Evolution of the Anther Gland in Early-Branching Papilionoids (ADA Clade, Papilionoideae, Leguminosae)
Abstract
1. Introduction
2. Results
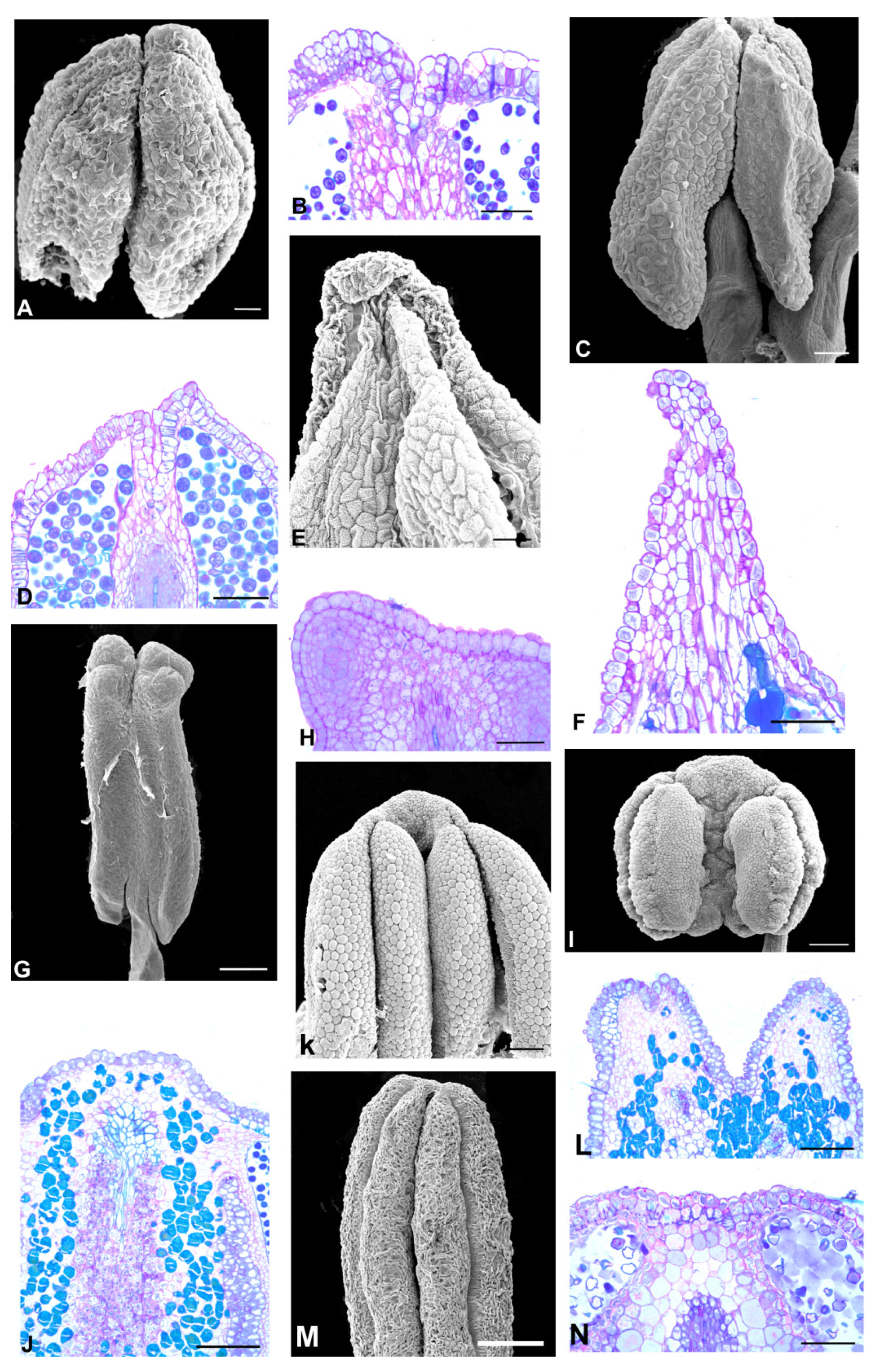
2.1. Flower Morphology
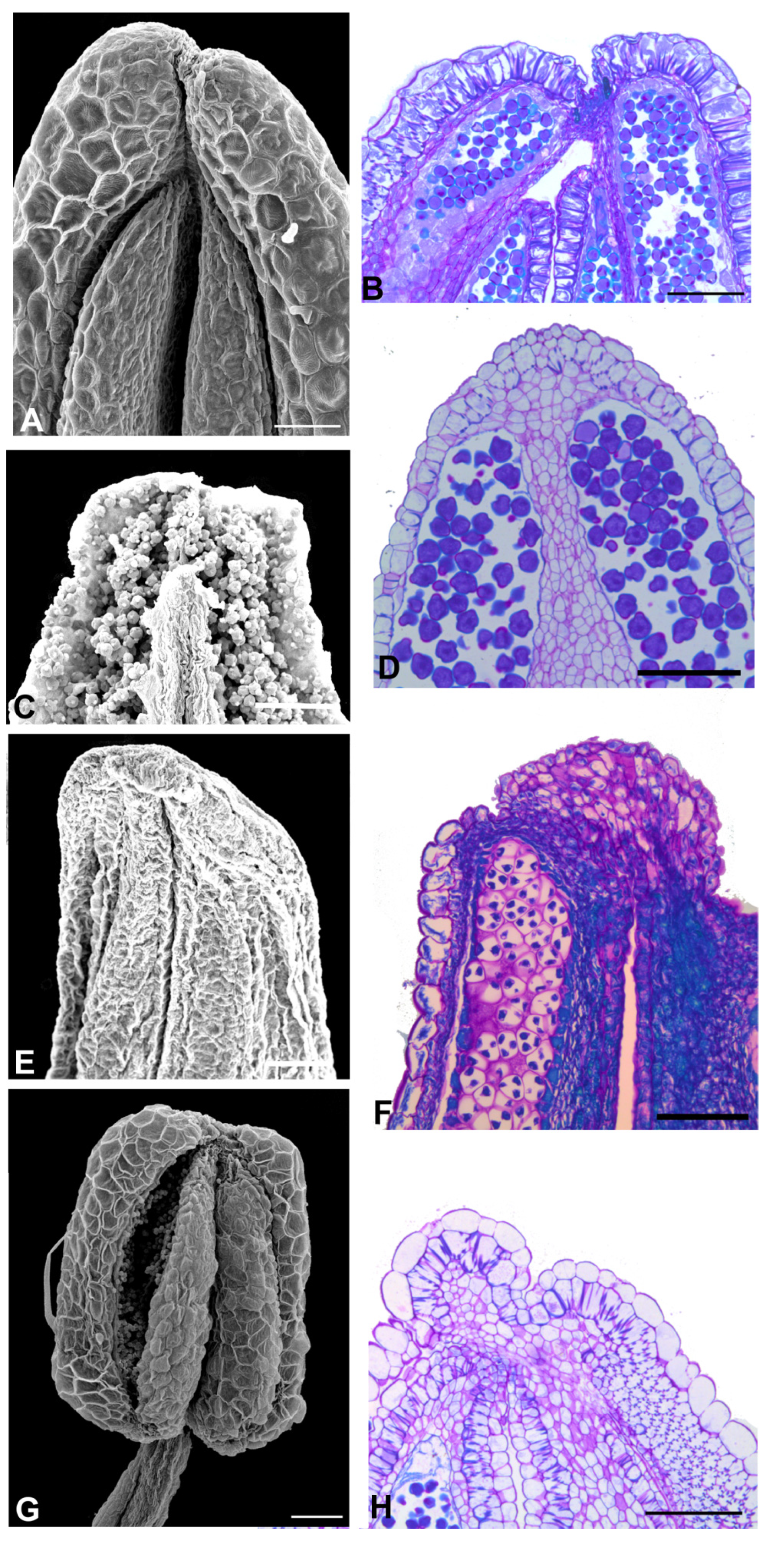
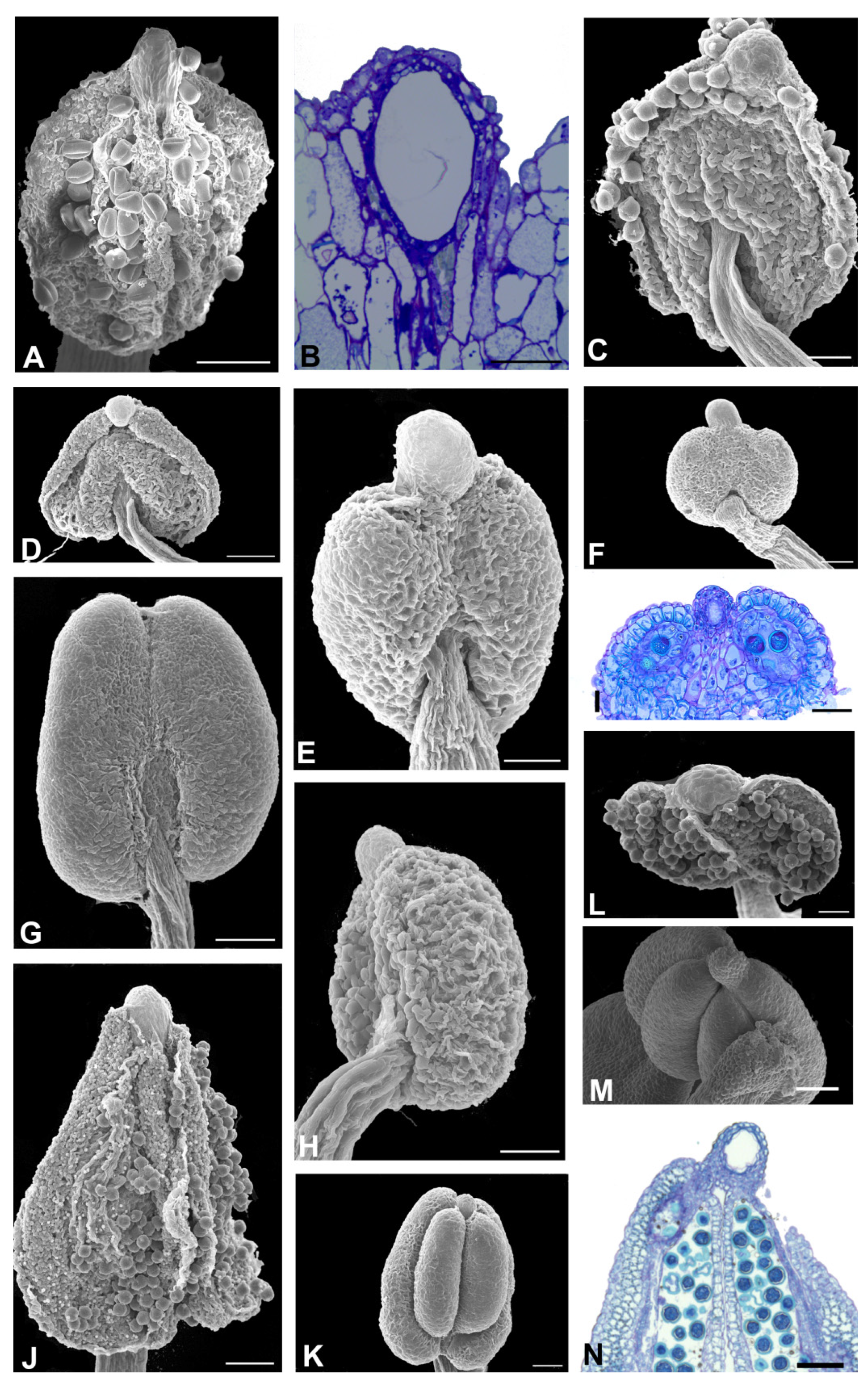
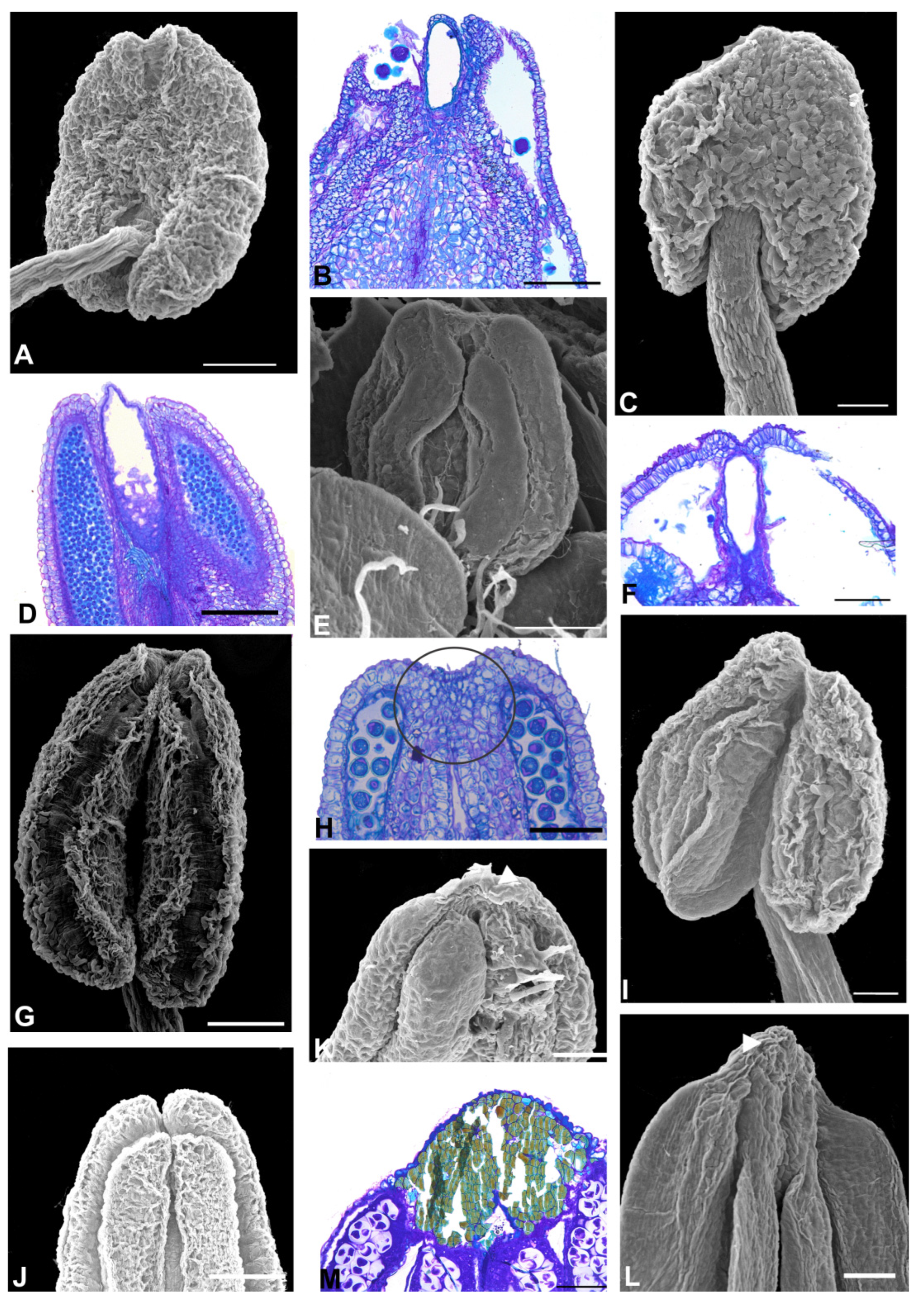
2.2. Anther Glands
2.3. Distribution of Secretory Cavities in the Anther of the Amburaneae and Dipterygeae Subclades
2.4. Correlations between Character -States
3. Discussion
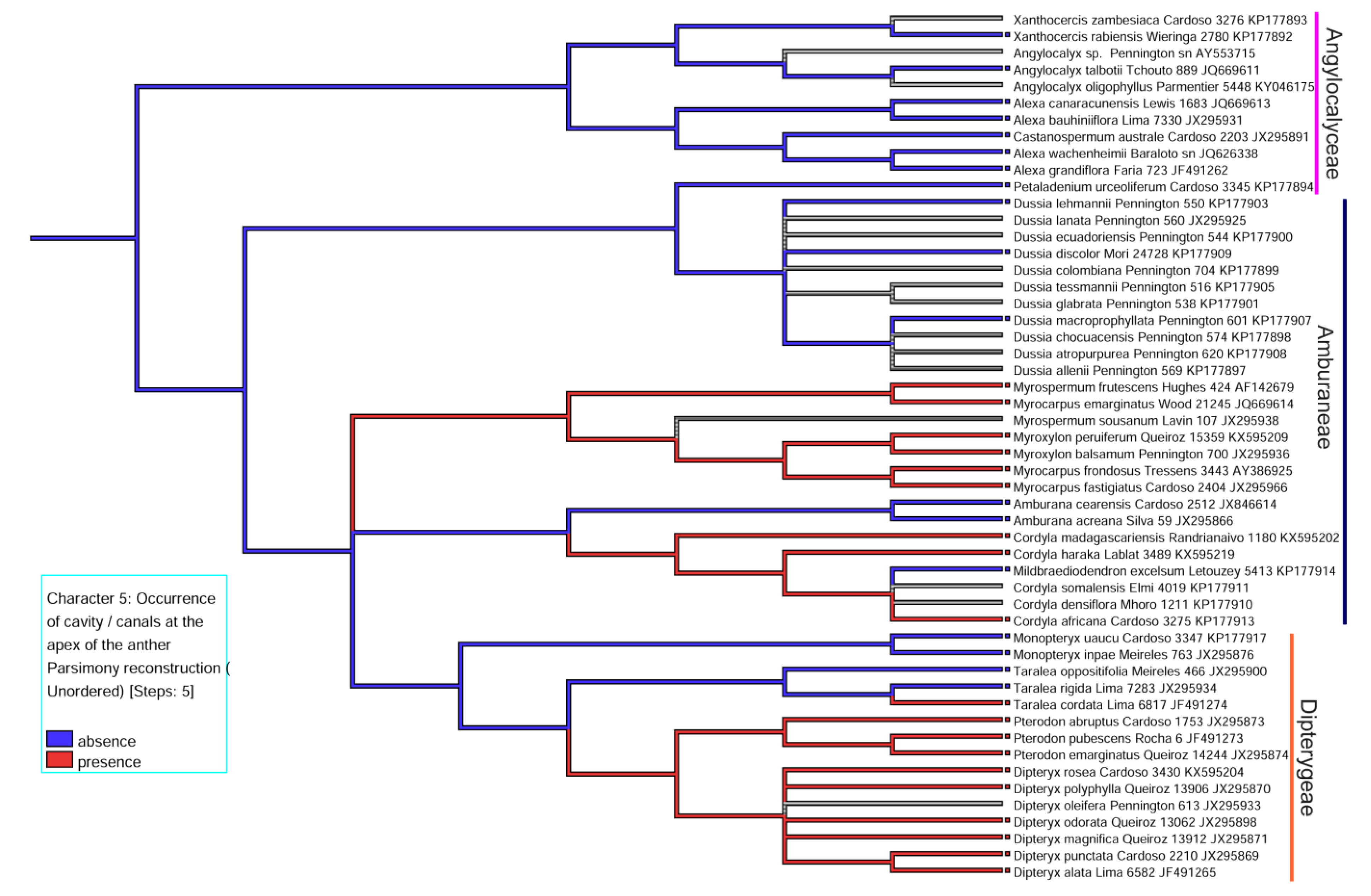
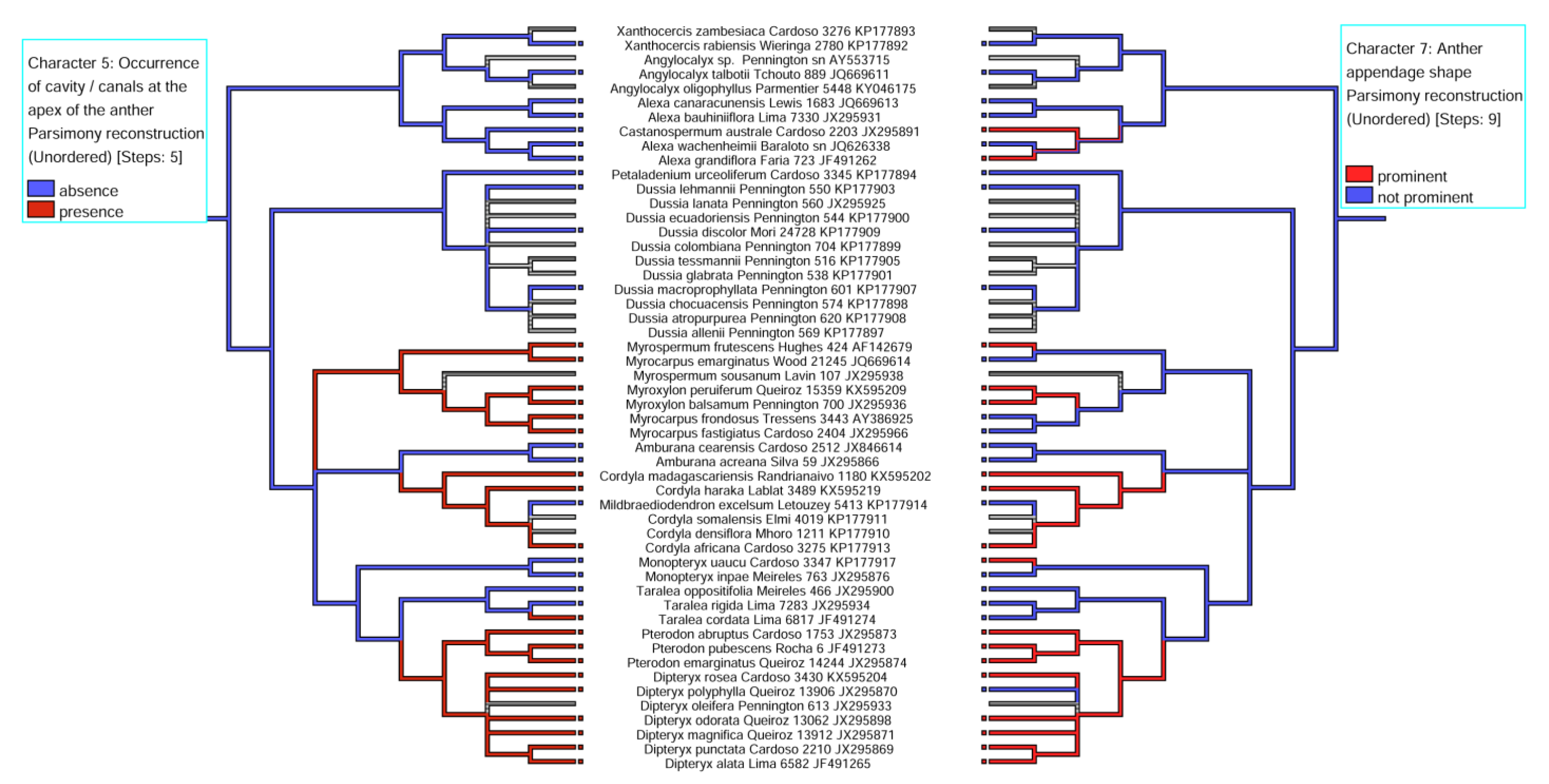
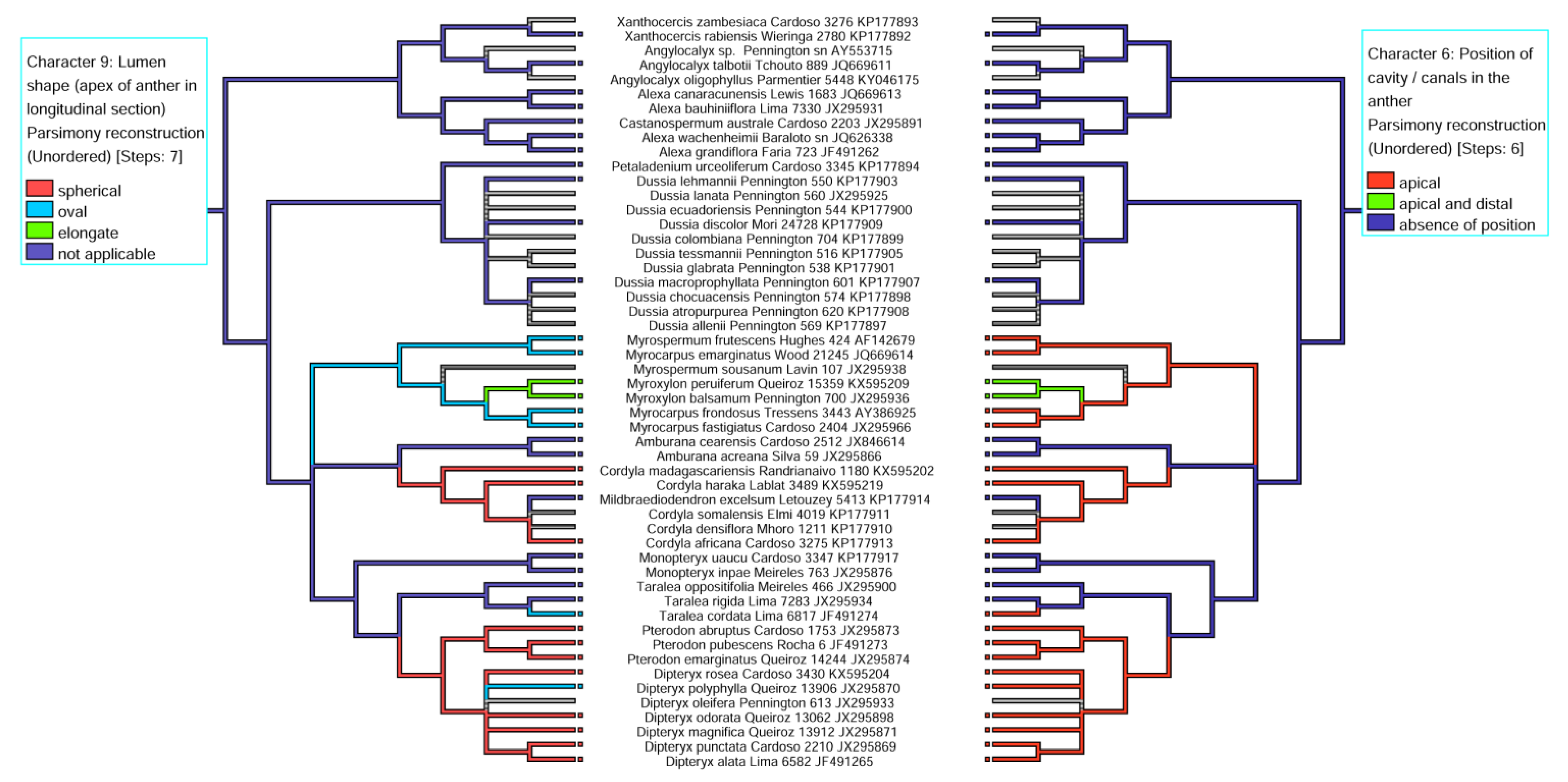
3.1. Distribution and Location of Secretory Cavities/Ducts
3.2. Evolutionary History of the Presence of a Secretory Cavity in the Anther of the ADA Clade
3.3. Evolutionary Significance of the Corolla, Type of Androecium vs. Presence of a Secretory Cavity/Duct in the Anther
3.4. Outlook
4. Materials and Methods
4.1. Sampling Plant Material
4.2. Scanning Electron Microscopy
4.3. Light Microscopy
4.4. Phylogenetic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LPWG (The Legume Phylogeny Working Group). A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef]
- LPWG (The Legume Phylogeny Working Group). Legume phylogeny and classification in the 21st century: Progress, prospects and lessons for other species-rich clades. Taxon 2013, 62, 217–248. [Google Scholar] [CrossRef]
