NLP2-NR Module Associated NO Is Involved in Regulating Seed Germination in Rice under Salt Stress
Abstract
1. Introduction
2. Results
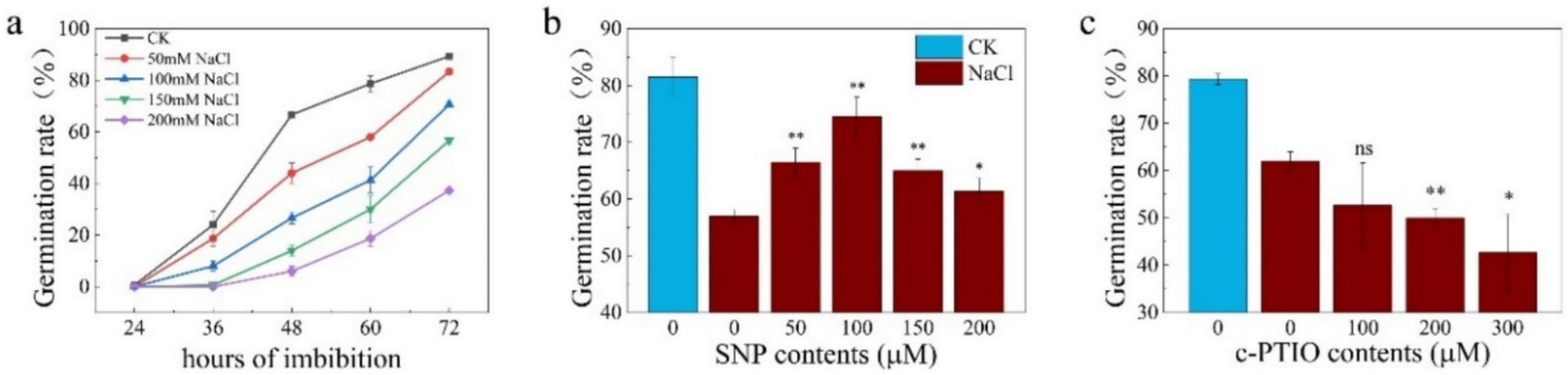
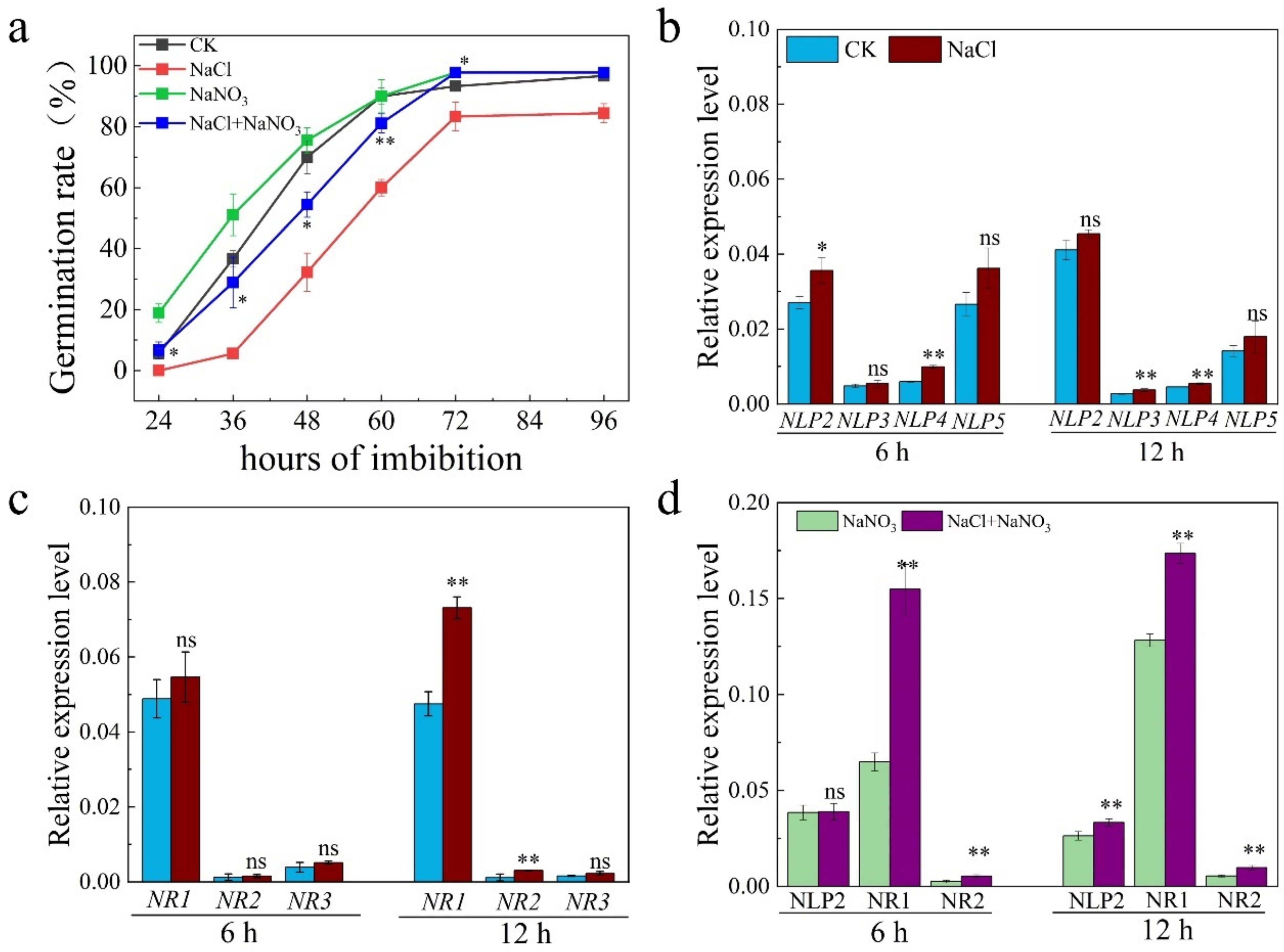
2.1. NR-Associated NO Is Involved in Enhancing Seed Germination under Salt Stress
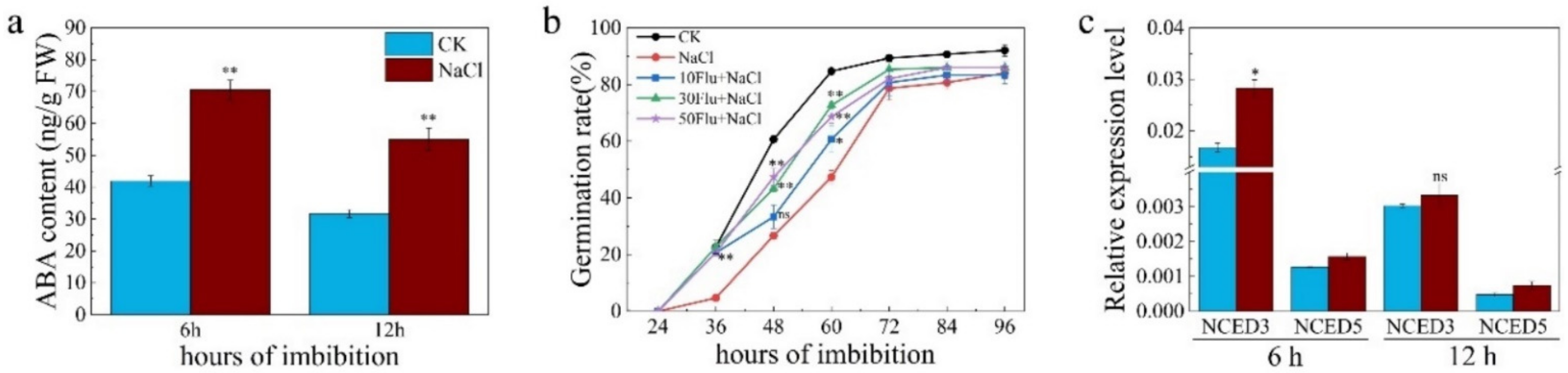
2.2. NR-Associated NO Increased Salt Tolerance by Promoting Expression of ABA Catabolism Genes during Seed Germination under Salt Stress
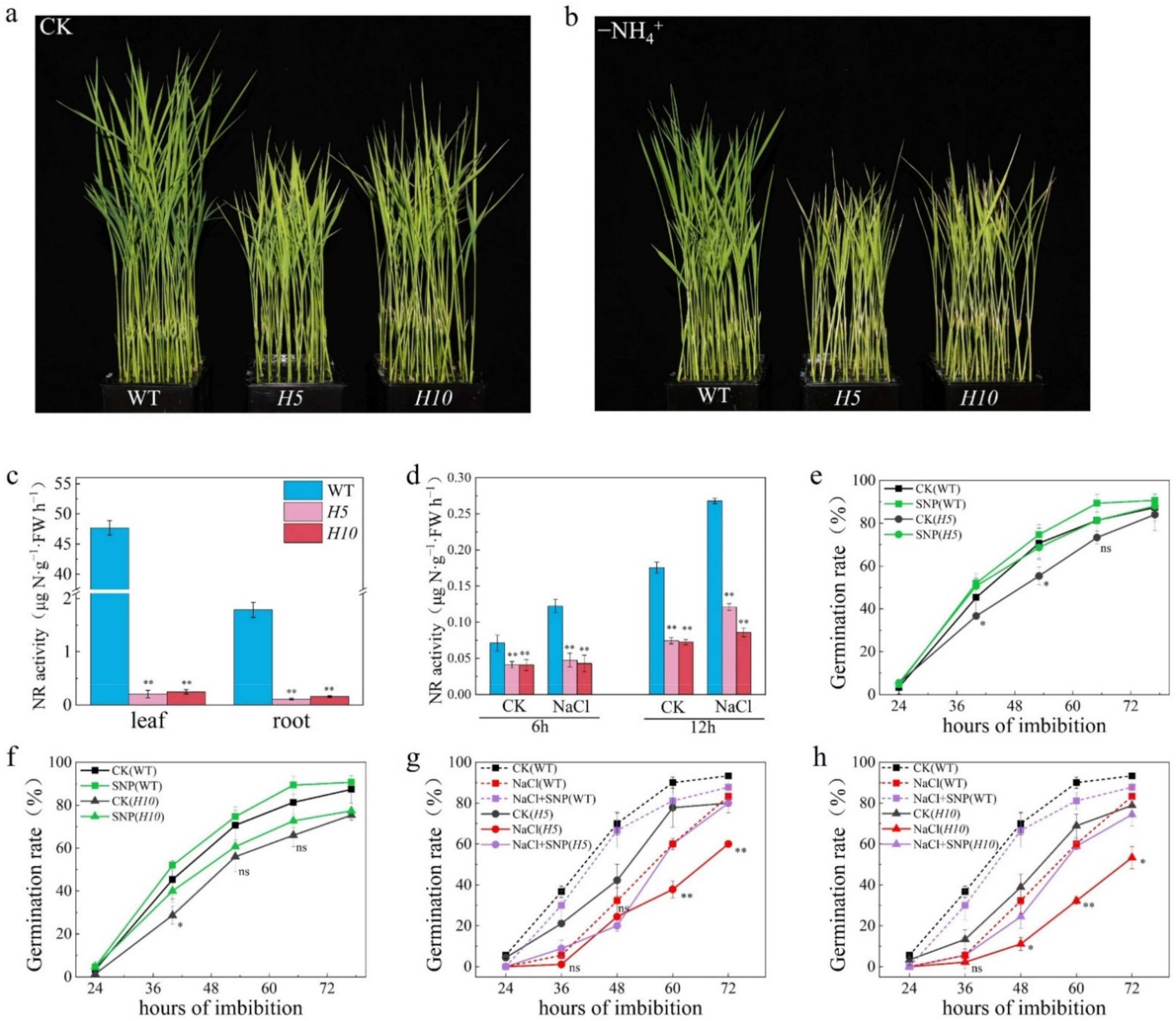
2.3. OsNLP2 Was Induced by Salt Stress to Upregulate Expression of OsNRs and Therefore Enhance the NR Activity
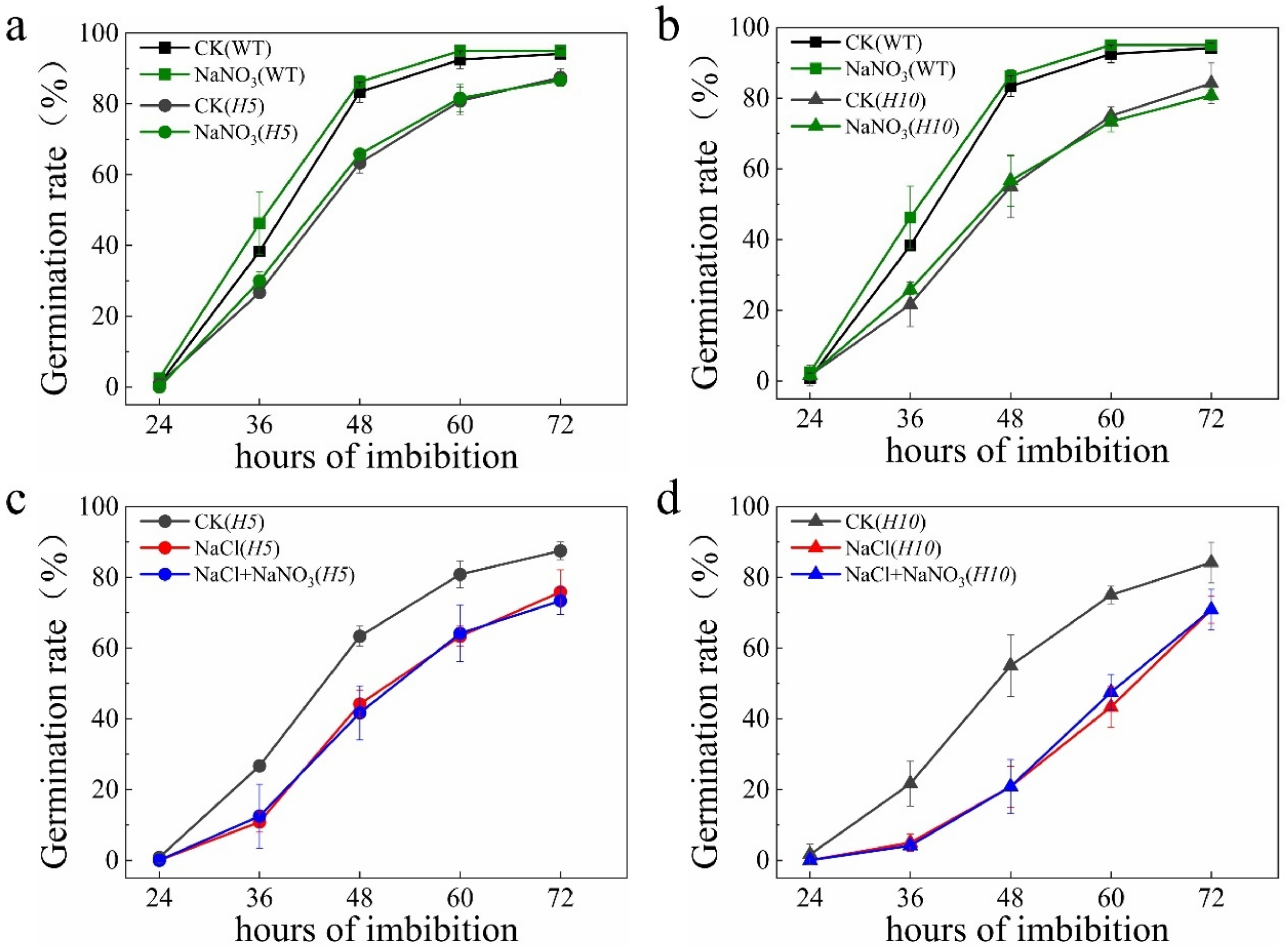
2.4. Nitrate Promoted Seed Germination under Salt Stress Was Partially Blocked in ABA Catabolism Mutants
3. Discussion
3.1. NR-Associated NO Plays a Key Role in Regulating Seed Germination and Salt Tolerance
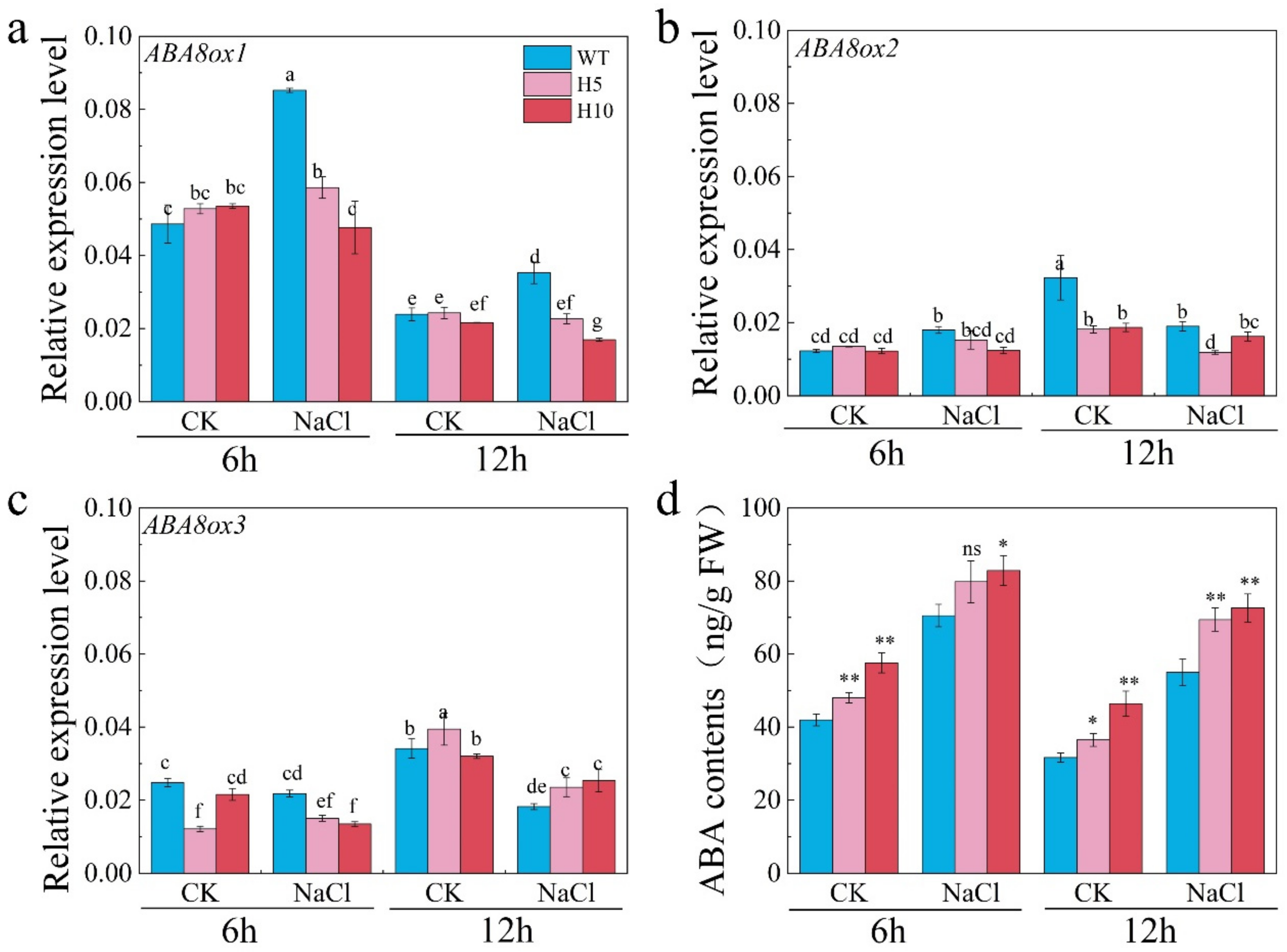
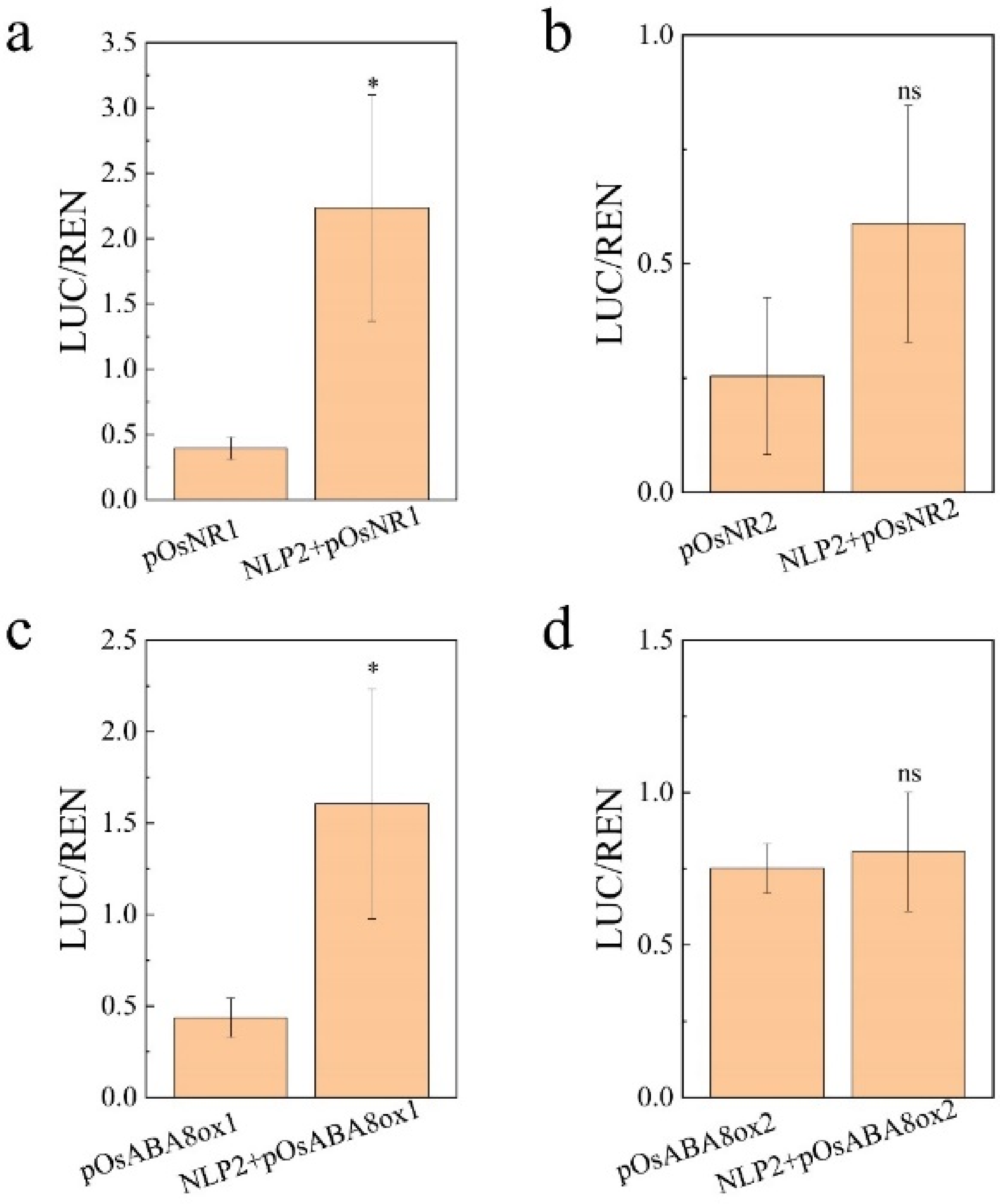
3.2. OsNLP2 Was Involved in Salt Defense by Up-Regulating Expression of Both OsNR1 and OsABA8ox1 Genes
4. Materials and Methods
4.1. Plant Materials and Germination Condition
4.2. RNA Isolation and Quantitative Real-Time PCR
4.3. NR Enzyme Activity Assay
4.4. Detection of ABA Content
4.5. Analysis of Luciferase In Vivo
4.6. Statistical Analysis
4.7. Accession Numbers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.J.L.; Awlia, M.; Al-Tamimi, N.; Saade, S.; Pailles, Y.; Negrão, S.; Tester, M. Salt stress under the scalpel-dissecting the genetics of salt tolerance. Plant J. 2019, 97, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.J.; Xiong, L.Z. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Martinez-Atienza, J.; Jiang, X.Y.; Garciadeblas, B.; Mendoza, I.; Zhu, J.K.; Pardo, J.M.; Quintero, F.J. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 2007, 143, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, B.; Liu, L.; Duan, W.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Li, G.; et al. Melatonin promotes seed germination under salt stress by regulating ABA and GA3 in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2021, 162, 506–516. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2021, 1, 25. [Google Scholar] [CrossRef]
- Ye, N.; Jia, L.; Zhang, J. ABA signal in rice under stress conditions. Rice 2012, 5, 1. [Google Scholar] [CrossRef]
- Shu, K.; Qi, Y.; Chen, F.; Meng, Y.; Luo, X.; Shuai, H.; Zhou, W.; Ding, J.; Du, J.; Liu, J.; et al. Salt stress represses soybean seed germination by negatively regulating GA biosynthesis while positively mediating ABA biosynthesis. Front. Plant Sci. 2017, 8, 1372. [Google Scholar] [CrossRef]
- González-Guzmán, M.; Apostolova, N.; Bellés, J.M.; Barrero, J.M.; Piqueras, P.; Ponce, M.R.; Micol, J.L.; Serrano, R.; Rodríguez, P.L. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 2002, 14, 1833–1846. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, J.; Zhang, J.; Ye, N.; Zhang, H.; Tan, M.; Jiang, M. Nitric oxide mediates brassinosteroid-induced ABA biosynthesis involved in oxidative stress tolerance in maize leaves. Plant Cell Physiol. 2011, 52, 181–192. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Fujita, M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci. 2012, 6, 1314–1323. [Google Scholar]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 2016, 126, 245–255. [Google Scholar] [CrossRef]
