pachón, C. Effect of salinity caused by NaCl on hybrid tomato plants (Lycopersicon esculentum Miller). Orinoquia 2013, 17, 23–29. [Google Scholar] [CrossRef]
- Hrubý, M.; Cígler, P.; Kuzel, S. Contribution to understanding the mechanism of titanium action in plant. J. Plant Nutr. 2002, 25, 577–598. [Google Scholar] [CrossRef]
- Kuzel, S.; Hruby, M.; Cígler, P.; Tlustos, P.; Van, N.P. Mechanism of physiological effects of titanium leaf sprays on plants grown on soil. Biol. Trace Elem. Res. 2003, 91, 179–190. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, J.C., Jr.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.; Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2020, 207, 111225. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Agathokleous, E. Hormesis: Transforming disciplines that rely on the dose response. IUBMB Life 2022, 74, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Cherchi, C.; Lin, Y.; Gu, A.Z. Nano-titanium dioxide exposure impacts nitrogen metabolism pathways in cyanobacteria. Environ. Engin. Sci. 2021, 38, 469–480. [Google Scholar] [CrossRef]
- Chahardoli, A.; Sharifan, H.; Karimi, N.; Shiva Najafi Kakavand, S.N. Uptake, translocation, phytotoxicity, and hormetic effects of titanium dioxide nanoparticles (TiO2NPs) in Nigella arvensis L. Sci. Total Environ. 2022, 806, 151222. [Google Scholar] [CrossRef]
- Haghighi, M.; da Silva, J.A.T. The effect of N-TiO2 on tomato, onion, and radish seed germination. J. Crop Sci. Biotechnol. 2014, 17, 221–227. [Google Scholar] [CrossRef]
- Ma, C.; Liu, H.; Chen, G.; Zhao, Q.; Eitzer, B.; Wang, Z.; Cai, W.; Newman, L.A.; White, J.C.; Dhankher, O.P.; et al. Effects of titanium oxide nanoparticles on tetracycline accumulation and toxicity in Oryza sativa (L.). Environ. Sci. Nano 2017, 4, 1827–1839. [Google Scholar] [CrossRef]
- Castiglione, M.R.; Giorgetti, L.; Geri, C.; Cremonini, R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J. Nanopart. Res. 2011, 13, 2443–2449. [Google Scholar] [CrossRef]
- Yang, F.; Hong, F.; Yang, P.; You, W.; Liu, C.; Gao, F.; Wu, C.; Yang, P. Influences of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006, 110, 179–190. [Google Scholar] [CrossRef]
- Calderón-Paniagua, N.; Estrada-Luna, A.A.; Martínez-Hernández, J.D.J. Efecto de la salinidad en el crecimiento y absorción nutrimental de plantas micropropagadas de nopal (Opuntia spp.). Rev. Chapingo Ser. Cienc. For. Ambient. 2001, 7, 127–132. [Google Scholar]
- Castorena, M.V.; Valencia, E.A.C.; Ibarra, M.A.I.; Ulery, A.L. Absorción y traslocación de sodio y cloro en plantas de chile fertilizadas con nitrógeno y crecidas con estrés salino. Rev. Fitotec. Mex. 2006, 29, 79–88. [Google Scholar]
- Gonzalez, P.; Syvertsen, J.P.; Etxeberria, E. Sodium distribution in salt-stressed citrus rootstock seedlings. HortScience 2012, 47, 1504–1511. [Google Scholar] [CrossRef]
- Quintana-Blanco, W.A.; Pinzón-Sandoval, E.H.; Torres, D.F. Evaluación del crecimiento de frijol (Phaseolus vulgaris L.) cv. Ica Cerinza, bajo estrés salino. Rev. U.D.C.A Act. Div. Cient. 2016, 19, 87–95. [Google Scholar] [CrossRef]
- Argentel, L.; López, D.R.; González, L.M.; López, R.C.; Gómez, E.; Girón, R.; Fonseca, I. Contenido de clorofila e iones en la variedad de trigo harinero Cuba-C-204 en condiciones de estrés salino. Cult. Trop. 2009, 30, 32–37. [Google Scholar]
- Africano, P.K.L.; Pinzón, S.E.H. Comportamiento Fisiológico de plantas de rábano (Raphanus sativus L.) sometidas a estrés por salinidad. Conex. Agropecu. 2014, 4, 13–24. [Google Scholar]
- Soussi, M.; Ocaña, A.; Lluch, C. Effects of salt stress on growth, photosynthesis and nitrogen fixation in chick-pea (Cicer arietinum L.). J. Exp. Bot. 1998, 49, 1329–1337. [Google Scholar] [CrossRef]
