Assessment of Tolerance to Lanthanum and Cerium in Helianthus Annuus Plant: Effect on Growth, Mineral Nutrition, and Secondary Metabolism
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Culture and Exposure Experiments
2.2. Dry Matter, Water Status, and Heavy Metal Tolerance Index Determination
2.3. REEs and Minerals Analysis
2.4. Determination of Phenolic Compounds and Flavonoids
2.5. Statistical Analysis
3. Results and Discussion
3.1. Lanthanum and Cerium Influence on H. Annuus
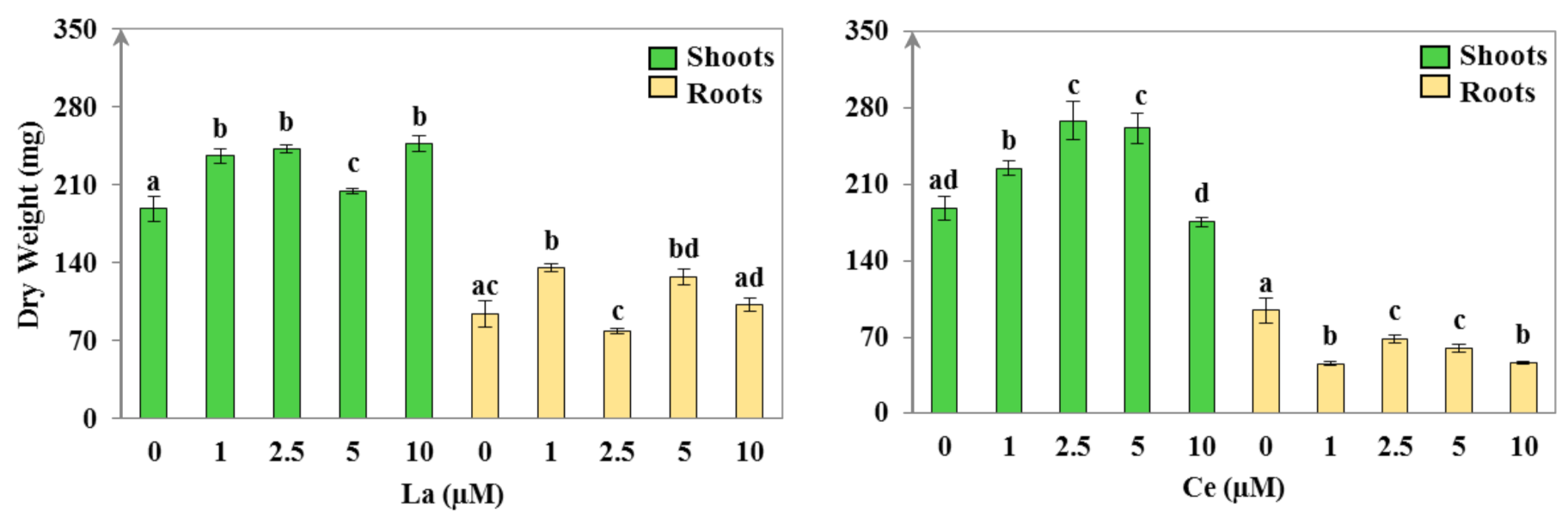
3.1.1. Plant Morphology and Growth
3.1.2. Tolerance Index
3.1.3. Water Status
3.2. REE and Mineral Uptake by H. Annuus
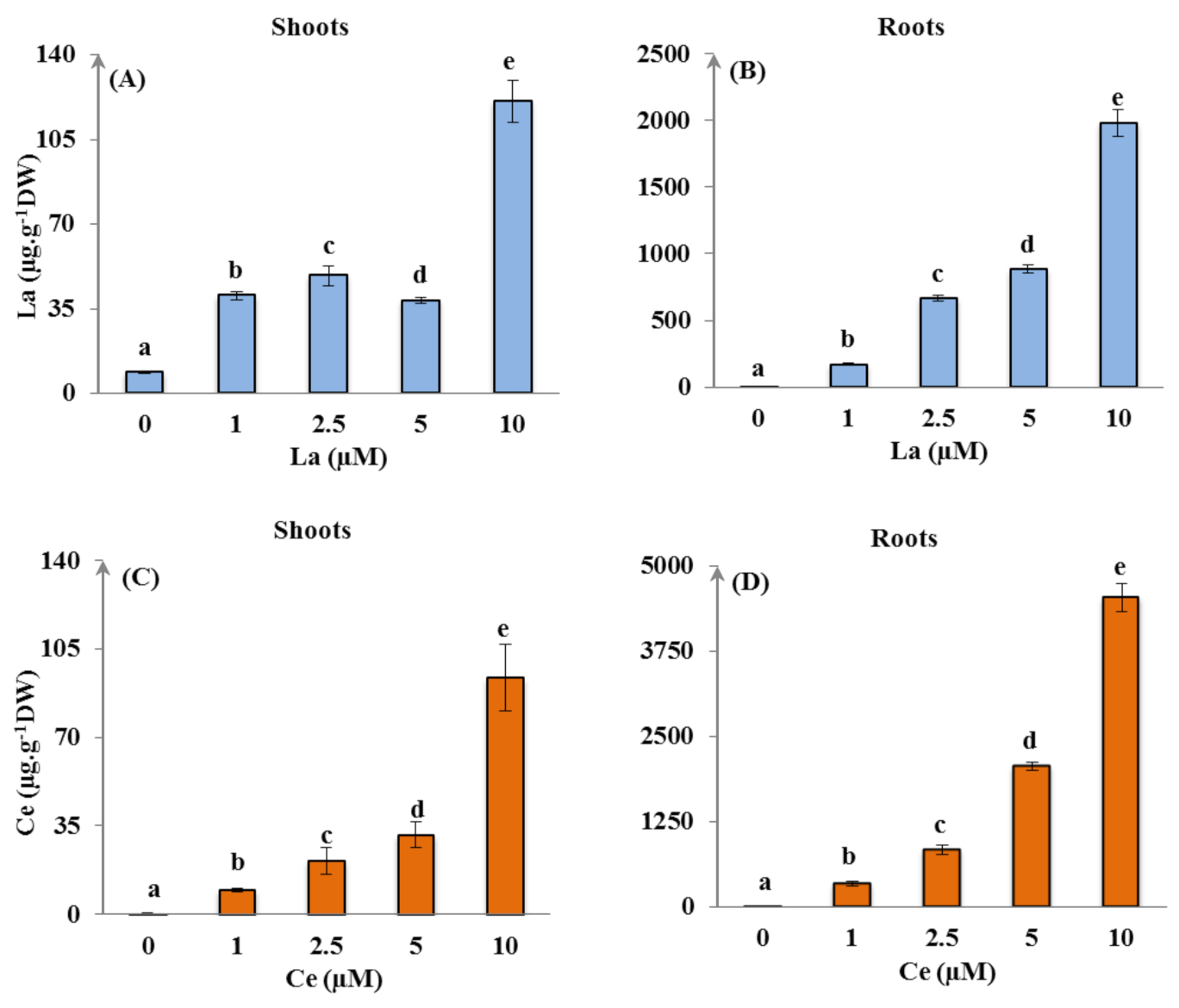
3.2.1. La and Ce Accumulation
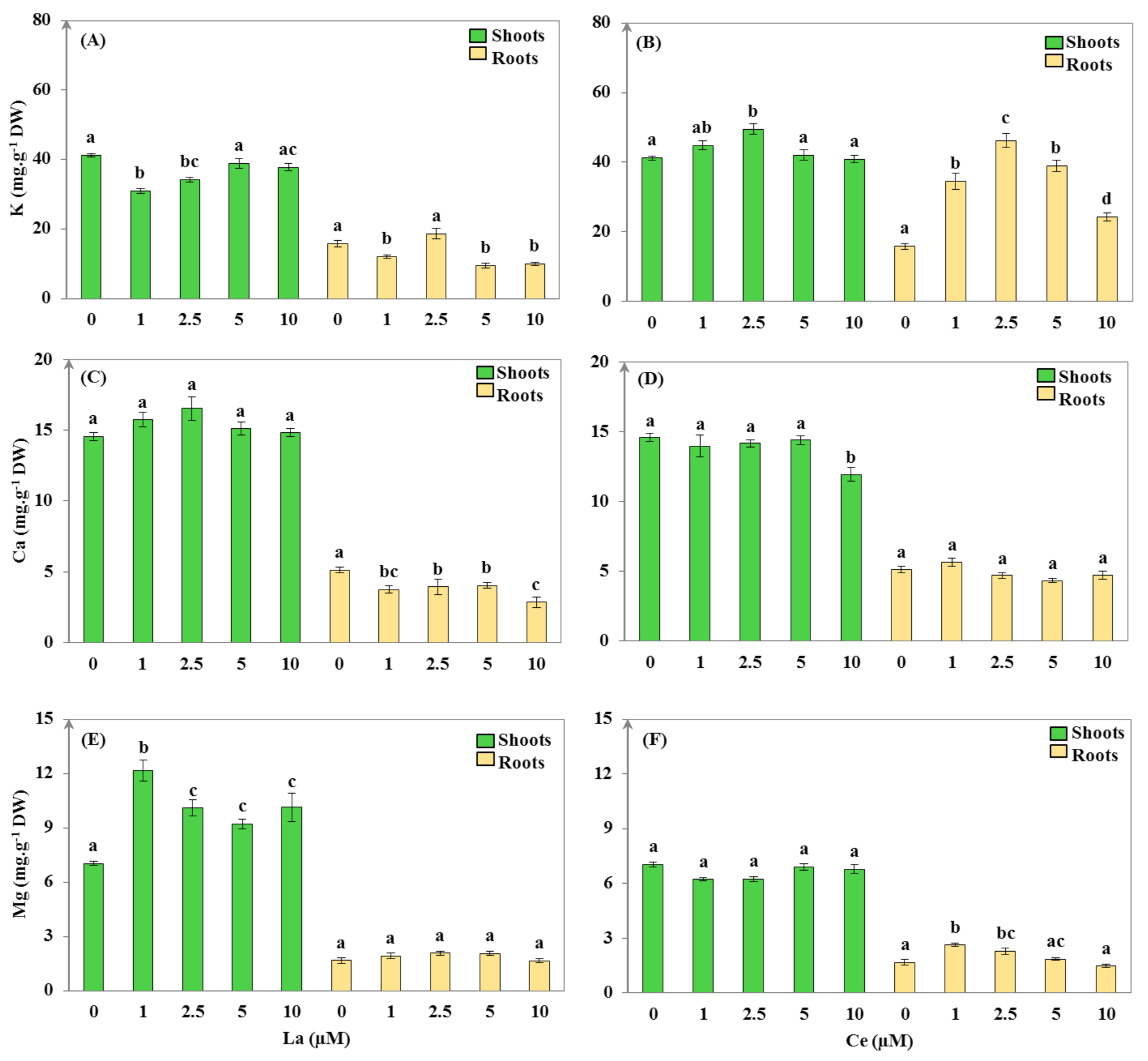
3.2.2. Mineral Uptake
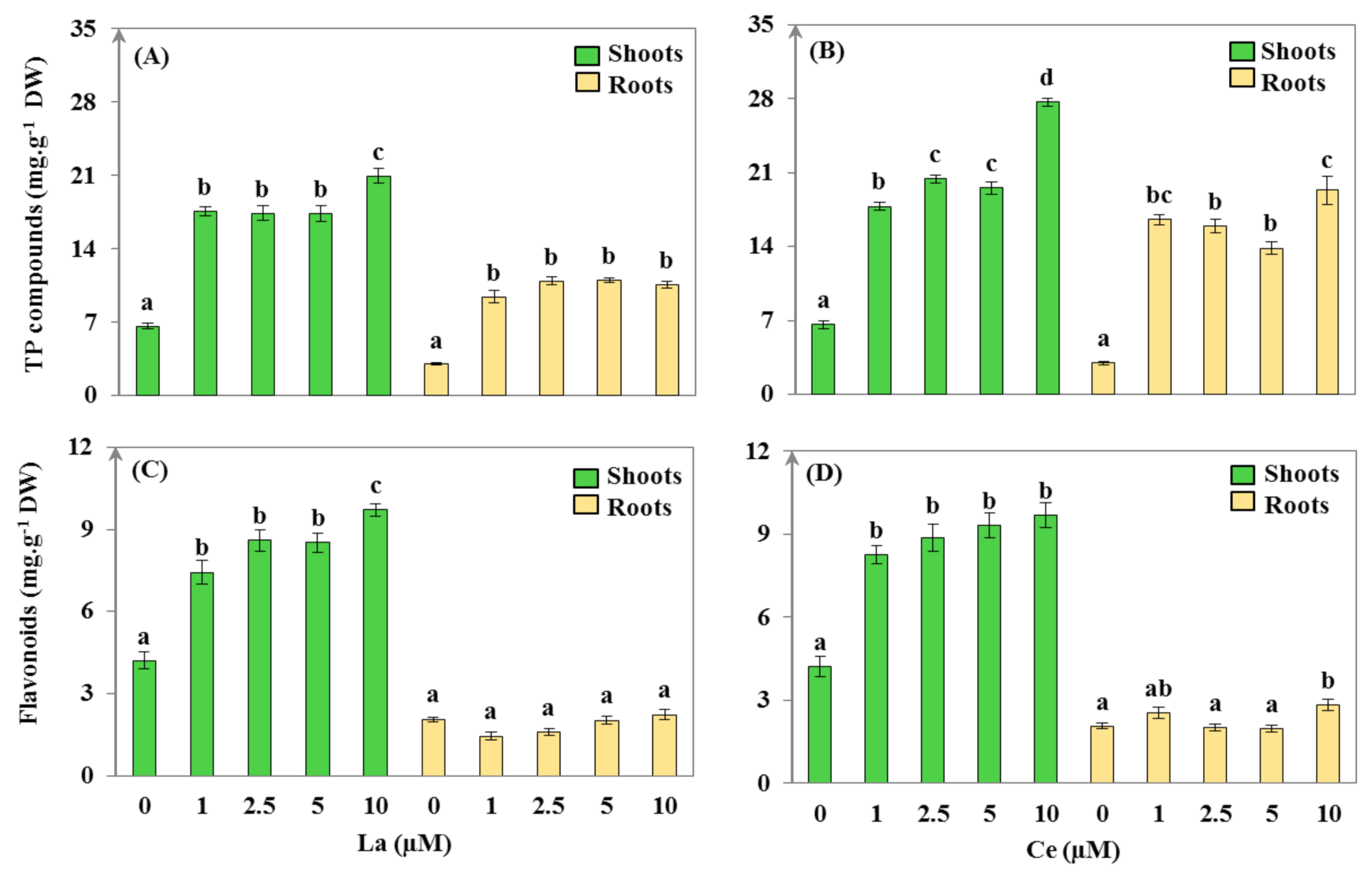
3.3. Secondary Metabolism Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tyler, G. Rare earth elements in soil and plant systems—A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, P.; Zhang, Z.; He, X.; Li, Y.; Zhang, J.; Zheng, L.; Chu, S.; Yang, K.; Zhao, Y.; et al. Origin of the different phytotoxicity and biotransformation of cerium and lanthanum oxide nanoparticles in cucumber. Nanotoxicology 2015, 9, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.S.; Ent, A.V.D.; Erskine, P.D.; Morel, J.L.; Echevarria, G.; Spiers, K.M.; Montargès-Pelletier, E.; Qiu, R.L.; Tang, Y.T. Spatially Resolved Localization of Lanthanum and Cerium in the Rare Earth Element Hyperaccumulator Fern Dicranopteris linearis from China. Environ. Sci. Technol. 2020, 54, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, X.; Zhang, X.; Gao, Z. Effects of lanthanum on growth and accumulation in roots of rice seedlings. Plant Soil Environ. 2013, 59, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.; Ramos, S.J.; Siqueira, J.O.; Faquin, V.; Castro, E.M.; Amaral, D.C.; Techio, V.H.; Coelho, L.C.; Silva, P.H.P.; Schnug, E.; et al. Bioaccumulation and effects of lanthanum on growth and mitotic index in soybean plants. Ecotox. Environ. Saf. 2015, 122, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Salgado, O.G.G.; Teodoro, J.C.; Alvarenga, J.P.; Oliveira, C.; Carvalho, T.S.; Domiciano, D.; Marchiori, P.E.R.; Guilherme, L.R.G. Cerium alleviates drought-induced stress in Phaseolus vulgaris. J. Rare Earths 2019, 38, 324–331. [Google Scholar] [CrossRef]
