Effects Induced by the Agricultural Application of Probiotics on Antioxidant Potential of Strawberries
Abstract
:1. Introduction
2. Results
2.1. The Impact of ProbioHumus and NaturGel on the Strawberry Fruit Biometric Parameters
2.2. The Impact of Probiotics on the Content and Activity of Antioxidants in Strawberry Fruits
2.2.1. Total Phenols
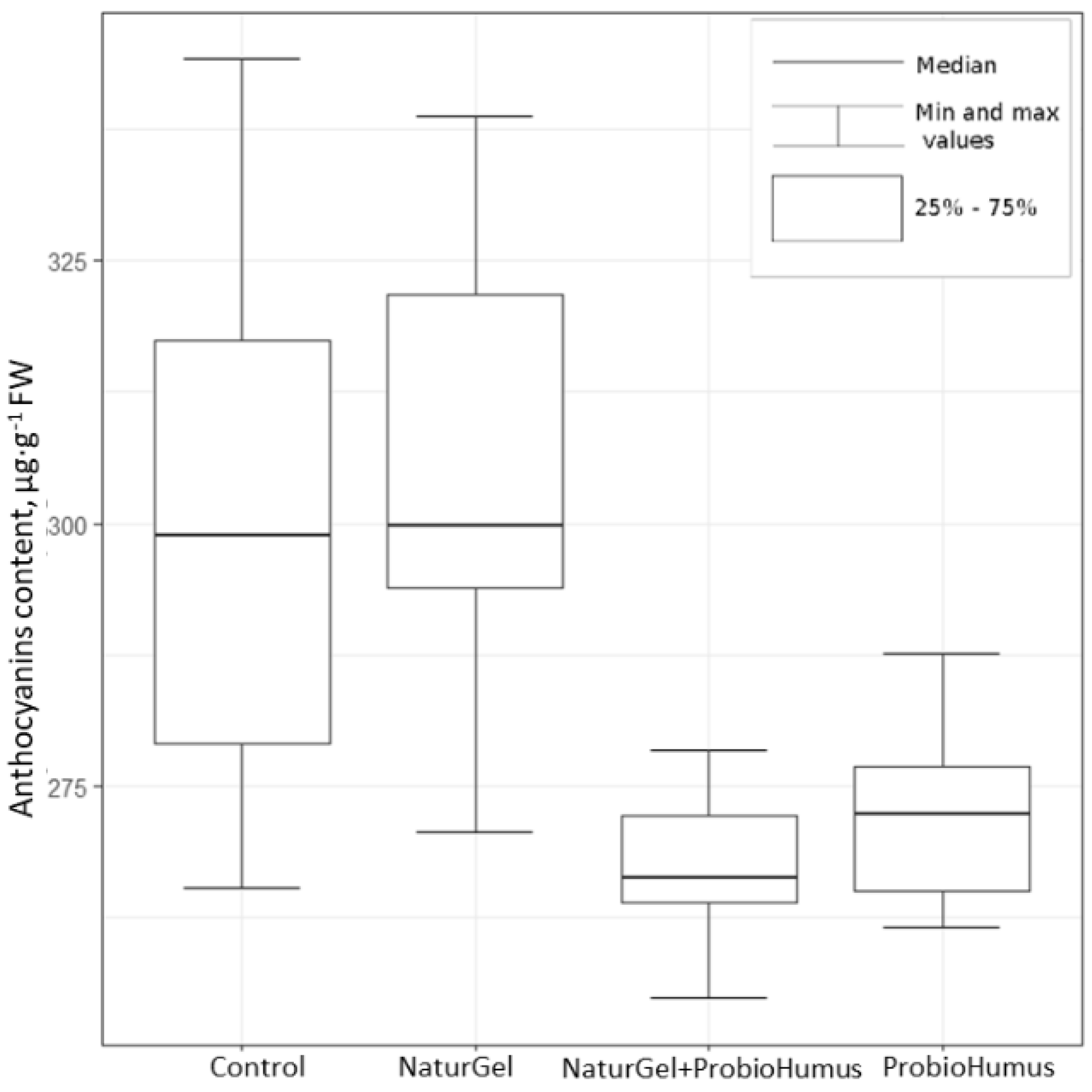
2.2.2. Total Anthocyanin Content
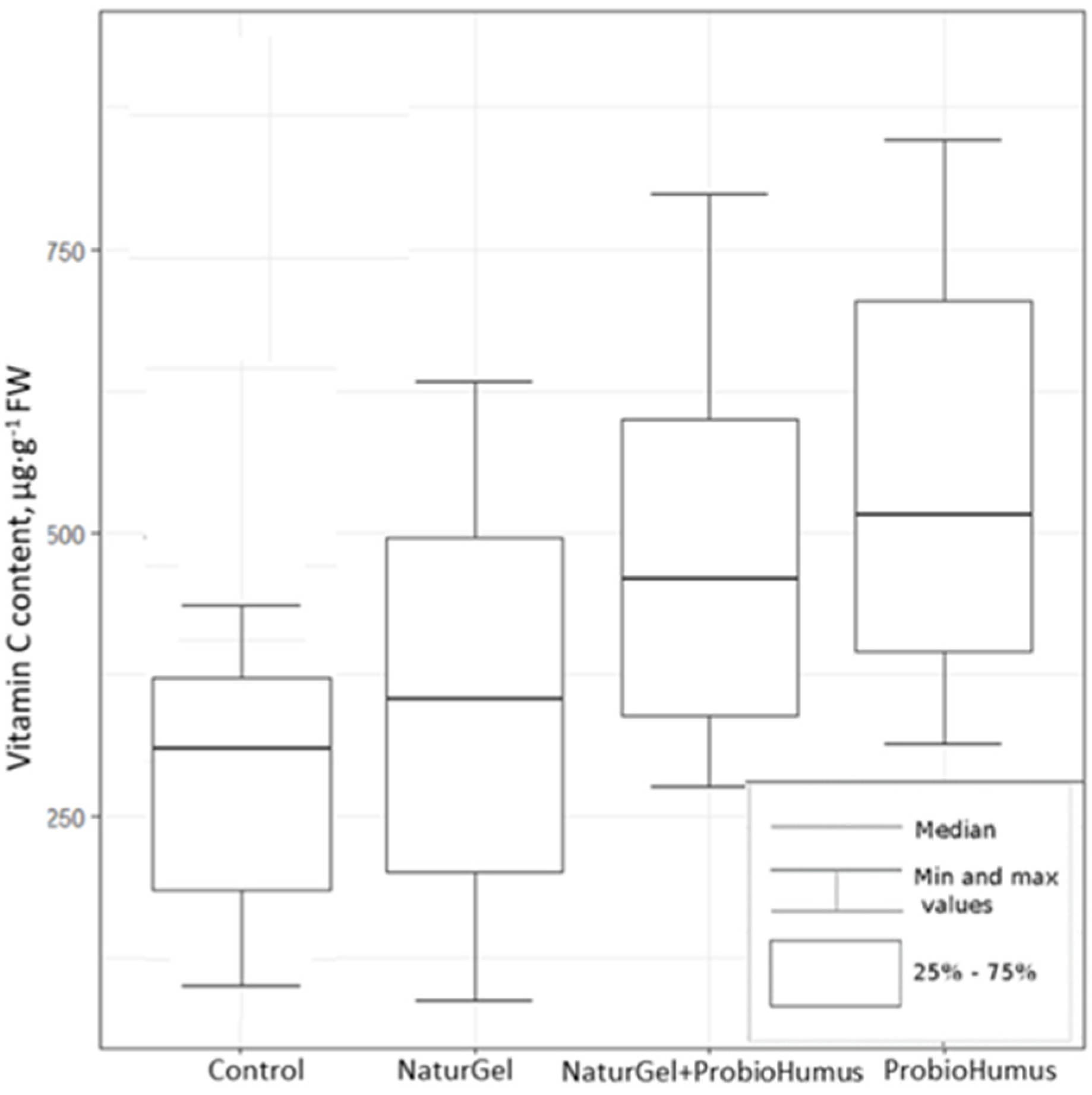
2.2.3. Ascorbic Acid
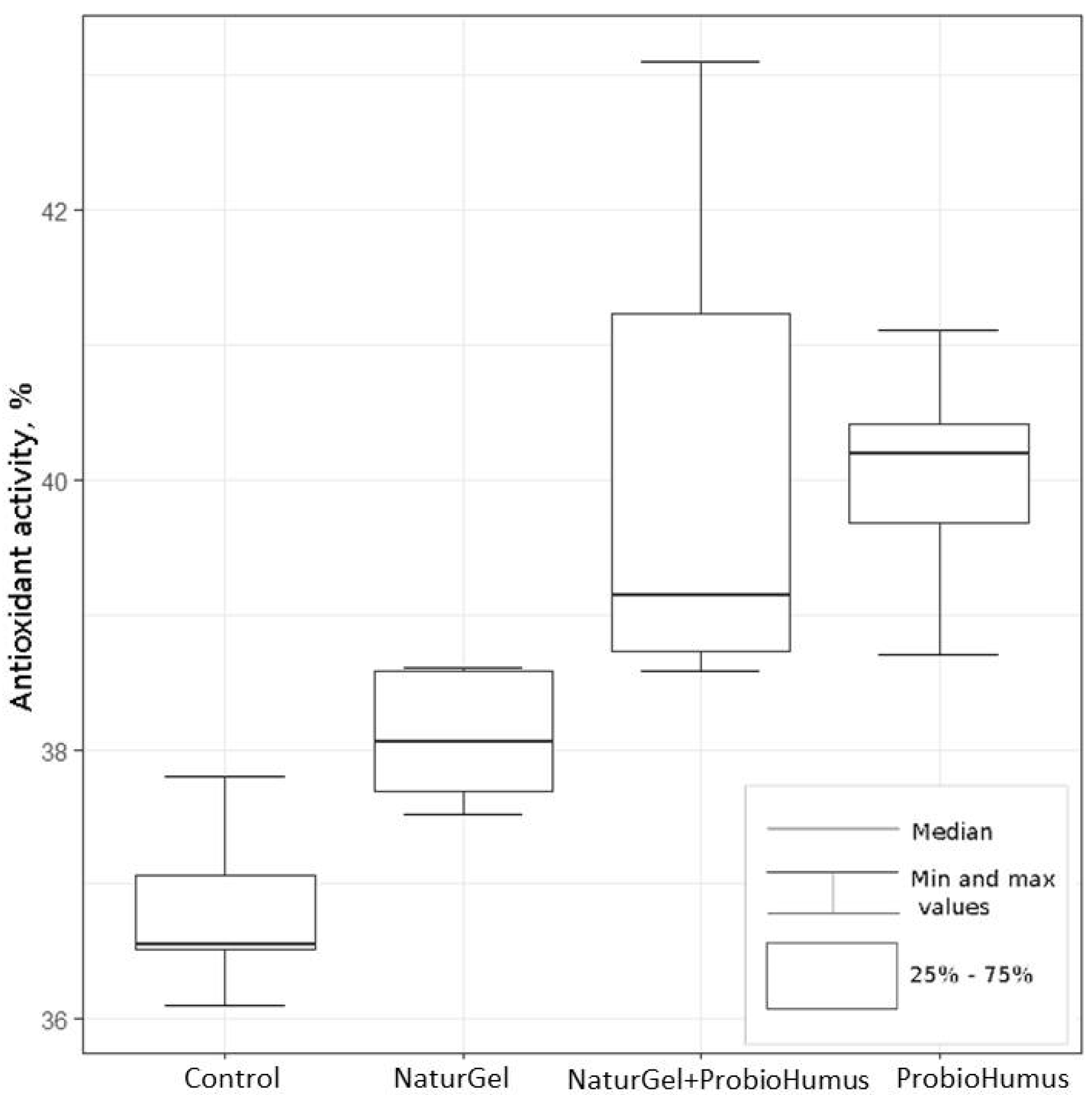
2.2.4. Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Probiotic Preparations ProbioHumus and NaturGel
4.3. Plant Treatment with Probiotic Preparations
4.4. Extraction
4.5. Antioxidant Activity Determination
4.6. Determination of Total Phenols
4.7. Determination of Anthocyanin Content
4.8. Ascorbic Acid Determination
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darrow, G.M. The Strawberry. History, Breeding and Physiology, 1st ed.; Holt, Rinehart and Winston: New York, NY, USA; Chicago, IL, USA; San Francisco, CA, USA, 1966; p. 447. [Google Scholar]
- Basu, A.; Nguyen, A.; Betts, N.; Lyons, T.J. Strawberry as a functional food: An evidence-based review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790806. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, V.C.; Calvete, E.; Reginatto, F.H. Quality properties and antioxidant activity of seven strawberry (Fragaria × ananassa Duch.) cultivars. Sci. Hortic. 2017, 225, 293–298. [Google Scholar] [CrossRef]
- Ganhão, R.; Pinheiro, J.; Tino, C.; Faria, H.; Gil, M.M. Characterization of nutritional, physicochemical, and phytochemical composition and antioxidant capacity of three strawberry “Fragaria × ananassa Duch.” cultivars (“Primoris”, “Endurance”, and “Portola”) from Western Region of Portugal. Foods 2019, 8, 0682. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Olsson, M.E.; Andersson, C.S.; Oredsson, S.; Berglund, R.H.; Gustavsson, K.E. Antioxidant levels and inhibition of cancer cell proliferation in vitro by extracts from organically and conventionally cultivated strawberries. J. Agric. Food Chem. 2006, 54, 1248–1255. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Buren, L.; van Wagner, E.; Wiseman, S.; Van De Put, F.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Tomlinson, B.; Benzie, I.F. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: Implications for dietary planning and food preservation. Br. J. Nutr. 2002, 87, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Aaby, K.; Ekeberg, D.; Skrede, G. Characterization of phenolic compounds in strawberry (Fragaria × ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J. Agric. Food Chem. 2007, 55, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; De Vos, R.C.H.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.E.; Ekvall, J.; Gustavsson, K.E.; Nilsson, J.; Pillai, D.; Sjo¨holm, I.; Svensson, U.; Akesson, B.; Nyman, M.G.L. Antioxidants, low molecular weight carbohydrates, and total antioxidant capacity in strawberries (Fragaria ananassa): Effects of cultivar, ripening, and storage. J. Agric. Food Chem. 2004, 52, 2490–2498. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.M.; Garufi, F.; Nitto, S.; Di Mauro, R.; Longhitano, R.C.; Magrì, G.; Catalfo, A.; Serrentino, M.E.; De Guidi, G. Antioxidant activity and phenolic content of strawberry genotypes from Fragaria × ananassa. Pharm. Biol. 2009, 47, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Jin, P.; Wang, S.Y.; Wang, C.Y.; Zheng, Y. Effect of cultural system and storage temperature on antioxidant capacity and phenolic compounds in strawberries. Food Chem. 2011, 124, 262–270. [Google Scholar] [CrossRef]
- Crecente-Campo, J.; Nunes-Damaceno, M.; Romero-Rodríguez, M.A.; Vázquez-Odériz, M.L. Color, anthocyanin pigment, ascorbic acid and total phenolic compound determination in organic versus conventional strawberries (Fragaria × ananassa Duch, cv Selva). J. Food Compos. Anal. 2012, 28, 23–30. [Google Scholar] [CrossRef]
- Lind, K.; Lafer, G.; Schloffer, K.; Innerhoffer, G.; Meister, H. Organic Fruit Growing; CABI Publishing: UK, Wallingford, 2003; p. 304. [Google Scholar]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef]
- Esitken, A.; Yildiz, H.E.; Ercisli, S.; Donmez, M.F.; Turan, M.; Gunes, A. Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Sci. Hortic. 2010, 124, 62–66. [Google Scholar] [CrossRef]
- Mohammadi, K.; Sohrabi, Y. Bacterial biofertilizers for sustainable crop production: A review. ARPN J. Agric. Biol. Sci. 2012, 7, 307–316. [Google Scholar]