- Stepien, P.; Johnson, G.N. Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: Role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol. 2009, 149, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Willadino, L.; Camara, T. Origen y naturaleza de los ambientes salinos. In La Ecofisiología Vegetal, una Ciencia de Síntesis; Reigosa, M., Pedrol, N., Sánchez, A., Eds.; Thomson Editores: Madrid, Spain, 2004; pp. 300–303. [Google Scholar]
- Romero, C.A.; Espinosa, R.M.C.; Cutanda, C.; Cortina, P.; Hernández, F.A.; Culiañez, M. La osmorregulación: Mecanismos y significado. In La ecofisiología vegetal, una ciencia de síntesis; Reigosa, M., Pedrol, N., Sánchez, A., Eds.; Thomson Editores: Madrid, Spain, 2004; pp. 603–620. [Google Scholar]
- Munns, R.; James, R.A.; Lauchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Mohammed, A.H.M.A. Physiological Aspects of mungbean plant (Vigna radiata L. Wilezek) in response to salt stress and gibberellic acid treatment. Res. J. Agric. Biol. Sci. 2007, 3, 200–213. [Google Scholar]
- Kühn, C.; Franceschi, V.R.; Schulz, A.; Lemoine, R.; Frommer, W.B. Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 1997, 275, 1298–1300. [Google Scholar] [CrossRef]
- Yin, Y.G.; Kobayashi, Y.; Sanuki, A.; Kondo, S.; Fukuda, N.; Ezura, H.; Sugaya, S.; Matsukura, C. Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) fruits in an ABA-and osmotic stress-independent manner. J. Exp. Bot. 2010, 61, 563–574. [Google Scholar] [CrossRef]
- Camejo, D.; Torres, W. La salinidad y su efecto en los estadios iniciales del desarrollo de dos cultivares de tomate (Lycopersicon esculentum Mill). Cult. Trop. 2000, 21, 23–26. [Google Scholar]
- Kleiber, T.; Markiewicz, B. Application of “Tytanit” in greenhouse tomato growing. Acta Sci. Pol. Hortorum Cultus 2013, 12, 117–126. [Google Scholar]
- Guil-Guerrero, J.L.; Rebolloso-Fuentes, M.M. Nutrient composition and antioxidant activity of eight tomato (Lycopersicon esculentum) varieties. J. Food Compos. Anal. 2009, 22, 123–129. [Google Scholar] [CrossRef]
- Bailly, C.; Benamar, A.; Corbineau, F.; Come, D. Antioxidant systems in sunflower (Helianthus annuum L.) seeds as affected by priming. Seed Sci. Res. 2000, 10, 35–42. [Google Scholar] [CrossRef]
- Bybordi, A.; Mamedov, G. Evaluation of application methods efficiency of zinc and iron for canola (Brassica napus L.). Not. Sci. Biol. 2010, 2, 94–103. [Google Scholar] [CrossRef]
- Qi, M.; Liu, Y.; Li, T. Nano-TiO2 improve the photosynthesis of tomato leaves under mild heat stress. Biol. Trace Elem. Res. 2013, 156, 323–328. [Google Scholar] [CrossRef]
- Carvajal, M.; Martínez-Sánchez, F.; Alcaraz, C.F. Effect of Ti(IV) application on some enzymatic activities in several developing stagesof Capsicum annuum L. plants. J. Plant Nutr. 1994, 17, 243–253. [Google Scholar] [CrossRef]
- Skupień, K.; Oszmiański, J. Influence of titanium treatment on antioxidants content and antioxidant activity of strawberries. Acta Sci. Pol. Technol. Aliment. 2007, 6, 83–94. [Google Scholar]
- Ghorbanpour, M.; Hatami, M.; Hatami, M. Activating antioxidant enzymes, hyoscyamine and scopolamine biosynthesis of Hyoscyamus niger L. plants with nano-sized titanium dioxide and bulk application. Acta Agric. Slov. 2015, 105, 23–32. [Google Scholar] [CrossRef]
- Moghaddam, H.; Madani, A. Influence of titanium foliar application on antioxidant enzyme activity and some biochemical attributes of corn. Maydica 2018, 61, 5. Available online: https://journals-crea.4science.it/index.php/maydica/article/view/1541 (accessed on 31 January 2022).