- Lavin, M.; Pennington, R.T.; Klitgaard, B.B.; Sprent, J.I.; Lima, H.C.; Gasson, P.E. The dalbergioid legumes (Fabaceae): Delimitation of a pantropical monophyletic clade. Am. J. Bot. 2001, 88, 503–533. [Google Scholar] [CrossRef]
- Schrire, B.D.; Lavin, M.; Lewis, G.P. Global distribution patterns of the Leguminosae: Insights from recent phylogenies. In Plant Diversity and Complexity Patterns: Local, Regional and Global Dimensions; Biologiske Skrifter 55; Friis, I., Balslev, H., Eds.; Kgl. Danske Videnskabernes Selskab: Copenhagen, Denmark, 2005; pp. 375–422. [Google Scholar]
- Tucker, S.C. Floral ontogeny in Sophoreae (Leguminosae: Papilionoideae). I. Myroxylon (Myroxylon Group) and Castanospermum (Angylocalyx Group). Am. J. Bot. 1993, 80, 65–75. [Google Scholar] [CrossRef]
- Prenner, G. New Aspects In Floral Development of Papilionoideae: Initiated but Suppressed Bracteoles and Variable Initiation of Sepals. Ann. Bot. 2004, 93, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Leite, V.G.; Mansano, V.F.; Teixeira, S.P. Floral ontogeny in Dipterygeae (Fabaceae) reveals new insights into one of the earliest branching tribes in papilionoid legumes. Bot. J. Linn. Soc. 2014, 174, 529–550. [Google Scholar] [CrossRef]
- Leite, V.G.; Teixeira, S.P.; Mansano, V.F.; Prenner, G. Floral development of the early branching papilionoid legume Amburana cearensis (Leguminosae) reveals rare and novel characters. Int. J. Plant Sci. 2015, 176, 94–106. [Google Scholar] [CrossRef]
