Cell Wall Components and Extensibility Regulate Root Growth in Suaeda salsa and Spinacia oleracea under Salinity
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Materials
2.2. Seedling Growth and Salt Treatment
2.3. Mechanical Parameters of the Root Cell Wall
2.4. Isolation of Root Cell Wall Polysaccharides
2.5. Statistical Analysis
3. Results
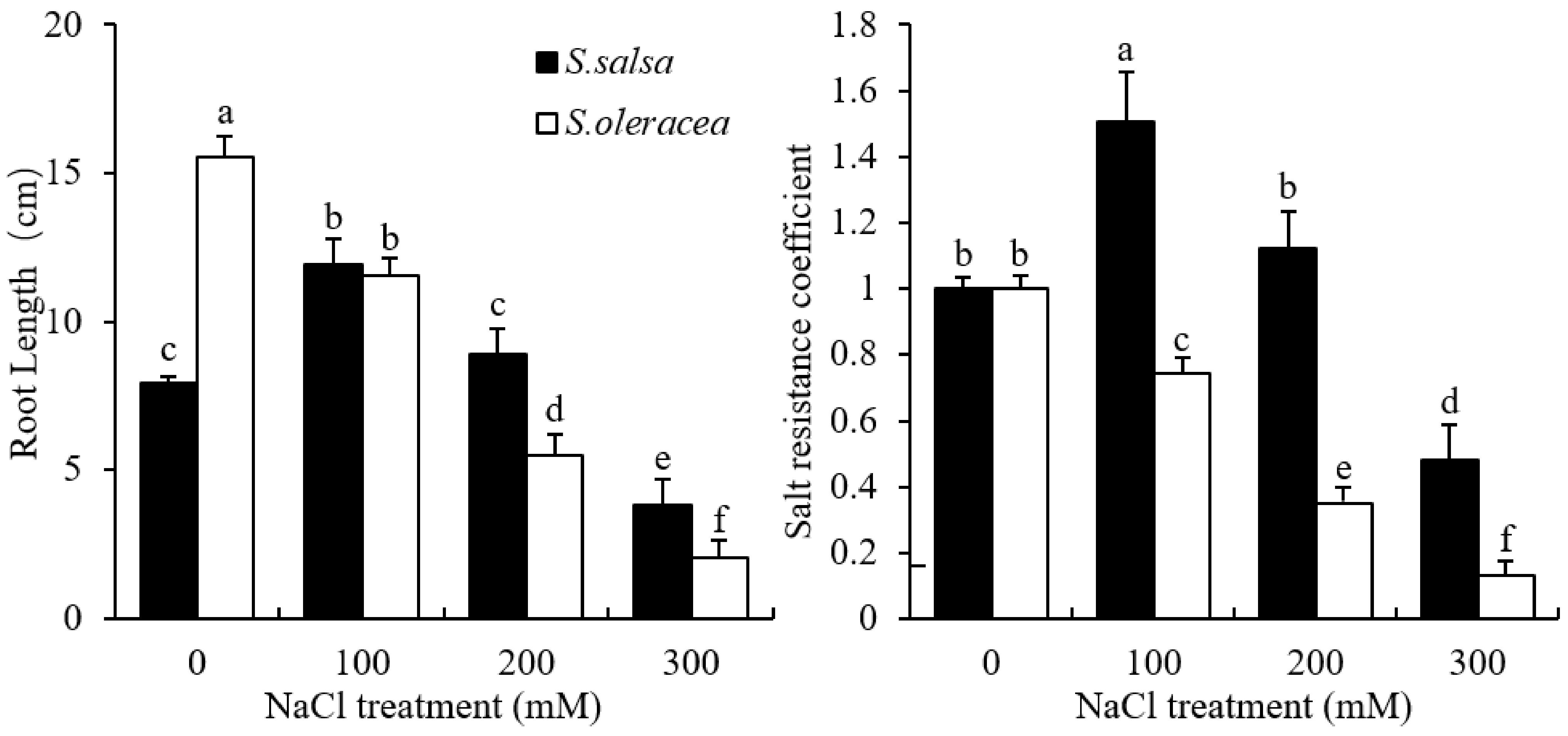
3.1. Root Growth
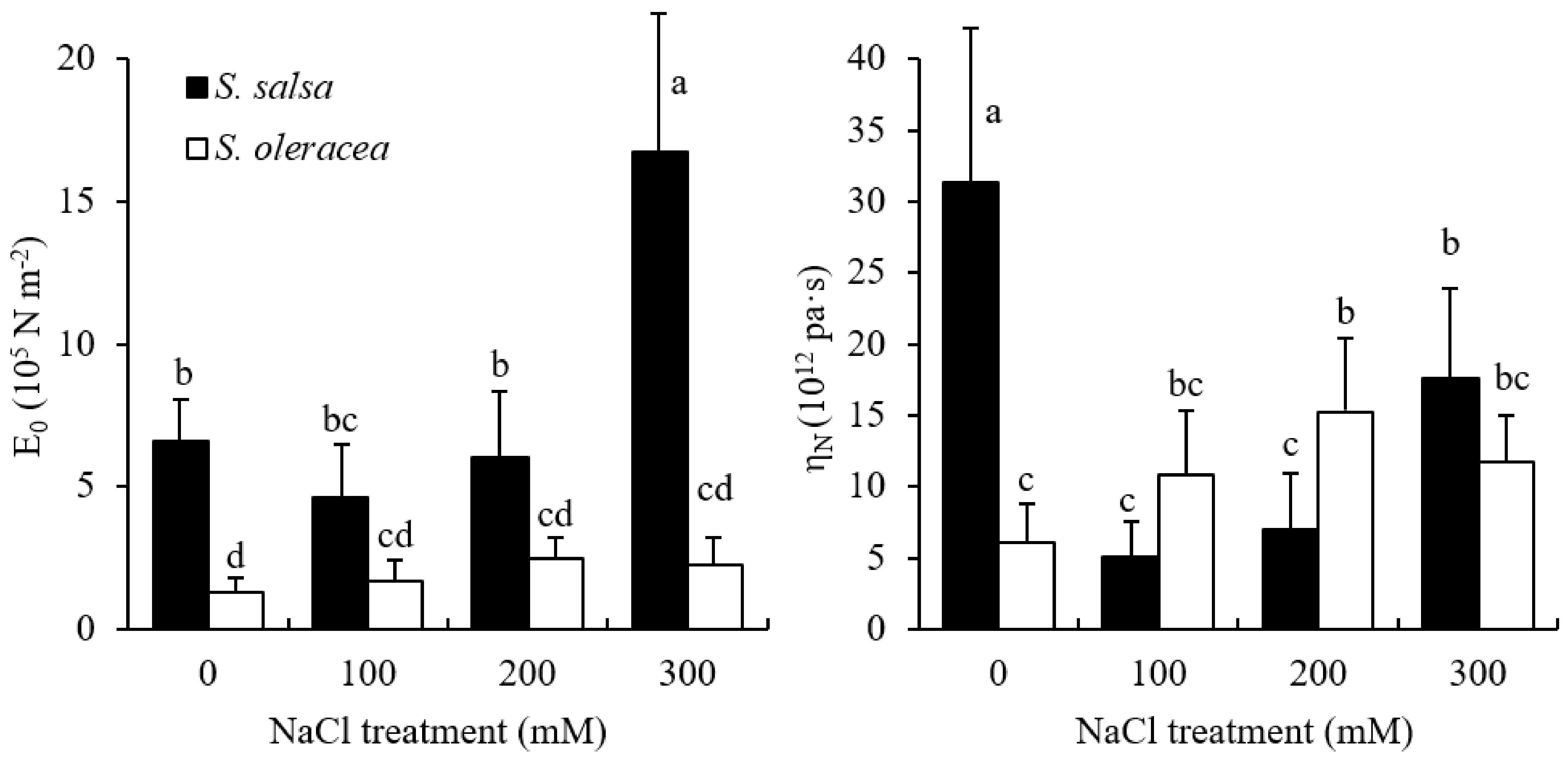
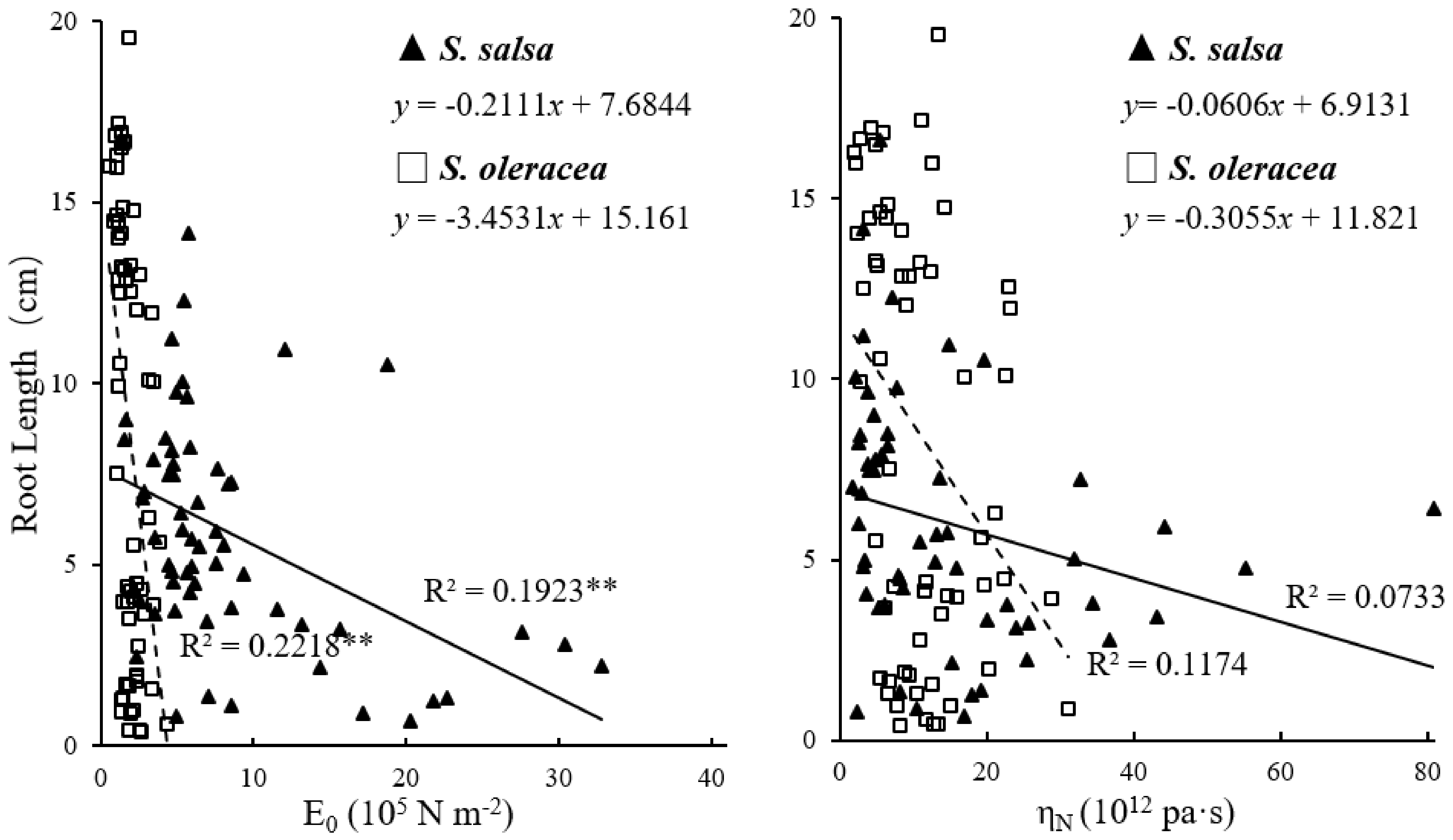
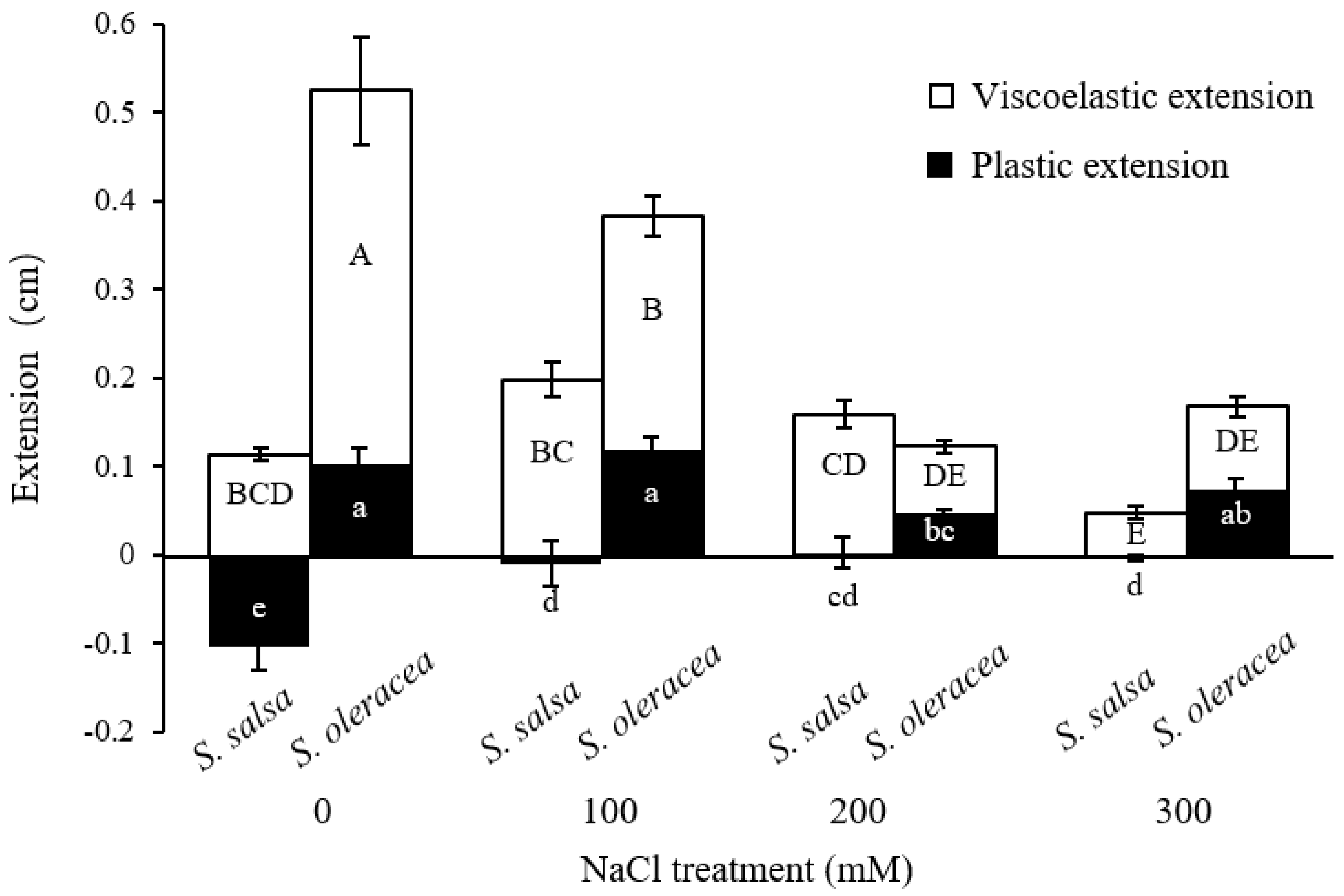
3.2. Mechanical Properties of Root Cell Wall
3.3. Chemical Composition of the Root Cell Wall
4. Discussion
Chemical Composition of the Root Cell Wall
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozema, J.; Schat, H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ. Exp. Bot. 2013, 92, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.-F.; LI, F.-Z.; Fan, S.-J.; Feng, L.-T. Halophytes in china. Chin. Bull. Bot. 1999, 16, 201. [Google Scholar]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosgrove, D.J. Plant cell wall extensibility: Connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Diffuse Growth of Plant Cell Walls. Plant Physiol. 2018, 176, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.; Doan, V.; Duan, Q.; Liu, M.-C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA Receptor Kinase Maintains Cell-Wall Integrity during Salt Stress through Ca2+ Signaling. Curr. Biol. 2018, 28, 666–675.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hocq, L.; Pelloux, J.; Lefebvre, V. Connecting Homogalacturonan-Type Pectin Remodeling to Acid Growth. Trends Plant Sci. 2017, 22, 20–29. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.A.; Ishii, T.; Albersheim, P.; Darvill, A.G. Rhamnogalacturonan II: Structure and Function of a Borate Cross-Linked Cell Wall Pectic Polysaccharide. Annu. Rev. Plant Biol. 2004, 55, 109–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proseus, T.E.; Boyer, J.S. Calcium deprivation disrupts enlargement of Chara corallina cells: Further evidence for the calcium pectate cycle. J. Exp. Bot. 2012, 63, 3953–3958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, H.; Lauchli, A. Changes of Cell Wall Composition and Polymer Size in Primary Roots of Cotton Seedlings Under High Salinity. J. Exp. Bot. 1993, 44, 773–778. [Google Scholar] [CrossRef]
- An, P.; Li, X.; Zheng, Y.; Matsuura, A.; Abe, J.; Eneji, A.E.; Tanimoto, E.; Inanaga, S. Effects of NaCl on Root Growth and Cell Wall Composition of Two Soya bean Cultivars with Contrasting Salt Tolerance. J. Agron. Crop Sci. 2014, 200, 212–218. [Google Scholar] [CrossRef]
- Corrêa-Ferreira, M.L.; Viudes, E.B.; de Magalhães, P.M.; de Santana-Filho, A.P.; Sassaki, G.; Pacheco, A.C.; Petkowicz, C.L.D.O. Changes in the composition and structure of cell wall polysaccharides from Artemisia annua in response to salt stress. Carbohydr. Res. 2019, 483, 107753. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.S.; Grativol, C.; Mourão, P.A.S. Rising from the Sea: Correlations between Sulfated Polysaccharides and Salinity in Plants. PLoS ONE 2011, 6, e18862. [Google Scholar] [CrossRef] [Green Version]