- Ashraf, M.; Shahzad, S.M.; Imitaz, M.; Rizwan, M.S. Salinity effects on nitrogen metabolism in plants focusing on the activities of nitrogen metabolizing enzymes: A Review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Effects of NaCl stress on nitrogen and phosphorous metabolism in a true mangrove Bruguiera parviflora grown under hydroponic culture. J. Plant Physiol. 2004, 161, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Abdelgadir, E.M.; Oka, M.; Fujiyama, H. Characteristics of nitrate uptake by plants under salinity. J. Plant Nutr. 2005, 28, 33–46. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Michailidi, E.; Tzortzakis, N. Physiological and biochemical responses of Lavandula angustifolia to salinity under mineral foliar application. Front. Plant Sci. 2018, 9, 489. [Google Scholar] [CrossRef]
- Sonneveld, C.; de Kreij, C. Response of cucumber (Cucumis sativus L.) to an unequal distribution of salts in the root environment. Plant Soil. 1999, 209, 47–56. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Giménez, J.L.; Martínez-Sánchez, F.; Moreno, A.; Fuentes, J.L.; Alcaraz, C.F. Titanium in plant nutrition. III. Effect of Ti(IV) on yield of Capsicum annuum L. In Proceedings of the III Simposium Nacional de Nutrición Mineral de las Plantas, Nutrición Mineral bajo Condiciones de Estrés; SPIC-UIB-Universitat de les Illes: Balears, Spain, 1990; pp. 123–128. [Google Scholar]
- Martínez-Sánchez, F.; Carvajal, M.; Frutos, M.J.; Giménez, J.L.; Alcaraz, C.F. Titanium in the nutrition of Capsicum annuum L. plants. Cien. Agron. 1991, 11, 73–78. [Google Scholar]
- Frutos, M.J.; Pastor, J.J.; Martínez-Sánchez, F.; Alcaraz, C.F. Improvement of the nitrogen uptake induced by titanium (iv) leaf supply in nitrogen-stressed pepper seedlings. J. Plant Nutr. 1996, 19, 771–783. [Google Scholar] [CrossRef]
- López-Moreno, J.L.; Giménez, J.L.; Moreno, A.; Fuentes, J.L.; Alcaraz, C.F. Plant biomass and fruit yield induction by Ti(IV) in P-stressed pepper crops. Fert. Res. 1996, 43, 131–136. [Google Scholar] [CrossRef]
- Carvajal, M.; Martínez-Sánchez, F.; Pastor, J.J.; Alcaraz, C.F. Leaf spray with Ti(IV) ascorbate improves the iron uptake and iron activity in Capsicum annuum L. plants. In Iron Nutrition in Soils and Plants; Abadía, J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 1–5. [Google Scholar]
- Carvajal, M.; Alcaraz, C.F. Why titanium is a beneficial element for plants. J. Plant Nutr. 1998, 21, 655–664. [Google Scholar] [CrossRef]
- Alcaraz-López, C.; Botía, M.; Alcaraz, C.F.; Riquelme, F. Effect of foliar sprays containing calcium, magnesium and titanium on peach (Prunus persica L.) fruit quality. J. Sci. Food Agric. 2004, 84, 949–954. [Google Scholar] [CrossRef]