- Gong, X.L.; Hong, M.M.; Wang, Y.; Zhou, M.; Cai, J.W.; Liu, C. Cerium relieves the inhibition of photosynthesis of maize caused by manganese deficiency. Biol. Trace Elem. Res. 2010, 141, 305–316. [Google Scholar] [CrossRef]
- Li, W.J.; Tang, Z.H.; Lv, J.S.; Bao, J.; Liu, Z.Y.; Song, Q. Protective effect of cerium against salinity-induced oxidative stress in Jerusalem Artichoke (Helianthus tuberosus). Int. J. Agric. Biol. 2017, 19, 1201. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R.G. Rare Earth Elements in the Soil Environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Luo, Y.; Qiu, N.; Hu, F.; Sheng, L.; Wang, R.; Cao, F. Ce3+ induces flavonoids accumulation by regulation of pigments, ions, chlorophyll fluorescence and antioxidant enzymes in suspension cells of Ginkgo biloba L. Plant Cell Tissue Organ Cult. 2015, 123, 283–296. [Google Scholar] [CrossRef]
- Ramirez-Olvera, S.M.; Trejo-Tellez, L.I.; Garcia-Morales, S.; Perez-Sato, J.A.; Gomez-Merino, F.C. Cerium enhances germination and shoot growth, and alters mineral nutrient concentration in rice. PLoS ONE 2018, 13, e0194691. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Chen, Y.; Deng, X. Effects of redox potential and pH value on the release of rare earth elements from soil. Chemosphere 2001, 44, 655–661. [Google Scholar] [CrossRef]
- Milania, Z.M.; Charbgoob, F.; Darroudi, M. Impact of physicochemical properties of cerium oxide nanoparticles on their toxicity effects: Review. Ceram. Int. 2017, 43, 14572–14581. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.F.; Lutts, S.; Caia, G.; Guerrierod, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2018, 161, 98–106. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid—A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Jiang, W.; Yu, M.; Zhou, Y.; Zhao, Y.; Chai, Z. Direct measurement of lanthanum uptake and distribution in internodal cells of Chara. Plant Sci. 2008, 174, 496–501. [Google Scholar] [CrossRef]