- Ricardi, M.M.; González, R.M.; Zhong, S.; Domínguez, P.G.; Duffy, T.; Turjanski, P.G.; Salgado Salter, J.D.; Alleva, K.; Carrari, F.; Giovannoni, J.J. Genome-wide data (ChIP-seq) enabled identification of cell wall-related and aquaporin genes as targets of tomato ASR1, a drought stress-responsive transcription factor. BMC Plant Biol. 2014, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Flowers, T.; Hajibagheri, M.A. Salinity tolerance in Hordeum vulgare: Ion concentrations in root cells of cultivars differing in salt tolerance**. Plant Soil 2001, 231, 1–9. [Google Scholar] [CrossRef]
- Neumann, P.M.; Azaizeh, H.; Leon, D. Hardening of root cell walls: A growth inhibitory response to salinity stress. Plant Cell Environ. 1994, 17, 303–309. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Wu, Y.; Cosgrove, D.J. Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J. Exp. Bot. 2000, 51, 1543–1553. [Google Scholar] [CrossRef] [Green Version]
- Nonami, H.; Tanimoto, K.; Tabuchi, A.; Fukuyama, T.; Hashimoto, Y. Salt stress under hydroponic conditions causes changes in cell wall extension during growth. Hydroponics Transpl. Prod. 1994, 396, 91–98. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Linker, R.; Gepstein, S.; Tanimoto, E.; Yamamoto, R.; Neumann, P.M. Progressive Inhibition by Water Deficit of Cell Wall Extensibility and Growth along the Elongation Zone of Maize Roots Is Related to Increased Lignin Metabolism and Progressive Stelar Accumulation of Wall Phenolics. Plant Physiol. 2006, 140, 603–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, T.; Inanaga, S.; Tanimoto, E.; Lux, A.; Luxová, M.; Sugimoto, Y. Silicon-Induced Changes in Viscoelastic Properties of Sorghum Root Cell Walls. Plant Cell Physiol. 