- Alcaraz-López, C.; Botía, M.; Alcaraz, C.F.; Riquelme, F. Effects of calcium-containing foliar sprays combined with titanium and algae extract on plum fruit quality. J. Plant Nutr. 2004, 27, 713–729. [Google Scholar] [CrossRef]
- Wojcik, P.; Wojcik, M. Growth and nutrition of M.26 EMLA apple rootstock as influenced by titanium fertilization. J. Plant Nutr. 2001, 24, 1575–1588. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 105, 83–91. [Google Scholar] [CrossRef]
- Saxena, M.; Loza-Rosas, S.A.; Gaur, K.; Sharma, S.; Pérez-Otero, S.C.; Tinoco, A.D. Exploring titanium(IV) chemical proximity to iron(III) to elucidate a function for Ti(IV) in the human body. Coord. Chem. Rev. 2018, 363, 109–125. [Google Scholar] [CrossRef]
- Steiner, A. The Universal Nutrient Solution. In Proceedings of the 6th International Congress on Soilless Culture, Lunteren, The Netherlands, 29 April–5 May 1984; I.S.O.S.C.: Wageningen, The Netherlands, 1984; pp. 633–649. [Google Scholar]
- Sumanta, N.; Choudhury, I.H.; Jaishee, N.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Bailey, R.W. The reaction of pentoses with anthrone. Biochem. J. 1958, 68, 669–672. [Google Scholar] [CrossRef]
- Ibarra, E.E.; Pacheco, S.M.; García, M.R.; San Miguel, C.R.; Ramírez, V.G.; Soto, H.R.M. Actividad antioxidante de alcaloides de Erythrina americana Miller. Rev. Fitotec. Mex. 2011, 34, 241–246. [Google Scholar]
- Alcántar, G.G.; Sandoval, V.M. Handbook of Chemical Analysis of Plant Tissues, 1st ed.; Special Publication No. 10. Mexican Society of Soil Science: Chapingo, Mexico, 1999. [Google Scholar]
| Source of Variation | Seedling Height (cm) | Stem Diameter (mm) | |||||
|---|---|---|---|---|---|---|---|
| 10 DAT | 20 DAT | 30 DAT | 10 DAT | 20 DAT | 30 DAT | ||
| NaCl (mM) | |||||||
| 0 | 36.2 ± 1.4 a | 46.4 ± 5.4 a | 59.1 ± 3.6 a | 6.0 ± 0.3 a | 6.5 ± 0.2 a | 6.9 ± 0.3 a | |
| 50 | 32.5 ± 1.9 b | 37.4 ± 1.5 b | 39.7 ± 2.1 b | 5.5 ± 0.2 b | 5.7 ± 0.2 b | 5.9 ± 0.2 b | |
| 100 | 27.9 ± 1.3 c | 31.6 ± 1.2 c | 32.9 ± 1.2 c | 5.2 ± 0.2 b | 5.4 ± 0.1 b | 5.8 ± 0.2 b | |
| Ti (mg L−1) | |||||||
| 0 | 32.2 ± 2.1 a | 39.7 ± 3.8 a | 45.1 ± 6.8 a | 5.6 ± 0.2 a | 5.9 ± 0.2 a | 6.2 ± 0.3 a | |
| 500 | 33.0 ± 2.6 a | 37.6 ± 5.5 a | 44.3 ± 5.0 a | 5.6 ± 0.3 a | 5.9 ± 0.4 a | 6.2 ± 0.3 a | |
| 1000 | 31.3 ± 2.2 a | 38.1 ± 3.9 a | 44.3 ± 5.1 a | 5.5 ± 0.2 a | 5.7 ± 0.3 a | 6.1 ± 0.4 a | |
| NaCl (mM) | Ti (mg L−1) | ||||||
| 0 | 0 | 35.8 ± 1.1 a | 48.9 ± 1.1 a | 60.6 ± 5.7 a | 5.8 ± 0.2 ab | 6.2 ± 0.2 abc | 6.6 ± 0.4 abc |