- Prenner, G.; Cardoso, D.; Zartman, C.E.; Queiroz, L.P. Flowers of the early-branching papilionoid legume Petaladenium urceoliferum display unique morphological and ontogenetic features. Am. J. Bot. 2015, 102, 1780–1793. [Google Scholar] [CrossRef]
- Cardoso, D.; São-Mateus, W.M.B.; Cruz, D.T.; Zartman, C.E.; Komura, D.L.; Kite, G.; Prenner, G.; Wieringa, J.J.; Clark, A.; Lewis, G.; et al. Filling in the gaps of the papilionoid legume phylogeny: The enigmatic Amazonian genus Petaladenium is a new branch of the early-diverging Amburaneae clade. Mol. Phylogenetics Evol. 2015, 84, 112–124. [Google Scholar] [CrossRef]
- Lewis, G.; Schrire, B.; Mackinder, B.; Lock, M. Legumes of the World; Royal Botanic Gardens, Kew: London, UK, 2005. [Google Scholar]
- Cardoso, D.; Pennington, R.T.; Queiroz, L.P.; Boatwright, J.S.; Van Wyk, B.-E.; Wojciechowski, M.F.; Lavin, M. Reconstructing the deep-branching relationships of the papilionoid legumes. S. Afr. J. Bot. 2013, 89, 58–75. [Google Scholar] [CrossRef]
- Leite, V.G.; Mansano, V.F.; Pansarin, E.; Teixeira, S.P. Presence of the anther gland is a key feature in pollination of the early-branching papilionoids Dipteryx alata and Pterodon pubescens (Leguminosae). Plant Biol. 2019, 21, 1016–1023. [Google Scholar] [CrossRef]