- Costa-Broseta, Á.; Perea-Resa, C.; Castillo, M.C.; Ruíz, M.F.; Salinas, J.; León, J. Nitric oxide controls constitutive freezing tolerance in Arabidopsis by attenuating the levels of osmoprotectants, stress-related hormones and anthocyanins. Sci. Rep. 2018, 8, 9268. [Google Scholar] [CrossRef]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the origins of nitric oxide. Trends Plant Sci. 2011, 16, 160–168. [Google Scholar] [CrossRef]
- Lozano-Juste, J.; León, J. Enhanced abscisic acid-mediated responses in nia1nia2noa1-2 triple mutant impaired in NIA/NR-and AtNOA1-dependent nitric oxide biosynthesis in Arabidopsis. Plant Physiol. 2010, 152, 891–903. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.B.; Mahmud, J.A.; Baluska, F.; Fujita, M. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Liu, M.; Zhi, X.; Wang, Y.; Wang, Y. Genome-wide survey and expression analysis of NIN-like Protein (NLP) genes reveals its potential roles in the response to nitrate signaling in tomato. BMC Plant Biol. 2021, 21, 347. [Google Scholar] [CrossRef]
- Hayat, S.; Yadav, S.; Wani, A.S.; Irfan, M.; Alyemini, M.N.; Ahmad, A. Impact of sodium nitroprusside on nitrate reductase, proline content, and antioxidant system in tomato under salinity stress. Hortic. Environ. Biotechnol. 2012, 53, 362–367. [Google Scholar] [CrossRef]
- Marvasi, M. Potential use and perspectives of nitric oxide donors in agriculture. J. Sci. Food Agric. 2017, 97, 1065–1072. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, W.; He, J.; Zhang, L.; Wei, Y.; Yang, M. Nitric oxide alleviates salt stress in seed germination and early seedling growth of pakchoi (Brassica chinensis L.) by enhancing physiological and biochemical parameters. Ecotoxicol. Environ. Saf. 2020, 187, 109785. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Yang, J.L.; Qin, C.; Jin, C.W.; Mo, J.H.; Ye, T.; Zheng, S.J. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol. 2010, 154, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Manganaris, G.A.; Fotopoulos, V. Systemic mitigation of salt stress by hydrogen peroxide and sodium nitroprusside in strawberry plants via transcriptional regulation of enzymatic and non-enzymatic antioxidants. Environ. Exp. Bot. 2014, 107, 46–54. [Google Scholar] [CrossRef]
- Ahmad, P.; Latef, A.A.; Hashem, A.; Abd_Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Li, X.; Zhang, H.; Zhang, G.; Liu, Y.; Zhou, Y.; Xia, X.; Chen, Z.; Yu, J. Guard cell hydrogen peroxide and nitric oxide mediate elevated CO2-induced stomatal movement in tomato. New Phytol. 2015, 208, 342–353. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen peroxide signaling in plant development and abiotic responses: Crosstalk with nitric oxide and calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Ye, N.; Liu, R.; Jia, W.; Zhang, J. Nitric oxide-induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Phytol. 2009, 183, 1030–1042. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055. [Google Scholar] [CrossRef]
- Penfield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, 874–878. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Alboresi, A.; Gestin, C.; Leydecker, M.T.; Bedu, M.; Meyer, C.; Truong, H.N. Nitrate, a signal relieving seed dormancy in Arabidopsis. Plant Cell Environ. 2005, 28, 500–512. [Google Scholar] [CrossRef]