| 0 | 500 | 36.9 ± 2.1 a | 41.9 ± 9.3 abc | 57.0 ± 1.9 a | 6.4 ± 0.2 a | 6.8 ± 0.3 a | 7.0 ± 0.1 a |
| 0 | 1000 | 35.8 ± 0.7 a | 48.3 ± 1.2 ab | 59.7 ± 2.7 a | 5.7 ± 0.2 ab | 6.4 ± 0.2 ab | 7.0 ± 0.3 ab |
| 50 | 0 | 31.3 ± 2.4 abc | 37.6 ± 2.3 abc | 41.2 ± 2.9 b | 5.6 ± 0.2 ab | 5.9 ± 0.2 bcd | 6.2 ± 0.3 abc |
| 50 | 500 | 34.3 ± 2.2 ab | 38.9 ± 0.8 abc | 41.6 ± 0.8 b | 5.2 ± 0.1 b | 5.5 ± 0.1 dc | 5.8 ± 0.2 c |
| 50 | 1000 | 31.9 ± 0.6 abc | 35.9 ± 0.8 bc | 36.2 ± 1.0 bc | 5.7 ± 0.3 ab | 5.7 ± 0.2 bcd | 5.7 ± 0.2 c |
| 100 | 0 | 29.5 ± 1.6 bc | 32.6 ± 1.6 c | 33.6 ± 1.1 bc | 5.5 ± 0.2 ab | 5.6 ± 0.1 bcd | 5.9 ± 0.1 bc |
| 100 | 500 | 27.9 ± 1.0 c | 32.1 ± 0.9 c | 34.4 ± 1.3 bc | 5.1 ± 0.2 b | 5.4 ± 0.2 cd | 5.7 ± 0.3 c |
| 100 | 1000 | 26.1 ± 1.0 c | 30.2 ± 0.5 c | 30.9 ± 0.5 c | 5.0 ± 0.1 b | 5.2 ± 0.1 d | 5.7 ± 0.2 c |
| Source of Variation | Leaf Area (cm2) | SPAD Units | |||
|---|---|---|---|---|---|
| 10 DAT | 20 DAT | 30 DAT | |||
| NaCl (mM) | |||||
| 0 | 518.7 ± 20 a | 49.3 ± 1.8 ab | 54.4 ± 2.0 a | 52.8 ± 2.9 a | |
| 50 | 276.2 ± 46 b | 50.4 ± 2.6 ab | 50.5 ± 3.6 ab | 45.8 ± 2.8 b | |
| 100 | 157.8 ± 25 c | 46.3 ± 2.3 b | 47.8 ± 3.2 b | 34.7 ± 5.4 c | |
| Ti (mg L−1) | |||||
| 0 | 340.6 ± 76 a | 49.3 ± 2.8 a | 49.8 ± 3.1 a | 43.1 ± 4.1 a | |
| 500 | 322.7 ± 90 a | 48.1 ± 2.0 a | 50.0 ± 2.8 a | 43.7 ± 5.1 a | |
| 1000 | 289.5 ± 88 a | 48.5 ± 2.4 a | 52.9 ± 3.7 a | 46.5 ± 6.8 a | |
| NaCl (mM) | Ti (mg L−1) | ||||
| 0 | 0 | 503.8 ± 21 ab | 47.9 ± 2.2 a | 51.3 ± 2.0 a | 49.2 ± 4.5 ab |
| 0 | 500 | 547.4 ± 19 a | 49.9 ± 1.6 a | 54.4 ± 1.8 a | 53.4 ± 1.6 a |
| 0 | 1000 | 504.9 ± 17 ab | 50.0 ± 1.6 a | 57.5 ± 0.7 a | 55.9 ± 0.5 a |
| 50 | 0 | 340.5 ± 51 bc | 52.3 ± 3.3 a | 50.5 ± 3.6 a | 41.9 ± 2.9 abc |
| 50 | 500 | 244.0 ± 48 cd | 48.2 ± 2.4 a | 49.6 ± 2.9 a | 44.9 ± 2.0 abc |
| 50 | 1000 | 244.2 ± 34 cd | 50.7 ± 2.0 a | 51.6 ± 4.7 a | 50.6 ± 1.8 ab |
| 100 | 0 | 177.4 ± 19 cd | 47.8 ± 2.7 a | 47.6 ± 3.6 a | 38.2 ± 3.0 bc |
| 100 | 500 | 176.6 ± 27 cd | 46.2 ± 1.7 a | 46.0 ± 2.3 a | 32.8 ± 4.3 c |
| 100 | 1000 | 119.2 ± 25 c | 44.7 ± 2.5 a | 49.8 ± 3.9 a | 33.1 ± 8.2 c |
| Source of Variation | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Carotenoids | |
|---|---|---|---|---|---|
| (µg g−1 FW) | |||||
| NaCl (mM) | |||||
| 0 | 36.8 ± 5 a | 15.9 ± 1 a | 52.8 ± 3.1 a | 9.0 ± 1.3 a | |
| 50 | 36.3 ± 4 a | 15.7 ± 1 a | 53.4 ± 2.3 a | 9.2 ± 0.8 a | |
| 100 | 31.9 ± 2 a | 12.1 ± 3 a | 44.1 ± 2.1 a | 7.7 ± 1.2 a | |
| Ti (mg L−1) | |||||
| 0 | 34.1 ± 3 a | 15.6 ± 0.6 a | 49.7 ± 3.2 a | 8.2 ± 0.9 a | |
| 500 | 38.4 ± 5 a | 15.6 ± 3.1 a | 54.1 ± 3.5 a | 9.2 ± 1.5 a | |