- Kotelnikova, A.; Fastovets, I.; Rogova, O.; Volkov, D.S.; Stolbova, V. Toxicity assay of lanthanum and cerium in solutions and soil. Ecotox. Environ. Saf. 2019, 167, 20–28. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, K.; He, M.; Jiang, C.; Tian, L.; Tian, Y.; Wang, X. Mineral nutrient imbalance, DNA lesion and DNA-protein crosslink involved in growth retardation of Viciafaba L. seedlings exposed to lanthanum ions. J. Environ. Sci. 2012, 24, 214–220. [Google Scholar] [CrossRef]
- Figueiredo, C.; Raimundo, J.; Lopes, A.R.; Lopes, C.; Rosa, N.; Brito, P.; Diniz, M.; Caetano, M.; Grilo, T.F. Warming enhances lanthanum accumulation and toxicity promoting cellular damage in glass eels (Anguilla anguilla). Environ. Res. 2020, 191, 110051. [Google Scholar] [CrossRef]
- Vilela, L.A.F.; Ramos, S.J.; Carneiro, M.A.C.; Faquin, V.; Guilherme, L.R.G.; Siqueira, J.O. Cerium (Ce) and Lanthanum (La) promoted plant growth and mycorrhizal colonization of maize in tropical soil. Aust. J. Crop Sci. 2018, 12, 704–710. [Google Scholar] [CrossRef]
- Brioschi, L.; Steinmann, M.; Lucot, E.; Pierret, M.C.; Stille, P.; Prunier, J.; Badot, P.M. Transfer of rare earth elements (REE) from natural soil to plant systems: Implications for the environmental availability of anthropogenic REE. Plant Soil 2012, 366, 143–163. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rizvi, H.; Rinklebe, J.; Tsang, D.C.W.; Meers, E.; Ok, Y.S.; Ishaque, W. Phytomanagement of heavy metals in contaminated soils using sunflower: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1498–1528. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussaind, M.; Ishfaq, M.; Ahmad, M.; Anjum, M.Z. Review Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.S.; Zhao, X.; Li, J.; Kim, D.; Kalia, V.C.; Kim, I.-W.; Kim, J.W.; Lee, J.K. Metal accumulation by sunflower (Helianthus annuus L.) and the efficacy of its biomass in enzymatic saccharification. PLoS ONE 2017, 12, e0175845. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Stolt, J.P.; Sneller, F.E.C.; Brynelsson, T.; Lundborg, T.; Schat, H. Phytochelatin and cadmium accumulation in wheat. Environ. Exp. Bot. 2003, 49, 21–28. [Google Scholar] [CrossRef]
- Labidi, O.; Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M.; Sleimi, N. Assessing of growth, antioxidant enzymes, and phytohormone regulation in Cucurbita pepo under cadmium stress. Food Sci. Nutr. 2021, 9, 2021–2031. [Google Scholar] [CrossRef]
- Sleimi, N.; Kouki, R.; Hadj-Ammar, M.; Ferreira, R.; Pérez-Clemente, R. Barium effect on germination, plant growth, and antioxidant enzymes in Cucumis sativus L. plants. Food Sci. Nutr. 2021, 9, 2086–2094. [Google Scholar] [CrossRef]
- Bankaji, I.; Pérez-Clemente, R.M.; Caçador, I.; Sleimi, N. Accumulation potential of Atriplex halimus to zinc and lead combined with NaCl: Effects on physiological parameters and antioxidant enzymes activities. S. Afr. J. Bot. 2019, 123, 51–61. [Google Scholar] [CrossRef]
- Sleimi, N.; Abdelly, C. Salt-tolerance strategy of two halophyte species: Spartina alterniflora and Suaeda fruticosa. In Tasks for Vegetation Science (Cash Crop Halophytes); Lieth, H., Mochtchenko, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; Volume 38, pp. 79–85. [Google Scholar] [CrossRef]
- Sghaier, D.B.; Bankaji, I.; Pedro, S.; Caçador, I.; Sleimi, N. Photosynthetic behaviour and mineral nutrition of Tamarix gallica cultivated under Aluminium and NaCl combined stress. Phyton-Int. J. Exp. Bot. 2019, 88, 239–252. [Google Scholar] [CrossRef]
- Brito, P.; Ferreira, R.A.; Martins-Dias, S.; Azevedo, O.M.; Caetano, M.; Caçador, I. Cerium uptake, translocation and toxicity in the salt marsh halophyte Halimione portulacoides (L.) Aellen. Chemosphere 2021, 266, 128973. [Google Scholar] [CrossRef]