2003, 44, 743–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Li, Y.; Jin, Q.; Li, F. Silica nanoparticles inhibit arsenic uptake into rice suspension cells via improving pectin synthesis and the mechanical force of the cell wall. Environ. Sci. Nano 2020, 7, 162–171. [Google Scholar] [CrossRef]
- Ma, J.F.; Shen, R.; Nagao, S.; Tanimoto, E. Aluminum Targets Elongating Cells by Reducing Cell Wall Extensibility in Wheat Roots. Plant Cell Physiol. 2004, 45, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Tabuchi, A.; Matsumoto, H. Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiol. Plant. 2001, 112, 353–358. [Google Scholar] [CrossRef]
- Połeć-Pawlak, K.; Ruzik, R.; Lipiec, E.; Ciurzyńska, M.; Gawrońska, H. Investigation of Pb(ii) binding to pectin in Arabidopsis thaliana. J. Anal. At. Spectrom. 2007, 22, 968–972. [Google Scholar] [CrossRef]
- Hossain, M.T.; Soga, K.; Wakabayashi, K.; Hoson, T. Effects of lead toxicity on growth and cell wall extensibility in rice seedlings. Bangladesh J. Bot. 2015, 44, 333–336. [Google Scholar] [CrossRef]
- Muchate, N.S.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. NaCl induced salt adaptive changes and enhanced accumulation of 20-hydroxyecdysone in the in vitro shoot cultures of Spinacia oleracea (L.). Sci. Rep. 2019, 9, 12522. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Chen, M.; Feng, G.; Jia, Y.; Wang, B.; Zhang, F. Effect of salinity on growth, ion accumulation and the roles of ions in osmotic adjustment of two populations of Suaeda salsa. Plant Soil 2009, 314, 133–141. [Google Scholar] [CrossRef]