| 1000 | 33.9 ± 3 a | 12.5 ± 2.0 a | 46.4 ± 4.0 a | 8.6 ± 0.8 a | |
| NaCl (mM) | Ti (mg L−1) | ||||
| 0 | 0 | 32.4 ± 5 a | 14.8 ± 0.5 a | 47.3 ± 5.4 ab | 8.0 ± 1.7 a |
| 0 | 500 | 47.7 ± 2 a | 19.0 ± 0.3 a | 66.7 ± 2.1 a | 11.4 ± 0.4 a |
| 0 | 1000 | 30.4 ± 1 a | 13.8 ± 1.1 a | 44.3 ± 2.6 ab | 7.6 ± 0.7 a |
| 50 | 0 | 36.1 ± 2 a | 16.1 ± 0.7 a | 52.3 ± 2.1 ab | 8.4 ± 0.8 a |
| 50 | 500 | 36.4 ± 6 a | 14.9 ± 1.5 a | 51.3 ± 3.5 ab | 9.3 ± 1.0 a |
| 50 | 1000 | 40.3 ± 2 a | 16.0 ± 1.1 a | 56.4 ± 4.9 ab | 10.0 ± 0.7 a |
| 100 | 0 | 33.7 ± 1 a | 15.7 ± 0.7 a | 49.4 ± 1.9 ab | 8.1 ± 0.2 a |
| 100 | 500 | 31.1 ± 3 a | 13.0 ± 2.5 a | 44.2 ± 3.5 ab | 6.9 ± 2.2 a |
| 100 | 1000 | 30.9 ± 2 a | 7.7 ± 2.0 a | 38.6 ± 0.9 b | 8.1 ± 0.8 a |
| Source of Variation | Leaves | Stems | Roots | |
|---|---|---|---|---|
| (g 100 g−1 FW) | ||||
| NaCl (mM) | ||||
| 0 | 1.06 ± 0.10 a | 1.87 ± 0.2 b | 0.52 ± 0.06 a | |
| 50 | 0.78 ± 0.08 b | 2.43 ± 0.3 a | 0.43 ± 0.03 ab | |
| 100 | 1.01 ± 0.11 a | 1.61 ± 0.1 b | 0.36 ± 0.07 b | |
| Ti (mg L−1) | ||||
| 0 | 1.01 ± 0.07 a | 2.43 ± 0.3 a | 0.48 ± 0.06 a | |
| 500 | 0.75 ± 0.07 b | 1.83 ± 0.2 a | 0.40 ± 0.04 a | |
| 1000 | 1.08 ± 0.12 a | 1.73 ± 0.2 a | 0.44 ± 0.08 a | |
| NaCl (mM) | Ti (mg L−1) | |||
| 0 | 0 | 1.12 ± 0.07 ab | 1.90 ± 0.20 bc | 0.54 ± 0.07 a |
| 0 | 500 | 0.83 ± 0.06 bc | 1.41 ± 0.03 cd | 0.43 ± 0.04 a |
| 0 | 1000 | 1.23 ± 0.06 a | 2.29 ± 0.12 b | 0.58 ± 0.07 a |
| 50 | 0 | 0.90 ± 0.08 abc | 3.32 ± 0.03 a | 0.39 ± 0.04 a |
| 50 | 500 | 0.66 ± 0.07 c | 2.19 ± 0.21 b | 0.44 ± 0.04 a |
| 50 | 1000 | 0.77 ± 0.05 bc | 1.77 ± 0.15 bcd | 0.48 ± 0.03 a |
| 100 | 0 | 1.01 ± 0.03 abc | 1.82 ± 0.09 bcd | 0.51 ± 0.07 a |
| 100 | 500 | 0.77 ± 0.06 bc | 1.87 ± 0.10 bcd | 0.32 ± 0.03 a |
| 100 | 1000 | 1.24 ± 0.06 a | 1.14 ± 0.06 d | 0.27 ± 0.04 a |
| Source of Variation | 15 min | 30 min | 60 min | 15 min | 30 min | 60 min | |
|---|---|---|---|---|---|---|---|
| (mg g−1 FW) | (mg g−1 FW) | ||||||
| NaCl (mM) | Leaves | Stems | |||||
| 0 | 0.70 ± 0.09a | 0.73 ± 0.08 a | 0.75 ± 0.07 a | 0.11 ± 0.04 a | 0.17 ± 0.05 a | 0.26 ± 0.06 b | |
| 50 | 0.63 ± 0.12 ab | 0.66 ± 0.10 a | 0.69 ± 0.08 a | 0.28 ± 0.09 a | 0.38 ± 0.11 a | 0.48 ± 0.10 ab | |
| 100 | 0.51 ± 0.21b | 0.57 ± 0.21 a | 0.62 ± 0.20 a | 0.32 ± 0.11 a | 0.41 ± 0.10 a | 0.52 ± 0.09 a | |
| Ti (mg L−1) | |||||||
| 0 | 0.67 ± 0.11a | 0.71 ± 0.10 a | 0.73 ± 0.08 a | 0.18 ± 0.07 a | 0.27 ± 0.08 a | 0.40 ± 0.09 a | |
| 500 | 0.64 ± 0.15a | 0.69 ± 0.13 a | 0.72 ± 0.10 a | 0.21 ± 0.08 a | 0.29 ± 0.09 a | 0.40 ± 0.05 a | |
| 1000 | 0.54 ± 0.0.20 a | 0.58 ± 0.19 a | 0.61 ± 0.19 a | 0.32 ± 0.07 a | 0.41 ± 0.08 a | 0.47 ± 0.06 a | |