- Mattina, M.J.I.; Lannucci-Berger, W.; Musante, C.; White, J.C. Concurrent plant uptake of heavy metals and persistent organic pollutants from soil. Environ. Pollut. 2003, 124, 375–378. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Bouslimi, H.; Ferreira, R.; Dridi, N.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Effects of Barium stress in Brassica juncea and Cakile maritima: The indicator role of some antioxidant enzymes and secondary metabolites. Phyton 2021, 90, 145–158. [Google Scholar] [CrossRef]
- Quittier-Deleu, C.; Gressier, B. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and four. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Ma, J.J.; Ren, Y.J.; Yan, L.Y. Effects of Spray Application of Lanthanum and Cerium on Yield and Quality of Chinese Cabbage (Brassica chinensis L) Based on Different Seasons. Biol. Trace Elem. Res. 2014, 160, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Santos, C.; Pinho, S.; Oliveira, H.; Pedrosa, T.; Dias, M.C. Cadmium-induced cyto-and genotoxicity are organ-dependent in lettuce. Chem. Res. Toxicol. 2012, 25, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Wang, C.; Chen, D.; Björn, L.O.; Li, S. Copper-induced root growth inhibition of Allium cepa var. agrogarum L. involves disturbances in cell division and DNA damage. Environ. Toxicol. Chem. 2015, 34, 1045–1055. [Google Scholar] [CrossRef]
- Hu, X.; Ding, Z.; Chen, Y.; Wang, X.; Dai, L. Bioaccumulation of lanthanum and cerium and their effects on the growth of wheat (Triticum aestivum L.) seedlings. Chemosphere 2002, 48, 621–629. [Google Scholar] [CrossRef]
- Vachirapatama, N.; Jirakiattikul, Y.; Dicinoski, G.; Townsend, A.T.; Haddad, P.R. Effect of vanadium on plant growth and its accumulation in plant tissues. J. Sci. Technol. 2011, 33, 255–261. [Google Scholar]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 2017, 225, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Ma, C.; Zhan, X.; White, J.; Xing, B. Molecular mechanisms of maize seedling response to La2O3 NPs exposure: Water uptake, aquaporin gene expression and signal transduction. Environ. Sci. Nano. 2017, 4, 843–855. [Google Scholar] [CrossRef]