- Tanimoto, E.; Fujii, S.; Yamamoto, R.; Inanaga, S. Measurement of viscoelastic properties of root cell walls affected by low pH in lateral roots of Pisum sativum L. Plant Soil 2000, 226, 21–28. [Google Scholar] [CrossRef]
- Nonami, H.; Boyer, J.S. Wall extensibility and cell hydraulic conductivity decrease in enlarging stem tissues at low water po-tentials. Plant Physiol. 1990, 93, 1610–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanimoto, E.; Huber, D.J. Effect of GA3 on the Molecular Mass of Polyuronides in the Cell Walls of Alaska Pea Roots. Plant Cell Physiol. 1997, 38, 25–35. [Google Scholar] [CrossRef]
- Ahmed, A.E.R.; Labavitch, J.M. A Simplified method for accurate determination of cell wall uronide content. J. Food Biochem. 1978, 1, 361–365. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F.G. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Mühling, K.H.; Kutschera, U.; Geilfus, C.-M. Salinity stiffens the epidermal cell walls of salt-stressed maize leaves: Is the epidermis growth-restricting? PLoS ONE 2015, 10, e0118406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peura, M.; Grotkopp, I.; Lemke, H.; Vikkula, A.; Laine, J.; Müller, M.; Serimaa, R. Negative Poisson Ratio of Crystalline Cellulose in Kraft Cooked Norway Spruce. Biomacromolecules 2006, 7, 1521–1528. [Google Scholar] [CrossRef]
- Park, Y.B.; Cosgrove, D.J. A revised architecture of primary cell walls based on biomechanical changes induced by sub-strate-specific endoglucanases. Plant Physiol. 2012, 158, 1933–1943. [Google Scholar] [CrossRef] [Green Version]