- Rodrigues, T.M.; Machado, S.R. Anatomia comparada do pulvino, pecíolo e raque de Pterodon pubescens Benth (Fabaceae-Faboideae). Acta Bot. Bras. 2004, 18, 381–390. [Google Scholar] [CrossRef][Green Version]
- Palermo, F.; Teixeira, S.P.; Mansano, V.F.; Leite, V.G.; Rodrigues, T.M. Secretory spaces in species of clade Dipterygeae (Leguminosae, Papilionoideae). Acta Bot. Bras. 2017, 31, 374–381. [Google Scholar] [CrossRef]
- Silva, N.F.; Arruda, R.C.O.; Alves, F.M.; Sartori, A.L.B. Leaflet anatomy of the Dipterygeae clade (Faboideae: Fabaceae): Evolutionary implications and systematics. Bot. J. Linn. Soc. 2018, 187, 99–117. [Google Scholar] [CrossRef]
- Rodrigues, T.M.; Santos, D.C.; Machado, S.R. The role of the parenchyma sheath and PCD during the development of oil cavities in Pterodon pubescens (Leguminosae-Papilionoideae). Comptes Rendus Biol. 2011, 334, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Paiva, E.A.S.; Oliveira, D.M.T.; Machado, S.R. Anatomy and ontogeny of the pericarp of Pterodon emarginatus Vogel (Fabaceae, Faboideae), with emphasis on secretory ducts. An. Acad. Bras. Ciênc. 2008, 80, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.M.; Machado, S.R. Oil glands in Pterodon pubescens Benth. (Leguminosae-Papilionoideae): Distribution, structure and secretion mechanisms. Int. J. Plant Sci. 2012, 173, 984–992. [Google Scholar] [CrossRef]
- Sartori, A.L.B.; Tozzi, A.M.G.A. Comparative leaflet anatomy in Myrocarpus Allemão, Myroxylon L. f and Myrospermum Jacq. (Leguminosae–Papilionoideae–Sophoreae) species. Bot. J. Linn. Soc. 2002, 140, 249–259. [Google Scholar] [CrossRef][Green Version]