- Jiménez-Gómez, A.; García-Fraile, P.; Flores-Félix, J.D.; Rivas, R. Plants probiotics as a tool to produce highly functional fruits. In Bioactive Molecules in Food. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–13. [Google Scholar]
- Rahman, M.; Sabir, A.A.; Mukta, J.A.; Khan, M.M.A.; Mohi-Ud-Din, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 2504. [Google Scholar] [CrossRef] [Green Version]
- Berlec, A. Novel techniques and findings in the study of plant microbiota: Search for plant probiotics. Plant Sci. 2012, 193–194, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Lingua, G.; Bona, E.; Manassero, P.; Marsano, F.; Todeschini, V.; Cantamessa, S.; Copetta, A.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria × ananassa var. Selva) in conditions of reduced fertilization. Int. J. Mol. Sci. 2013, 14, 16207–16225. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; Celador-Lera, L.; Fradejas-Bayón, M.; Rivas, R. Plant probiotic bacteria enhance the quality of fruit and horticultural crops. AIMS Microbiol. 2017, 3, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Onyia, C.O.; Okoh, A.M.; Okoh, I. Production of plant growth-promoting bacteria biofertilizer from organic waste material and evaluation of its performance on the growth of corn (Zea mays). Am. J. Plant Sci. 2020, 11, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Hossain, M.M. Plant probiotics in phosphorus nutrition in crops, with special reference to rice. In Bacteria in Agrobiology: Plant Probiotics; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 325–363. [Google Scholar]
- Spence, C.; Alff, E.; Shantharaj, D.; Bais, H. Probiotics for plants: Importance of Rhizobacteria on aboveground fitness in plants. In Bacteria in Agrobiology: Plant Probiotics; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–14. [Google Scholar]
- Bona, E.; Lingua, G.; Manassero, P.; Cantamessa, S.; Marsano, F.; Todeschini, V.; Copetta, A.; Massa, N.; Avidano, L.; Gamalero, E.; et al. AM fungi and PGP pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza 2015, 25, 181–193. [Google Scholar] [CrossRef]
- Flores-Félix, J.D.; Silva, L.R.; Rivera, L.P.; Marcos-Garcia, M.; García-Fraile, P.; Martínez-Molina, E.; Mateos, P.F.; Velázquez, E.; Andrade, P.; Rivas, R. Plants probiotics as a tool to produce highly functional fruits: The case of phyllobacterium and vitamin C in strawberries. PLoS ONE 2015, 10, e0122281. [Google Scholar] [CrossRef]