- Rezaee, A.; Hale, B.; Santos, R.M.; Chiang, Y.W. Accumulation and Toxicity of Lanthanum and Neodymium in Horticultural Plants (Brassica chinensis L. and Helianthus annuus L.). Can. J. Chem. Eng. 2017, 96, 2263–2272. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Hernandez-Viezcas, J.A.; Zhao, L.J.; Corral-Diaz, B.; Ge, Y.; Priester, J.H.; Holde, P.A.; Gardea-Torresdey, J.L. Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol. Biochem. 2014, 80, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, L.; Li, Y.; Sun, J.; Zhou, Q.; Huang, X. Insight into the mechanism of lanthanum (III) induced damage to plant photosynthesis. Ecotox. Environ. Saf. 2016, 127, 43–50. [Google Scholar] [CrossRef]
- Dridi, N.; Romdhane, L.; Ferreira, R.; Sleimi, N. Fertilizer effect of composted sewage sludge and cattle manure on Pelargonium growth. J. Water Sanit. Hyg. Dev. 2020, 10, 1019–1025. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Chen, Z. Effects of rare earth elements and REE-binding proteins on physiological responses in plants. Protein Pept. Lett. 2012, 1, 198–205. [Google Scholar] [CrossRef]
- Henderson, P. Rare Earth Element Geochemistry; Elsevier: New York, NY, USA, 2013. [Google Scholar]
- Paoli, E.; Fiorini, S.; Munzi, S.; Sorbo, A.; Basile, S.; Loppi, S. Uptake and acute toxicity of cerium in the lichen Xanthoria parietina L. Ecotox. Environ. Saf. 2014, 104, 379–385. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Lu, Q.; Cai, S.; Chu, W.; Qiu, H.; Xu, T.; Li, F.; Xu, Q. Oxidative effects, nutrients and metabolic changes in aquatic macrophyte, Elodea nuttallii following exposure to lanthanum. Ecotox. Environ. Saf. 2015, 115, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Shtangeeva, I. Europium and cerium accumulation in wheat and rye seedlings. Water Air Soil Pollut. 2014, 225, 1964. [Google Scholar] [CrossRef]
- Shyam, R.; Aery, N.C. Effect of cerium on growth, dry matter production, biochemical constituents and enzymatic activities of cowpea plants [Vignaun guiculata (L.) Walp.]