- Voragen, F.; Schols, H.; Visser, R.G. Advances in Pectin and Pectinase Research; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 978-90-481-6229-1. [Google Scholar]
- Peaucelle, A.; Braybrook, S.; Le Guillou, L.; Bron, E.; Kuhlemeier, C.; Höfte, H. Pectin-Induced Changes in Cell Wall Mechanics Underlie Organ Initiation in Arabidopsis. Curr. Biol. 2011, 21, 1720–1726. [Google Scholar] [CrossRef] [Green Version]
- Peaucelle, A.; Braybrook, S.; Höfte, H. Cell wall mechanics and growth control in plants: The role of pectins revisited. Front. Plant Sci. 2012, 3, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szatanik-Kloc, A.; Szerement, J.; Józefaciuk, G. The role of cell walls and pectins in cation exchange and surface area of plant roots. J. Plant Physiol. 2017, 215, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Reboul, R.; Geserick, C.; Pabst, M.; Frey, B.; Wittmann, D.; Lütz-Meindl, U.; Léonard, R.; Tenhaken, R. Down-regulation of UDP-glucuronic Acid Biosynthesis Leads to Swollen Plant Cell Walls and Severe Developmental Defects Associated with Changes in Pectic Polysaccharides. J. Biol. Chem. 2011, 286, 39982–39992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrt, C.; Munns, R.; Burton, R.; Gilliham, M.; Wege, S. Root cell wall solutions for crop plants in saline soils. Plant Sci. 2018, 269, 47–55. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Kesten, C.; Menna, A.; Sánchez-Rodríguez, C. Regulation of cellulose synthesis in response to stress. Curr. Opin. Plant Biol. 2017, 40, 106–113. [Google Scholar] [CrossRef]