- Vandenberghe, L.P.S.; Garcia, L.M.B.; Rodrigues, C.; Camara, M.C.; Pereira, G.V.M.; Oliveira, J.; Soccol, C.R. Potential applications of plant probiotic microorganisms in agriculture and forestry. AIMS Microbiol. 2017, 3, 629–648. [Google Scholar] [CrossRef]
- Ali, L.; Alsanius, B.W.; Rosberg, A.K.; Svensson, B.; Nielsen, T.; Olsson, M.E. Effects of nutrition strategy on the levels of nutrients and bioactive compounds in blackberries. Eur. Food Res. Technol. 2011, 234, 33–44. [Google Scholar] [CrossRef]
- Capanoglu, E. The potential of priming in food production. Trends Food Sci. Technol. 2010, 21, 399–407. [Google Scholar] [CrossRef]
- Chamam, A.; Sanguin, H.; Bellvert, F.; Meiffren, G.; Comte, G.; Wisniewski-Dye, F.; Bertrand, C.; Prigent-Combaret, C. Plant secondary metabolite profiling evidences strain-dependent effect in the Azospirillum-Oryza sativa assotiation. Phytochemistry 2013, 87, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Oksman-Caldentey, K.M.; Inzé, D. Plant cell factories in the post-genomic era: New ways to produce designer secondary metabolites. Trends Plant Sci. 2004, 9, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Reddy, M.S.; Kloepper, J.W. Tobacco growth enhancement and blue mold disease protection by rhizobacteria: Relationship between plant growth promotion and systemic disease protection by PGPR strain 90-166. Plant Soil 2004, 262, 277–288. [Google Scholar] [CrossRef]
- García-Seco, D.; Bonilla, A.; Algar, E.; García-Villaraco, A.; Gutierrez-Mañero, J.; Ramos-Solano, B. Enhanced blackberry production using Pseudomonas fluorescens as elicitor. Agron. Sustain. Dev. 2013, 33, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Albanchez, E.; Kirakosyan, A.; Bolling, S.F.; García-Villaraco, A.; Gutierrez-Mañero, J.; Ramos-Solano, B. Biotic elicitation as a tool to improve berry (Strawberry and Raspberry) extract potential on metabolic syndrome related enzymes in vitro. J. Sci. Food Agric. 2018, 99, 2939–2946. [Google Scholar] [CrossRef]
- Jurkonienė, S.; Mockevičiūtė, R.; Jankauskienė, J.; Jankovska-Bortkevič, E.; Armalytė, G.; Gavelienė, V. Aplication of commercial plant probiotics improvesberry yield and quality of field-grown blackcurrant. ACS Agric. Sci. Technol. 2021, 1, 615–622. [Google Scholar] [CrossRef]