- Bento, J.P.S.P.; Scremin-Dias, E.; Alves, F.M.; Mansano, V.F.; Sartori, A.L.B. Phylogenetic implications of the anatomical study of the Amburaneae clade (Fabaceae: Faboideae). Bot. J. Linn. Soc. 2020, 192, 69–83. [Google Scholar] [CrossRef]
- Cardoso, D.; Queiroz, L.P.; Pennington, R.T.; Lima, H.C.; Fonty, E.; Wojciechowski, M.F.; Lavin, M. Revisiting the phylogeny of Papilionoid Legumes: New insights from comprehensively sampled early-branching lineages. Am. J. Bot. 2012, 99, 1991–2013. [Google Scholar] [CrossRef] [PubMed]
- Seleme, E.P.; Lewis, G.P.; Stirton, C.H.; Sartori, A.L.B.; Mansano, V.F. A Taxonomic review and a new species of the south American woody genus Amburana (Leguminosae, Papilionoideae). Phytotaxa 2015, 212, 249–263. [Google Scholar] [CrossRef]
- Rudd, V.E. The Genus Dussia (Leguminosae); United States National Museum Contributions from the United States National Herbarium, Smithsonian Institution: Washington, DC, USA, 1963; pp. 247–277. [Google Scholar]
- Cowan, R.S. Swartzieae. In Advances in Legume Systematics, Part 1; Polhill, R.M., Raven, P.H., Eds.; Royal Botanic Gardens, Kew: London, UK, 1981; pp. 209–212. [Google Scholar]
- WFO. Mildbraediodendron excelsum Harms. Available online: http://www.worldfloraonline.org/taxon/wfo-0000171559 (accessed on 7 February 2022).