| NaCl (mM) | Ti (mg L−1) | ||||||
| 0 | 0 | 0.71 ± 0.02a | 0.75 ± 0.01 a | 0.77 ± 0.01 a | 0.10 ± 0.05 a | 0.15 ± 0.07 a | 0.15 ± 0.09 a |
| 0 | 500 | 0.75 ± 0.01a | 0.77 ± 0.01 a | 0.78 ± 0.01 a | 0.05 ± 0.02 a | 0.13 ± 0.01 a | 0.13 ± 0.01 a |
| 0 | 1000 | 0.64 ± 0.07a | 0.68 ± 0.07 a | 0.70 ± 0.06 a | 0.17 ± 0.03 a | 0.25 ± 0.04 a | 0.25 ± 0.05 a |
| 50 | 0 | 0.68 ± 0.04a | 0.68 ± 0.03 a | 0.74 ± 0.02 a | 0.26 ± 0.09 a | 0.38 ± 0.08 a | 0.38 ± 0.09 a |
| 50 | 500 | 0.61 ± 0.07a | 0.68 ± 0.06 a | 0.70 ± 0.05 a | 0.27 ± 0.08 a | 0.30 ± 0.07 a | 0.30 ± 0.11 a |
| 50 | 1000 | 0.60 ± 0.07a | 0.68 ± 0.06 a | 0.65 ± 0.05 a | 0.32 ± 0.08 a | 0.47 ± 0.10 a | 0.47 ± 0.09 a |
| 100 | 0 | 0.62 ± 0.10a | 0.68 ± 0.09 a | 0.69 ± 0.07 a | 0.17 ± 0.05 a | 0.29 ± 0.04 a | 0.29 ± 0.05 a |
| 100 | 500 | 0.54 ± 0.09 a | 0.68 ± 0.09 a | 0.68 ± 0.07 a | 0.31 ± 0.07 a | 0.43 ± 0.06 a | 0.43 ± 0.06 a |
| 100 | 1000 | 0.38 ± 0.10a | 0.68 ± 0.08 a | 0.48 ± 0.11 a | 0.48 ± 0.16 a | 0.51 ± 0.17 a | 0.51 ± 0.15 a |
| Source of Variation | 15 min | 30 min | 60 min | |
|---|---|---|---|---|
| (mg g−1 FW) | ||||
| NaCl (mM) | ||||
| 0 | 0.31 ± 0.02 b | 0.29 ± 0.02 b | 0.27 ± 0.02 b | |
| 50 | 0.43 ± 0.03 a | 0.41 ± 0.02 a | 0.40 ± 0.02 a | |
| 100 | 0.11 ± 0.01 c | 0.11 ± 0.01 c | 0.12 ± 0.01 c | |
| Ti( mg L−1) | ||||
| 0 | 0.27 ± 0.06 a | 0.27 ± 0.06 a | 0.26 ± 0.05 a | |
| 500 | 0.28 ± 0.06 a | 0.27 ± 0.06 a | 0.26 ± 0.05 a | |
| 1000 | 0.29 ± 0.08 a | 0.28 ± 0.08 a | 0.27 ± 0.07 a | |
| NaCl (mM) | Ti (mg L−1) | |||
| 0 | 0 | 0.27 ± 0.03 c | 0.25 ± 0.03 cd | 0.23 ± 0.03 cd |
| 0 | 500 | 0.33 ± 0.01 bc | 0.31 ± 0.01 bc | 0.29 ± 0.01 bc |
| 0 | 1000 | 0.32 ± 0.01 bc | 0.31 ± 0.01 bc | 0.30 ± 0.01 bc |
| 50 | 0 | 0.42 ± 0.04 ab | 0.40 ± 0.04 ab | 0.39 ± 0.04 ab |
| 50 | 500 | 0.40 ± 0.03 abc | 0.39 ± 0.02 ab | 0.37 ± 0.02 ab |
| 50 | 1000 | 0.47 ± 0.01 a | 0.45 ± 0.01 a | 0.44 ± 0.01 a |
| 100 | 0 | 0.13 ± 0.01 d | 0.14 ± 0.01 de | 0.15 ± 0.01 de |
| 100 | 500 | 0.11 ± 0.01 d | 0.11 ± 0.01 e | 0.12 ± 0.01 de |
| 100 | 1000 | 0.08 ± 0.01 d | 0.08 ± 0.02 e | 0.09 ± 0.02 e |
| Source of Variation | N | P | K | Ca | Mg | Ti | |
|---|---|---|---|---|---|---|---|
| (g kg−1 DW) | (mg kg−1 DW) | ||||||
| NaCl (mM) | |||||||
| 0 | 22.7 ± 1.7 a | 1.88 ± 0.10 b | 11.9 ± 0.9 a | 20.6 ± 1.5 a | 4.82 ± 0.4 a | 792 ± 77.3 b | |
| 50 | 22.5 ± 0.7 a | 1.99 ± 0.06 b | 8.6 ± 0.4 b | 19.1 ± 0.3 b | 4.80 ± 0.2 a | 895 ± 97.8 b | |
| 100 | 22.1 ± 0.6 a | 2.28 ± 0.19 a | 8.1 ± 0.8 b | 17.3 ± 0.8 c | 3.86 ± 0.2 b | 1445 ± 156.0 a | |
| Ti (mg L−1) | |||||||