- Yan, D.; Easwaran, V.; Chau, V.; Okamoto, M.; Ierullo, M.; Kimura, M.; Endo, A.; Yano, R.; Pasha, A.; Gong, Y.; et al. NIN-like protein 8 is a master regulator of nitrate-promoted seed germination in Arabidopsis. Nat. Commun. 2016, 7, 13179. [Google Scholar] [CrossRef]
- Yu, J.; Xuan, W.; Tian, Y.; Fan, L.; Sun, J.; Tang, W.; Chen, G.; Wang, B.; Liu, Y.; Wu, W.; et al. Enhanced OsNLP4-OsNiR cascade confers nitrogen use efficiency by promoting tiller number in rice. Plant Biotechnol. J. 2021, 19, 167–176. [Google Scholar] [CrossRef]
- Alfatih, A.; Wu, J.; Zhang, Z.S.; Xia, J.Q.; Jan, S.U.; Yu, L.H.; Xiang, C.B. Rice NIN-LIKE PROTEIN 1 rapidly responds to nitrogen deficiency and improves yield and nitrogen use efficiency. J. Exp. Bot. 2020, 71, 6032–6042. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Xia, J.Q.; Alfatih, A.; Song, Y.; Huang, Y.J.; Sun, L.Q.; Wan, G.Y.; Wang, S.M.; Wang, Y.P.; Hu, B.H.; et al. Rice NIN-LIKE PROTEIN 3 plays a significant role in nitrogen use efficiency and grain yield under nitrate-sufficient conditions. BioRxiv 2021. [Google Scholar] [CrossRef]
- Bethke, P.C.; Libourel, I.G.; Jones, R.L. Nitric oxide reduces seed dormancy in Arabidopsis. J. Exp. Bot. 2006, 57, 517–526. [Google Scholar] [CrossRef]
- Vidal, A.; Cantabella, D.; Bernal-Vicente, A.; Díaz-Vivancos, P.; Hernández, J.A. Nitrate-and nitric oxide-induced plant growth in pea seedlings is linked to antioxidative metabolism and the ABA/GA balance. J. Plant Physiol. 2018, 230, 13–20. [Google Scholar] [CrossRef]
- Kataria, S.; Jain, M.; Tripathi, D.K.; Singh, V.P. Involvement of nitrate reductase-dependent nitric oxide production in magnetopriming-induced salt tolerance in soybean. Physiol. Plant. 2020, 168, 422–436. [Google Scholar] [CrossRef]