- Polhill, R.M. Sophoreae. In Advances in Legume Systematics, Part 1; Polhill, R.M., Raven, P.H., Eds.; Royal Botanic Gardens, Kew: London, UK, 1981; pp. 213–230. [Google Scholar]
- Sartori, A.L.B.; Tozzi, A.M.G.A. Revisão taxonômica de Myrocarpus Allemão (Leguminosae, Papilionoideae, Sophoreae). Acta Bot. Bras. 2004, 18, 521–535. [Google Scholar] [CrossRef]
- Sartori, A.L.B. Revisão Taxonômica e Estudos Morfológicos de Myrocarpus Allemão, Myroxylon L.f. e Myrospermum Jacq. (Leguminosae Papilionoideae Sophoreae). Ph.D. Thesis, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, Brazil, 2000. [Google Scholar]
- Ramirez, N. Revision Taxonomica del Genero Alexa Moq. (Fabaceae, Sophoreae). Ann. Mo. Bot. Gard. 1995, 82, 549–569. [Google Scholar] [CrossRef]
- Taubert, P. Leguminosae africanae. Engl. Bot. Jahrb. 1896, 23, 172. [Google Scholar]
- Louppe, D.; Oteng-Amoako, A.A.; Brink, M. (Eds.) Plant Resources of Tropical Africa 7 (1) Timbers 1; PROTA Foundation: Wageningen, The Netherlands, 2008; p. 701. [Google Scholar]
- Rodrigues, W.A. Contribuição para o estudo do gênero Monopteryx Spr. ex Benth. (Leguminosae) da Amazônia. Acta Amaz. 1975, 5, 153–155. [Google Scholar] [CrossRef]
- Skubatz, H.; Kunkel, D.D.; Howald, W.N.; Trenkle, R.; Mookherjee, B. The Sauromatum guttatum appendix as an osmophore: Excretory pathways, composition of volatiles and attractiveness to insects. New Phytol. 1996, 134, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, A.; Dotterl, S.; Meve, A.U. The chemical nature of fetid floral odours in stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae). New Phytol. 2006, 172, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Castro, M.M.; Demarco, D. Phenolic compounds produced by secretory structures in plants: A brief review. Nat. Prod. Commun. 2008, 3, 1273–1284. [Google Scholar] [CrossRef]
- Du Puy, D.J.; Labat, J.N. Swartzieae. In The Leguminosae of Madagascar; Du Puy, D.J., Labat, J.N., Rabevohitra, R., Villiers, J.F., Bosser, J., Moat, J., Eds.; Royal Botanic Gardens, Kew: London, UK, 2002; pp. 294–297. [Google Scholar]
- Sartori, A.L.B.; Lewis, G.P.; Mansano, V.F.; Tozzi, A.M.G.A. A revision of the genus Myroxylon (Leguminosae, Papilionoideae). Kew Bull. 2015, 70, 48. [Google Scholar] [CrossRef]
- Kochanovski, F.J.; Paulino, J.V.; Teixeira, S.P.; Tozzi, A.M.G.A.; Mansano, V.F. Floral development of Hymenaea verrucosa: An ontogenetic approach to the unusual flower of Fabaceae subfamily Detarioideae. Bot. J. Linn. Soc. 2018, 1, 46–58. [Google Scholar] [CrossRef]
- De Barros, T.C.; Pedersoli, G.D.; Teixeira, S.P. Anther glands in Mimosoideae (Leguminosae) are emergences with a conserved meristematic origin. Flora 2017, 226, 1–9. [Google Scholar] [CrossRef]
- Frankie, G.W. Tropical forest phenology and pollinator plant coevolution. In Coevolution of Animals and Plants; Symposium V First International Congress of Systematic and Evolutionary Biology, Boulder, Colorado; Lawrence, E.G., Raven, P.H., Eds.; University of Texas Press: Austin, TX, USA, 1973. [Google Scholar]
- Pfeiffer, P.M.M. Plant-Bee Interactions and Pollen Flux in Restored Areas of Atlantic Forest. Ph.D. Thesis, Instituto de Biociências da Universidade de São Paulo, Sao Paulo, Brazil, 2018. [Google Scholar]
- Lemmens, R.H.M.J.; Nyunai, N. Plant Resources of Tropical 7(2). Timbers 2; Lemmens, R.H.M.J., Louppe, D., Oteng-Amoako, A.A., Eds.; PROTA (Plant Resources of Tropical Africa) Foundation: Wageningen, The Netherlands, 2012. [Google Scholar]
- Rech, A.R. Recursos Polínicos Utilizados por 23 Espécies de Meliponini Lepeletier, 1836 Para Coleta de Pólen ao Longo da Bacia do Rio Negro, Amazonas-Brasil. Master’s Dissertation, Instituto Nacional de Pesquisas da Amazônia, Manaus, Brazil, 2010. [Google Scholar]
- Vogel, S. Chiropterophilie in der neotropischen Flora. Neue Mitteilungen II. Flora 1969, 158, 185–222. [Google Scholar]
- von Hegnauer, R. Chemotaxonomie der Pflanzen Band XIb-1 Leguminosae Teil 2: Caesalpinoideae und Mimosoideae; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 1996. [Google Scholar]
- Carvalho, C.T. Sobre os hábitos alimentares de Phillostomideos (Mammalia, Chiroptera). Rev. Biol. Trop. 1961, 9, 53–60. [Google Scholar] [CrossRef]
- Mansano, V.F.; Tucker, S.C.; Tozzi, A.M.G.A. Floral ontogeny of Lecointea, Zollernia, Exostiles and Harleyodendron (Leguminosae: Papilionoideae: Swartzieae S.L.). Am. J. Bot. 2002, 89, 1553–1569. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Hufford, L. Evolution and development in the amorphoid clade (Amorpheae: Papilionoideae: Leguminosae): Petal loss and dedifferentiation. Int. J. Plant Sci. 2005, 166, 383–396. [Google Scholar] [CrossRef][Green Version]
- Paulino, J.V.; Mansano, V.F.; Teixeira, S.P. Elucidating the unusual floral features of Swartzia dipetala (Fabaceae). Bot. J. Linn. Soc. 2013, 173, 303–320. [Google Scholar] [CrossRef]
- Pennington, R.T.; Klitgaard, B.B.; Ireland, H.; Lavin, M. New insights into floral evolution of basal papilionoids from molecular phylogenies. In Advances in Legume Systematics, Part 9; Herendeen, P.S., Bruneau, A., Eds.; Royal Botanic Gardens, Kew: London, UK, 2000; pp. 233–248. [Google Scholar]
- Tucker, S.C. Loss of floral organs in Ateleia (Leguminosae: Papilionoideae: Sophoreae). Am. J. Bot. 1990, 77, 750–761. [Google Scholar] [CrossRef]
- Tucker, S.C. Floral ontogeny in Sophoreae (Leguminosae: Papilionoideae). III. Radial symmetry and random petal aestivation in Cadia purpurea. Am. J. Bot. 2002, 89, 748–757. [Google Scholar] [CrossRef]
- Tucker, S.C. Floral Ontogeny in Swartzia (Leguminosae: Papilionoideae: Swartzieae): Distribution and Role of the Ring Meristem. Am. J. Bot. 2003, 90, 1271–1292. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.H.; Smith, E.C. Anatomy of the inferior ovary of Darbya. Am. J. Bot. 1942, 29, 464–471. [Google Scholar] [CrossRef]
- Gerrits, P.O.; Horobin, R.W. The Application of Glycol Methacrylate in Histotechnology; Some Fundamental Principles; Faculteit der Geneeskunde, Rijksuniversiteit Groningen: Groningen, The Netherlands, 1991. [Google Scholar]
- O’ Brien, T.P.; Feder, N.; Mccully, M.E. Polychromatic staining of plant cell walls by Toluidine Blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis, Version 3.6. 2018. Available online: http://mesquiteproject.org (accessed on 19 December 2019).