. J. Soil Sci. Plant Nutr. 2012, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.J.; Peralta-Videa, J.R.; Peng, B.; Bandyopadhyay, S.; Corral-Diaz, B.; Osuna-Avila, P.; Montes, M.O.; Keller, A.A.; Gardea-Torresdey, J.L. Alginate modifies the physiological impact of CeO2 nanoparticles in corn seedlings cultivated in soil. J. Environ. Sci. 2014, 26, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Hermans, C.; Chen, J.; Coppens, F.; Inzé, D.; Verbruggen, N. Low magnesium status in plants enhances tolerance to cadmium exposure. New Phytol. 2011, 192, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Janicka-Russak, M.; Kabala, K.; Burzynski, M. Different effect of cadmium and copper on H+-ATPase activity in plasma membrane vesicles from Cucumis sativus roots. J. Exp. Bot. 2012, 63, 4133–4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babula, P.; Klejdus, B.; Kovacik, J.; Hedbavny, J.; Hlavna, M. Lanthanum rather than cadmium induces oxidative stress and metabolite changes in Hypericum perforatum. J. Hazard. Mater. 2015, 286, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress: A review. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Atmani, D.; Chaher, N.; Berboucha, M.; Ayouni, K.; Lounis, H.; Boudaoud, H.; Debbache, N.; Atmani, D. Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chem. 2009, 112, 303–309. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: An updated review of mechanisms and catalysing metals. Phytother. Res. PTR 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
| La (μM) | WC (mL·g−1 DW) | S/R | EP DW (mg) | TI (%) | |||
|---|---|---|---|---|---|---|---|
| Shoots | Roots | Shoots | Roots | EP | |||
| 0 | 0.927 ± 0.002 0.925 ± 0.001 0.920 ± 0.001 0.924 ± 0.002 0.922 ± 0.002 | 0.943 ± 0.002 | 2.1 ± 0.2 | 283.2 ± 21.5 | |||
| 1 | 0.939 ± 0.001 | 1.7 ± 0.0 | 371.4 * ± 8.8 | 180.6 ± 11.2 | 164.4 ± 5.7 | 172.2 ± 7.2 | |
| 2.5 | 0.959 ** ± 0.000 | 3.1 ± 0.1 | 321.4 * ± 3.4 | 191.1 ± 6.1 | 75.8 ± 3.6 | 131.3 ± 2.8 | |
| 5 | 0.939 ± 0.001 | 1.6 ± 0.1 | 332.2 * ± 7.4 | 127.4 ± 4.3 | 151.9 ± 11.1 | 140.1 ± 6.1 | |
| 10 | 0.949 * ± 0.001 | 2.4 ± 0.1 | 349.4 * ± 11.9 | 199.1 ± 11.6 | 112.5 ± 9.3 | 154.2 ± 9.7 | |
| Ce (μM) | WC (mL·g−1DW) | S/R | EP DW (mg) | TI (%) | |||