- Reda, M.; Golicka, A.; Kabała, K.; Janicka, M. Involvement of NR and PM-NR in NO biosynthesis in cucumber plants subjected to salt stress. Plant Sci. 2018, 267, 55–64. [Google Scholar] [CrossRef]
- Luo, X.; Dai, Y.; Zheng, C.; Yang, Y.; Chen, W.; Wang, Q.; Chandrasekaran, U.; Du, J.; Liu, W.; Shu, K. The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol. 2021, 229, 950–962. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef]
- Silva, N.C.; de Souza, G.A.; Pimenta, T.M.; Brito, F.A.; Picoli, E.A.; Zsögön, A.; Ribeiro, D.M. Salt stress inhibits germination of Stylosanthes humilis seeds through abscisic acid accumulation and associated changes in ethylene production. Plant Physiol. Biochem. 2018, 130, 399–407. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.S.; Xia, J.Q.; Alfatih, A.; Song, Y.; Huang, Y.J.; Wan, G.Y.; Sun, L.Q.; Tang, H.; Liu, Y.; et al. Rice NIN-LIKE PROTEIN 4 plays a pivotal role in nitrogen use efficiency. Plant Biotechnol. J. 2021, 19, 448–461. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.G.; Yun, B.W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Adamu, T.A.; Mun, B.G.; Lee, S.U.; Hussain, A.; Yun, B.W. Exogenously applied nitric oxide enhances salt tolerance in rice (Oryza sativa L.) at seedling stage. Agronomy 2018, 8, 276. [Google Scholar] [CrossRef]
- Fancy, N.N.; Bahlmann, A.K.; Loake, G.J. Nitric oxide function in plant abiotic stress. Plant Cell Environ. 2017, 40, 462–472. [Google Scholar] [CrossRef]
- Egbichi, I.; Keyster, M.; Ludidi, N. Effect of exogenous application of nitric oxide on salt stress responses of soybean. S. Afr. J. Bot. 2014, 90, 131–136. [Google Scholar] [CrossRef]