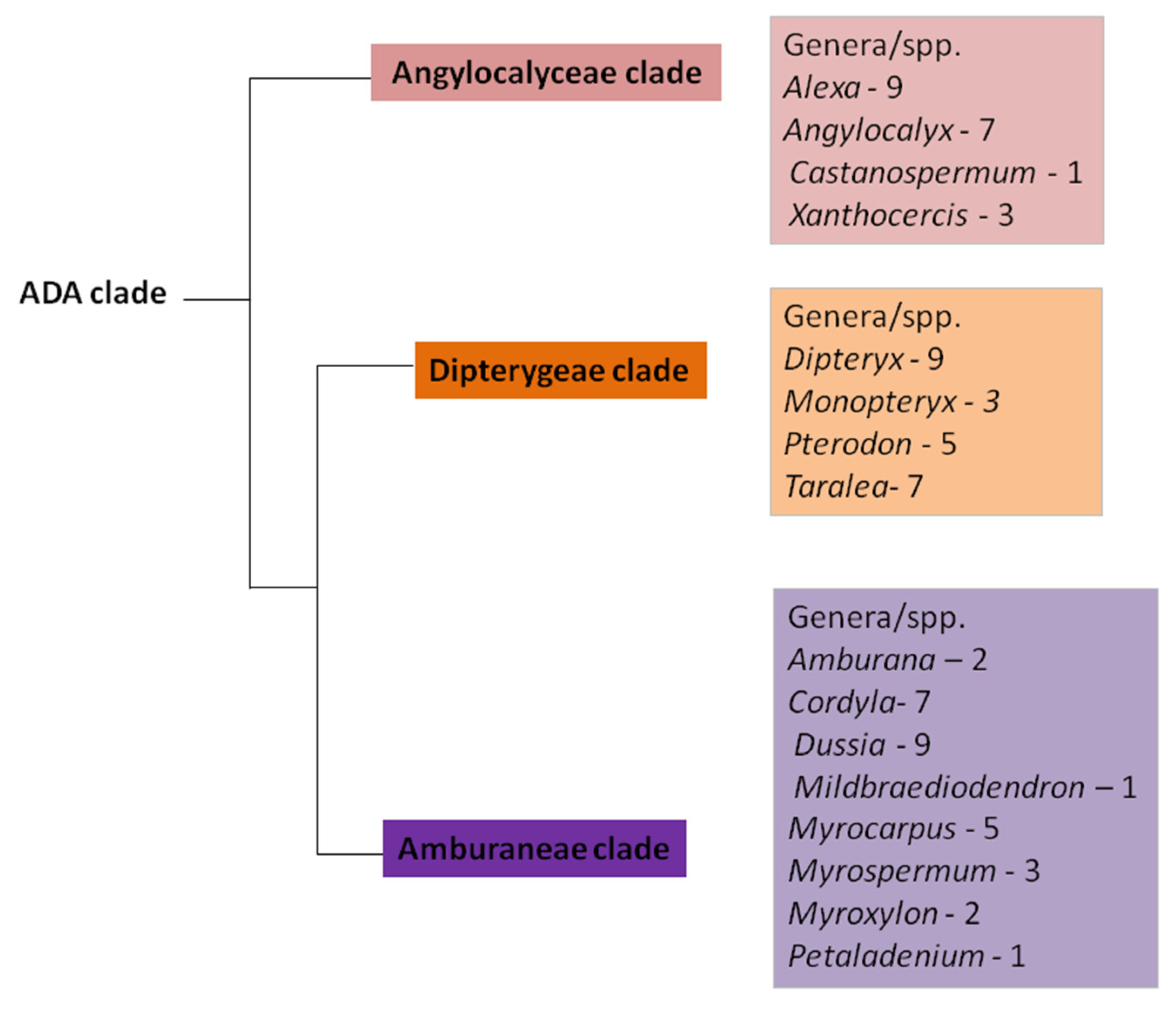



| Subclade | Species | Number of Sepals | Number of Petals | Number of Stamens | Stamen Connation | Occurrence of Cavity/Canal at the Anther | Position of Cavity/Canal at the Anther | Shape of Lumen of Cavity/Canal | Shape of the Anther Apex | Occurrence of Phenolic Cells at the Anther Apex | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amburaneae | Amburana acreana (Ducke) A.C. Sm. | 5 | 1 | 10 | free | absent | absence | [23], Present study | |||
| Amburana cearensis (Allemão) A.C. Sm. | 5 | 1 | 10 | free | absent | absence | [7] | ||||
| Amburana erythrosperma E. P. Seleme, C. H. Stirt. & V. F. Mansano | 5 | 1 | 10 | free | absent | absence | Present study | ||||
| Cordyla africana Lour | 3 | 0 | numerous | cavity | apical | spherical | prominent | Present study | |||
| Cordyla haraka Capuron | 3 | 0 | numerous | free | cavity | apical | spherical | prominent | Present study | ||
| Cordyla madagascariensis R. Vig. | 3 | 0 | numerous | free | cavity | apical | spherical | prominent | Present study | ||
| Dussia discolor (Benth.) Amshoff | 5 | 5 | 10 | fused at the base | absent | not prominent | absence | [6], Present study | |||
| Dussia lehmannii Harms | 5 | 5 | 10 | fused at the base | absent | not prominent | absence | [24], Present study | |||
| Dussia macroprophyllata (Donn. Sm.) Harms | 5 | 5 | 10 | fused at the base | absent | not prominent | absence | [24], Present study | |||
| Dussia martinicensis Krug & Urb. ex Taub | 5 | 5 | 10 | fused at the base | absent | not prominent | absence | [24], Present study | |||
| Dussia tessmannii Harms | 5 | 5 | 10 | absent | not prominent | absence | Present study | ||||
| Mildbraediodendron excelsum Harms | 5 | 0 | numerous | free | absent | not prominent | absence | [25,26], Present study | |||
| Myrocarpus emarginatus A.L.B. Sartori & A.M.G. Azevedo | 5 | 5 | 10 | fused at the base | cavity | apical | oval | not prominent | [27,28], Present study | ||
| Myrocarpus fastigiatus Allemão | 5 | 5 | 10 | fused at the base | cavity | apical | oval | not prominent | [27,28], Present study | ||
| Myrocarpus frondosus Allemão | 5 | 5 | 10 | fused at the base | cavity | apical | oval | not prominent | [20,27], Present study | ||
| Myrospermum frutescens Jacq | 5 | 5 | 10 | free | cavity | apical | oval | prominent | [27,29], Present study | ||
| Myroxylon balsamum (L.) Harms | 5 | 5 | 10 | free | duct | apical, distal | elongate | prominent | [5,27], Present study | ||
| Myroxylon peruiferum L. f. | 5 | 5 | 10 | free | duct | apical, distal | elongate | prominent | [27], Present study | ||
| Petaladenium urceoliferum Ducke | 5 | 5 | 10 | (fused at the base = nearly free) | absent | not prominent | absence | [9], Present study | |||
| Angylocalyceaee | Alexa bauhiniiflora Ducke | 5 | 5 | 8/10 | free | absent | not prominent | presence | [30], Present study | ||
| Alexa canaracunensis Pittier | 5 | 5 | 10 | free | absent | not prominent | presence | [30], Present study | |||
| Alexa cowanii Yakovlev | 5 | 5 | 10 | free | absent | not prominent | presence | [30], Present study | |||
| Alexa grandiflora Ducke | 5 | 5 | 10 | free | absent | prominent | presence | [30], Present study | |||
| Alexa imperatricis (R.H. Schomb.) Baill. | 5 | 5 | 8/10 | free | absent | not prominent | absence | [30], Present study | |||
| Alexa leiopetala Sandwith | 5 | 5 | absent | not prominent | presence | ||||||
| Alexa superba R.S. Cowan | 5 | 5 | 10/15 | free | absent | prominent | absence | [30], Present study | |||
| Alexa wachenheimii Benoist | 5 | 5 | 10 | free | absent | not prominent | presence | ||||
| Angylocalyx pynaertii De Wild. | 5 | 5 | 10 | monadelphous | absent | not prominent | absence | [31], Present study | |||
| Angylocalyx talbotii Hutch. & Dalziel | 5 | 5 | 10 | monadelphous | absent | not prominent | absence | Present study | |||
| Castanospermum australe A. Cunn. ex Mudie | 5 | 5 | 10 | free | absent | prominent | absence | [5], Present study | |||
| Xanthocercis zambesiaca (Baker) Dumaz-le-Grand | 5 | 5 | 10 | fused at the base | absent | not prominent | absence | [32], Present study | |||
| Dipterygeae | Dipteryx alata Vogel | 5 | 5 | 10 | monadelphous | cavity | apical | spherical | prominent | Present study | |
| Dipteryx lacunifera Ducke | 5 | 5 | 10 | monadelphous | cavity | apical | spherical | prominent | Present study | ||
| Dipteryx magnifica (Ducke) Ducke | 5 | 5 | 10 | monadelphous | cavity | apical | spherical | prominent | Present study | ||