- Pırlak, L.; Köse, M. Effects of plant growth promoting Rhizobacteria on yield and some fruit properties of strawberry. J. Plant Nutr. 2009, 32, 1173–1184. [Google Scholar] [CrossRef]
- Erturk, Y.; Ercisli, S.; Cakmakci, R. Yield and growth response of strawberry to plant growth-promoting rhizobacteria inoculation. J. Plant Nutr. 2012, 35, 817–826. [Google Scholar] [CrossRef]
- Mukta, A.J.; Rahman, M.; As Sabir, A.; Gupta, D.R.; Surovy, M.Z.; Rahman, M.; Islam, M.T. Chitosan and plant probiotics application enhance growth and yield of strawberry. Biocatal. Agric. Biotechnol. 2017, 11, 9–18. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.; Goncalves, O.; Teixeira, J.A.; Vicentesc, A.A. Comparative study of Selva and Camarosa strawberries for the commercial market. J. Food Sci. 2002, 67, 2132–2137. [Google Scholar] [CrossRef]
- Mandave, P.C.; Pawar, P.K.; Ranjekar, P.K.; Mantri, N.; Kuvalekar, A.A. Comprehensive evaluation of in vitro antioxidant activity, total phenols and chemical profiles of two commercially important strawberry varieties. Sci. Hortic. 2014, 172, 124–134. [Google Scholar] [CrossRef]
- Ramos-Solano, B.; Garcia-Villaraco, A.; Gutierrez-Mañero, F.J.; Lucas, J.A.; Bonilla, A.; Garcia-Seco, D. Annual changes in bioactive contents and production in field-grown blackberry after inoculation with Pseudomonas fluorescens. Plant Physiol. Biochem. 2013, 74, 1–8. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Fazeelat, T.; Afzal, W.; Asif, M.; Zamir, M.; Saleem, H. HPLC analysis of strawberry anthocyanins at partially ripe and ripe levels. J. Chem. Soc. Pak. 2007, 29, 243–246. [Google Scholar]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria × ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W.; Galletta, G.J. Cultural system affects fruit quality and antioxidant capacity in strawberries. J. Agric. Food Chem. 2002, 50, 6534–6542. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Bouchal, Y.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry puree functionalized with natural hydroxytyrosol: Effects on vitamin C and antioxidant activity. Molecules 2020, 25, 5829. [Google Scholar] [CrossRef]