- He, J.; Ren, Y.; Chen, X.; Chen, H. Protective roles of nitric oxide on seed germination and seedling growth of rice (Oryza sativa L.) under cadmium stress. Ecotoxicol. Environ. Saf. 2014, 108, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Daud, M.K.; Haider, M.Z.; Ali, S.; Rizwan, M.; Aslam, N.; Noman, A.; Iqbal, N.; Shahzad, F.; Deeba, F.; et al. Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol. Biochem. 2017, 119, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef]
- Tejada-Jimenez, M.; Llamas, A.; Galván, A.; Fernández, E. Role of nitrate reductase in NO production in photosynthetic eukaryotes. Plants 2019, 8, 56. [Google Scholar] [CrossRef]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- Sang, J.; Jiang, M.; Lin, F.; Xu, S.; Zhang, A.; Tan, M. Nitric oxide reduces hydrogen peroxide accumulation involved in water stress-induced subcellular antioxidant defense in maize plants. J. Integr. Plant Biol. 2008, 50, 231–243. [Google Scholar] [CrossRef]
- Konishi, M.; Yanagisawa, S. Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nat. Commun. 2013, 4, 1617. [Google Scholar] [CrossRef]
- Liu, K.H.; Niu, Y.; Konishi, M.; Wu, Y.; Du, H.; Chuang, H.S.; Li, L.; Boudsocq, M.; McCormack, M.; Maekawa, S.; et al. Discovery of nitrate–CPK–NLP signalling in central nutrient–growth networks. Nature 2017, 545, 311–316. [Google Scholar] [CrossRef]
- Duermeyer, L.; Khodapanahi, E.; Yan, D.; Krapp, A.; Rothstein, S.J.; Nambara, E. Regulation of seed dormancy and germination by nitrate. Seed Sci. Res. 2018, 28, 150–157. [Google Scholar] [CrossRef]