- Mustard, J.; Renault, S. Effects of NaCl on water relations and cell wall elasticity and composition of red-osier dogwood (Cornus stolonifera) seedlings. Physiol. Plant. 2004, 121, 265–271. [Google Scholar] [CrossRef]
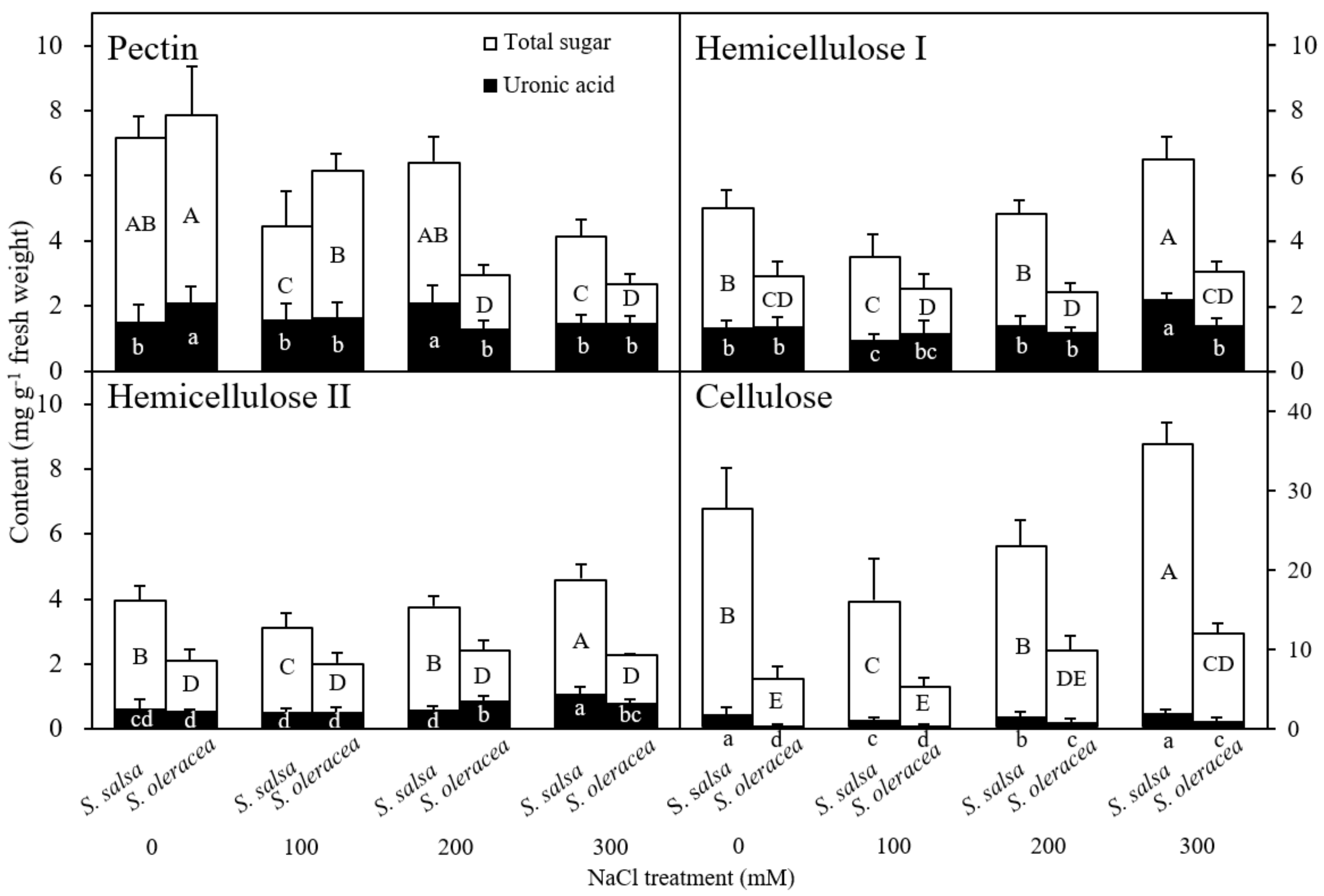
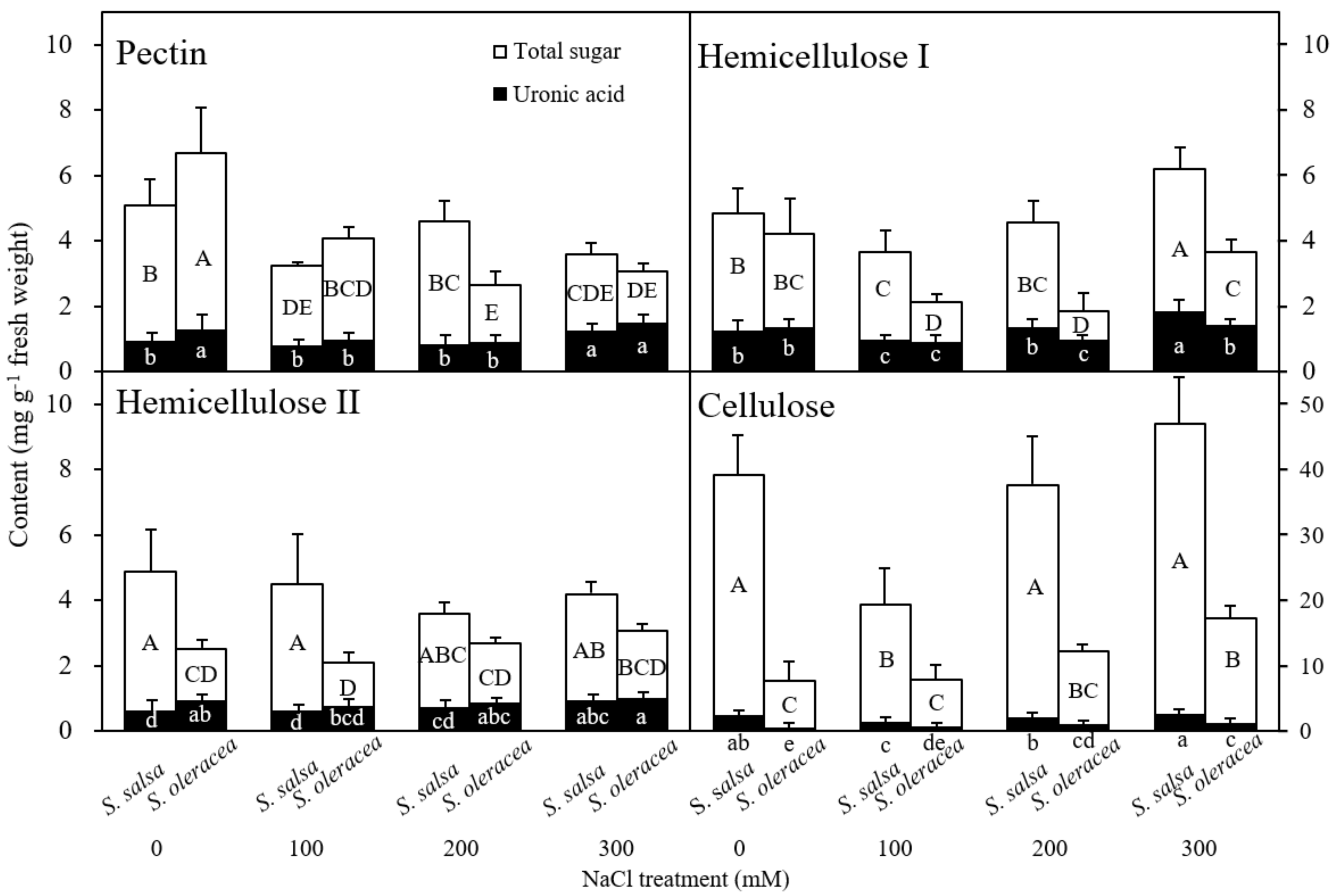
| Species | Root Region | Sugar Content (mg g−1 FW) | |||
|---|---|---|---|---|---|
| NaCl Treatment (mM) | |||||
| 0 | 100 | 200 | 300 | ||
| S. salsa | 0–5 mm | 43.5 ± 6.4 ab | 26.8 ± 7.3 c | 37.7 ± 3.7 bc | 50.7 ± 3.0 a |
| S. oleracea | 18.8 ± 2.3 a | 15.7 ± 1.1 b | 17.4 ± 1.7 a | 19.7 ± 1.1 a | |
| S. salsa | 5–10 mm | 53.9 ± 7.5 ab | 30.6 ± 7.9 b | 52.9 ± 13.7 ab | 61.0 ± 10.3 a |
| S. oleracea | 21.1 ± 4.5 ab | 16.1 ± 2.4 b | 19.3 ± 1.4 b | 27.0 ± 2.0 a | |
| RL | Pectin | HC-I | HC-II | Cellulose | Pectin (UA) | HC-I (UA) | HC-II (UA) | |
|---|---|---|---|---|---|---|---|---|
| Pectin | 0.121 | |||||||
| HC-I | −0.868 ** | −0.008 | ||||||
| HC-II | −0.814 ** | 0.005 | 0.915 ** | |||||
| Cellulose | −0.882 ** | 0.04 | 0.821 ** | 0.827 ** | ||||
| Pectin (UA) | 0.148 | 0.287 | −0.081 | −0.005 | −0.003 | |||
| HC I (UA) | −0.929 ** | −0.268 | 0.900 ** | 0.825 ** | 0.849 ** | −0.136 | ||
| HC II (UA) | −0.761 ** | −0.329 | 0.766 ** | 0.739 ** | 0.811 ** | 0.048 | 0.821 ** | |
| Cellulose (UA) | −0.724 ** | 0.268 | 0.786 ** | 0.854 ** | 0.875 ** | −0.016 | 0.657 ** | 0.647 ** |
| RL | Pectin | HC-I | HC-II | Cellulose | Pectin (UA) | HC-I (UA) | HC-II (UA) | |
|---|---|---|---|---|---|---|---|---|
| Pectin | 0.939 ** | |||||||