| Dipteryx micrantha Harms | 5 | 5 | 10 | monadelphous | cavity | apical | spherical | prominent | Present study | ||
| Dipteryx odorata (Aubl.) Willd. | 5 | 5 | 10 | monadelphous | cavity | apical | spherical | prominent | Present study | ||
| Dipteryx polyphylla (Huber) Ducke | 5 | 5 | 10 | monadelphous | cavity | apical | oval | not prominent | Present study | ||
| Dipteryx punctata (Blake) Amshoff | 5 | 5 | 10 | monadelphous | cavity | apical | spherical | prominent | Present study | ||
| Dipteryx rosea Spruce ex Benth | 5 | 5 | 10 | monadelphous | cavity | apical | spherical | prominent | Present study | ||
| Pterodon abruptus (Moric.) Benth. | 5 | 5 | 10 | monadelphous | cavity | apical | spherical | prominent | Present study | ||
| Pterodon emarginatus Vogel | 5 | 5 | 10 | monadelphous | cavity | apical | spherical | prominent | Present study | ||
| Pterodon pubescens (Benth.) Benth. | 5 | 5 | 10 | monadelphous | cavity | apical | spherical | prominent | Present study | ||
| Taralea cordata Ducke | 5 | 5 | 10 | monadelphous | cavity | apical | oval | not prominent | Present study | ||
| Taralea crassifolia (Benth.) Ducke | 5 | 5 | 10 | monadelphous | cavity | apical | oval | not prominent | Present study | ||
| Taralea nudipes (Tul.) Ducke | 5 | 5 | 10 | monadelphous | cavity | apical | oval | not prominent | Present study | ||
| Taralea oppositifolia Aubl. | 5 | 5 | 10 | monadelphous | absent | not prominent | Present study | ||||
| Taralea reticulata (Benth.) Ducke. | 5 | 5 | 10 | monadelphous | absent | not prominent | Present study | ||||
| Taralea rigida Schery | 5 | 5 | 10 | monadelphous | absent | not prominent | Present study | ||||
| Monopteryx inpae W.A.Rodrigues | 5 | 5 | 10 | free | absent | apical | not prominent | presence | [10,33], Present study | ||
| Monopteryx uaucu Spruce ex Benth. | 5 | 5 | 10 | free | absent | apical | prominent | presence | [10,33], Present study | ||
| Outgroup | Ateleia glazioveana Baill. | 5 | 1 | 10 | free | absent | not prominent | absence | Present study | ||
| Ateleia guaraya Herzog | 5 | 1 | 10 | free | absent | not prominent | absence | Present study | |||
| Candolleodendron brachystachyum (DC.) R.S. Cowan | 5 | 1 | numerous | free | absent | prominent | absence | Present study | |||
| Cyathostegia mathewsii (Benth.) Schery | 5 | 1 | numerous | free | absent | not prominent | absence | Present study | |||
| Swartzia langsdorffii Raddi | 4 | 0 | 2–3 (smaller stamens), numerous (larger stamens) | free | absent | prominent | presence | Present study | |||
| Uleanthus erythrinoides Harms | 5 | 4 | 10 | free | absent | not prominent | absence | Present study |
| Variables | |
|---|---|
| 1. Number of sepals on the mature flower | (0) five, (1) four, (2) three |
| 2. Number of petals on the mature flower | (0) zero, (1) one, (2) four, -(3) five |
| 3. Number of stamens | (0) up to 10, (1) 10, -(3) numerous (= over 10) |
| 4. Type of stamen connation | (0) free, (1) fused at the base, (2) monadelphous |
| 5. Occurrence of cavity /canals at the apex of the anther | (0) absence, (1) presence |
| 6. Position of cavity /canals in the anther | (0) apical, (1) distal, (2) apical and distal, (3) absence of position |
| 7. Anther appendage shape | (0) prominent, (1) not prominent |
| 8. Phenolic compound tissue at the apex of the anther | (0) absence, (1) presence |
| 9. Lumen shape (apex of anther in longitudinal section) | (0) spherical, (1) oval, (2) elongate, (3) not applicable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, V.G.; Teixeira, S.P.; Sartori, Â.L.B.; Mansano, V.F. Evolution of the Anther Gland in Early-Branching Papilionoids (ADA Clade, Papilionoideae, Leguminosae). Plants 2022, 11, 835. https://doi.org/10.3390/plants11070835
Leite VG, Teixeira SP, Sartori ÂLB, Mansano VF. Evolution of the Anther Gland in Early-Branching Papilionoids (ADA Clade, Papilionoideae, Leguminosae). Plants. 2022; 11(7):835. https://doi.org/10.3390/plants11070835
Chicago/Turabian StyleLeite, Viviane Gonçalves, Simone Pádua Teixeira, Ângela Lúcia Bagnatori Sartori, and Vidal Freitas Mansano. 2022. "Evolution of the Anther Gland in Early-Branching Papilionoids (ADA Clade, Papilionoideae, Leguminosae)" Plants 11, no. 7: 835. https://doi.org/10.3390/plants11070835
APA StyleLeite, V. G., Teixeira, S. P., Sartori, Â. L. B., & Mansano, V. F. (2022). Evolution of the Anther Gland in Early-Branching Papilionoids (ADA Clade, Papilionoideae, Leguminosae). Plants, 11(7), 835. https://doi.org/10.3390/plants11070835