- Matakiadis, T.; Alboresi, A.; Jikumaru, Y.; Tatematsu, K.; Pichon, O.; Renou, J.P.; Kamiya, Y.; Nambara, E.; Truong, H.N. The Arabidopsis abscisic acid catabolic gene CYP707A2 plays a key role in nitrate control of seed dormancy. Plant Physiol. 2009, 149, 949–960. [Google Scholar] [CrossRef]
- Scholl, R.L.; Harper, J.E.; Hageman, R.H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974, 53, 825–828. [Google Scholar] [CrossRef][Green Version]
- Wang, G.Q.; Li, H.X.; Feng, L.; Chen, M.X.; Meng, S.; Ye, N.H.; Zhang, J.H. Transcriptomic analysis of grain filling in rice inferior grains under moderate soil drying. J. Exp. Bot. 2019, 70, 1597–1611. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.; Peng, Y.; Song, T.; Lu, S.; Teng, Z.; Zheng, Q.; Zhao, F.; Meng, S.; Liu, B.; Peng, Y.; et al. NLP2-NR Module Associated NO Is Involved in Regulating Seed Germination in Rice under Salt Stress. Plants 2022, 11, 795. https://doi.org/10.3390/plants11060795
Yi Y, Peng Y, Song T, Lu S, Teng Z, Zheng Q, Zhao F, Meng S, Liu B, Peng Y, et al. NLP2-NR Module Associated NO Is Involved in Regulating Seed Germination in Rice under Salt Stress. Plants. 2022; 11(6):795. https://doi.org/10.3390/plants11060795
Chicago/Turabian StyleYi, Yake, Yaqiong Peng, Tao Song, Siqiong Lu, Zhenning Teng, Qin Zheng, Fankai Zhao, Shuan Meng, Bohang Liu, Yan Peng, and et al. 2022. "NLP2-NR Module Associated NO Is Involved in Regulating Seed Germination in Rice under Salt Stress" Plants 11, no. 6: 795. https://doi.org/10.3390/plants11060795
APA StyleYi, Y., Peng, Y., Song, T., Lu, S., Teng, Z., Zheng, Q., Zhao, F., Meng, S., Liu, B., Peng, Y., Chen, G., Zhang, J., & Ye, N. (2022). NLP2-NR Module Associated NO Is Involved in Regulating Seed Germination in Rice under Salt Stress. Plants, 11(6), 795. https://doi.org/10.3390/plants11060795