| HC-I | −0.055 | 0.113 | ||||||
| HC-II | −0.417 | −0.497 | 0.276 | |||||
| Cellulose | −0.826 ** | −0.784 ** | 0.118 | 0.513 * | ||||
| Pectin (UA) | 0.718 ** | 0.851 ** | 0.459 | −0.249 | −0.546 * | |||
| HC I (UA) | −0.033 | −0.068 | 0.666 ** | 0.535 * | 0.274 | 0.214 | ||
| HC II (UA) | −0.787 ** | −0.845 ** | 0.117 | 0.811 ** | 0.790 ** | −0.573 * | 0.339 | |
| Cellulose (UA) | −0.884 ** | −0.862 ** | −0.006 | 0.576 * | 0.958 ** | −0.650 ** | 0.196 | 0.857 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Shao, Y.; Feng, X.; Otie, V.; Matsuura, A.; Irshad, M.; Zheng, Y.; An, P. Cell Wall Components and Extensibility Regulate Root Growth in Suaeda salsa and Spinacia oleracea under Salinity. Plants 2022, 11, 900. https://doi.org/10.3390/plants11070900
Liu J, Shao Y, Feng X, Otie V, Matsuura A, Irshad M, Zheng Y, An P. Cell Wall Components and Extensibility Regulate Root Growth in Suaeda salsa and Spinacia oleracea under Salinity. Plants. 2022; 11(7):900. https://doi.org/10.3390/plants11070900
Chicago/Turabian StyleLiu, Jia, Yang Shao, Xiaohui Feng, Victoria Otie, Asana Matsuura, Muhammad Irshad, Yuanrun Zheng, and Ping An. 2022. "Cell Wall Components and Extensibility Regulate Root Growth in Suaeda salsa and Spinacia oleracea under Salinity" Plants 11, no. 7: 900. https://doi.org/10.3390/plants11070900
APA StyleLiu, J., Shao, Y., Feng, X., Otie, V., Matsuura, A., Irshad, M., Zheng, Y., & An, P. (2022). Cell Wall Components and Extensibility Regulate Root Growth in Suaeda salsa and Spinacia oleracea under Salinity. Plants, 11(7), 900. https://doi.org/10.3390/plants11070900