| Shoots | Roots | Shoots | Roots | EP | |||
| 0 | 0.927 ± 0.002 0.935 ± 0.003 0.937 ± 0.003 0.938 ± 0.001 0.907 ** ± 0.005 | 0.943 ± 0.002 | 2.1 ± 0.2 | 283.2 ± 21.5 | |||
| 1 | 0.964 ** ± 0.002 | 4.9 ± 0.2 | 271.0 ± 7.5 | 162.0 ± 10.9 | 23.3 ± 2.9 | 90.0 ± 6.2 | |
| 2.5 | 0.974 ** ± 0.002 | 4.0 ± 0.1 | 337.0 * ± 17.0 | 235.9 ± 29.3 | 58.9 ± 5.8 | 144.0 ± 13.9 | |
| 5 | 0.973 ** ± 0.001 | 4.5 ± 0.2 | 321.3 * ± 14.1 | 242.9 ± 14.2 | 45.3 ± 6.2 | 131.2 ± 11.5 | |
| 10 | 0.969 ** ± 0.002 | 3.8 ± 0.1 | 222.1* ± 4.2 | 77.7 ± 8.0 | 24.3 ± 1.7 | 50.0 ± 3.4 | |
| REE Concentration (µM) | TF | |
|---|---|---|
| La | Ce | |
| 1 | 0.238 ± 0.019 | 0.028 ± 0.003 |
| 2.5 | 0.073 ± 0.009 | 0.026 ± 0.009 |
| 5 | 0.044 ± 0.002 | 0.015 ± 0.003 |
| 10 | 0.061 ± 0.009 | 0.021 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dridi, N.; Ferreira, R.; Bouslimi, H.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Assessment of Tolerance to Lanthanum and Cerium in Helianthus Annuus Plant: Effect on Growth, Mineral Nutrition, and Secondary Metabolism. Plants 2022, 11, 988. https://doi.org/10.3390/plants11070988
Dridi N, Ferreira R, Bouslimi H, Brito P, Martins-Dias S, Caçador I, Sleimi N. Assessment of Tolerance to Lanthanum and Cerium in Helianthus Annuus Plant: Effect on Growth, Mineral Nutrition, and Secondary Metabolism. Plants. 2022; 11(7):988. https://doi.org/10.3390/plants11070988
Chicago/Turabian StyleDridi, Nesrine, Renata Ferreira, Houda Bouslimi, Pedro Brito, Susete Martins-Dias, Isabel Caçador, and Noomene Sleimi. 2022. "Assessment of Tolerance to Lanthanum and Cerium in Helianthus Annuus Plant: Effect on Growth, Mineral Nutrition, and Secondary Metabolism" Plants 11, no. 7: 988. https://doi.org/10.3390/plants11070988
APA StyleDridi, N., Ferreira, R., Bouslimi, H., Brito, P., Martins-Dias, S., Caçador, I., & Sleimi, N. (2022). Assessment of Tolerance to Lanthanum and Cerium in Helianthus Annuus Plant: Effect on Growth, Mineral Nutrition, and Secondary Metabolism. Plants, 11(7), 988. https://doi.org/10.3390/plants11070988