| 0 | 20.6 ± 0.9 c | 1.79 ± 0.06 c | 8.4 ± 0.60 b | 18.4 ± 0.6 b | 4.14 ± 0.1 b | 19 ± 1.7 c | |
| 500 | 22.1 ± 0.3 b | 2.05 ± 0.06 b | 10.0 ± 1.30 a | 19.9 ± 1.6 a | 4.73 ± 0.3 a | 1072 ± 144 b | |
| 1000 | 24.6 ± 0.8 a | 2.30 ± 0.17 a | 10.2 ± 1.20 a | 18.6 ± 1.1 b | 4.61 ± 0.4 a | 2042 ± 336 a | |
| NaCl (mM) | Ti (mg L−1) | ||||||
| 0 | 0 | 19.0 ± 1.2 c | 1.62 ± 0.01 d | 9.5 ± 0.05 b | 17.0 ± 0.04 cd | 3.73 ± 0.05 de | 16 ± 0.7 e |
| 0 | 500 | 22.8 ± 0.4 b | 1.97 ± 0.06 bc | 13.4 ± 0.11 a | 23.8 ± 0.80 a | 5.57 ± 0.23 a | 941 ± 30 d |
| 0 | 1000 | 26.3 ± 0.2 a | 2.06 ± 0.02 bc | 13.0 ± 0.26 a | 20.9 ± 0.09 b | 5.18 ± 0.03 ab | 1419 ± 3 c |
| 50 | 0 | 21.7 ± 0.3 bc | 1.84 ± 0.02 cd | 8.9 ± 0.25 bc | 19.0 ± 0.10 bc | 4.35 ± 0.17 cd | 19 ± 1 e |
| 50 | 500 | 22.1 ± 0.2 bc | 2.01 ± 0.03 bc | 9.5 ± 0.13 b | 18.9 ± 0.34 bc | 4.70 ± 0.07 bc | 848 ± 28 d |
| 50 | 1000 | 24.4 ± 0.1 ab | 2.10 ± 0.02 bc | 7.5 ± 0.18 cd | 19.2 ± 0.53 bc | 5.34 ± 0.07 ab | 1820 ± 49 b |
| 100 | 0 | 21.7 ± 0.4 bc | 1.90 ± 0.04 bcd | 7.0 ± 0.47 d | 19.3 ± 0.67 bc | 4.33 ± 0.08 cd | 23 ± 0.7 e |
| 100 | 500 | 21.6 ± 0.1 bc | 2.18 ± 0.03 b | 7.3 ± 0.27 d | 16.8 ± 0.22 cd | 3.93 ± 0.09 de | 1426 ± 98 c |
| 100 | 1000 | 23.1 ± 0.9 ab | 2.75 ± 0.12 a | 10.1 ± 0.16 b | 15.8 ± 0.09 d | 3.30 ± 0.02 e | 2887 ± 138 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbajal-Vázquez, V.H.; Gómez-Merino, F.C.; Alcántar-González, E.G.; Sánchez-García, P.; Trejo-Téllez, L.I. Titanium Increases the Antioxidant Activity and Macronutrient Concentration in Tomato Seedlings Exposed to Salinity in Hydroponics. Plants 2022, 11, 1036. https://doi.org/10.3390/plants11081036
Carbajal-Vázquez VH, Gómez-Merino FC, Alcántar-González EG, Sánchez-García P, Trejo-Téllez LI. Titanium Increases the Antioxidant Activity and Macronutrient Concentration in Tomato Seedlings Exposed to Salinity in Hydroponics. Plants. 2022; 11(8):1036. https://doi.org/10.3390/plants11081036
Chicago/Turabian StyleCarbajal-Vázquez, Víctor Hugo, Fernando Carlos Gómez-Merino, Ernesto Gabriel Alcántar-González, Prometeo Sánchez-García, and Libia Iris Trejo-Téllez. 2022. "Titanium Increases the Antioxidant Activity and Macronutrient Concentration in Tomato Seedlings Exposed to Salinity in Hydroponics" Plants 11, no. 8: 1036. https://doi.org/10.3390/plants11081036
APA StyleCarbajal-Vázquez, V. H., Gómez-Merino, F. C., Alcántar-González, E. G., Sánchez-García, P., & Trejo-Téllez, L. I. (2022). Titanium Increases the Antioxidant Activity and Macronutrient Concentration in Tomato Seedlings Exposed to Salinity in Hydroponics. Plants, 11(8), 1036. https://doi.org/10.3390/plants11081036