- Capocasa, F.; Diamanti, J.; Mezzetti, B.; Tulipani, S.; Battino, M. Breeding strawberry (Fragaria × ananassa Duch.) to increase fruit nutritional quality. BioFactors 2008, 34, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Meier, U. Growth Stages of Mono and Dicotyledonous Plants. In BBCH Monograph; Meier, U., Ed.; Julius Kühn-Institut: Quedlinburg, Germany, 2018; pp. 89–92. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV–visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Schwartz, S.J., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar]
- Chakraborthy, G. Quantitative estimation of ascorbic acid by HPTLC indifferent varieties of amla. J. Young Pharm. 2009, 1, 82–85. [Google Scholar] [CrossRef] [Green Version]



| Parameters | Control | NaturGel | NaturGel + ProbioHumus | ProbioHumus |
|---|---|---|---|---|
| Fresh weight, g | 5.9 ± 0.28 | 8.0 ± 0.51 a | 8.1 ± 0.39 a | 8.4 ± 0.50 a |
| Length, cm | 2.18 ± 0.03 a | 2.54 ± 0.05 b | 2.42 ± 0.04 ab | 2.28 ± 0.03 ab |
| Diameter, cm | 2.31 ± 0.02 a | 2.56 ± 0.03 b | 2.60 ± 0.05 b | 2.45 ± 0.04 ab |
| Dry biomass, % | 12.76 ± 0.50 | 13.74 ± 0.61 ab | 13.44 ± 0.59 b | 14.09 ± 0.74 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mockevičiūtė, R.; Jurkonienė, S.; Gavelienė, V.; Jankovska-Bortkevič, E.; Šocik, B.; Armalytė, G.; Budrys, R. Effects Induced by the Agricultural Application of Probiotics on Antioxidant Potential of Strawberries. Plants 2022, 11, 831. https://doi.org/10.3390/plants11060831
Mockevičiūtė R, Jurkonienė S, Gavelienė V, Jankovska-Bortkevič E, Šocik B, Armalytė G, Budrys R. Effects Induced by the Agricultural Application of Probiotics on Antioxidant Potential of Strawberries. Plants. 2022; 11(6):831. https://doi.org/10.3390/plants11060831
Chicago/Turabian StyleMockevičiūtė, Rima, Sigita Jurkonienė, Virgilija Gavelienė, Elžbieta Jankovska-Bortkevič, Božena Šocik, Gabija Armalytė, and Rimas Budrys. 2022. "Effects Induced by the Agricultural Application of Probiotics on Antioxidant Potential of Strawberries" Plants 11, no. 6: 831. https://doi.org/10.3390/plants11060831
APA StyleMockevičiūtė, R., Jurkonienė, S., Gavelienė, V., Jankovska-Bortkevič, E., Šocik, B., Armalytė, G., & Budrys, R. (2022). Effects Induced by the Agricultural Application of Probiotics on Antioxidant Potential of Strawberries. Plants, 11(6), 831. https://doi.org/10.3390/plants11060831








