The Halophyte Species Solanum chilense Dun. Maintains Its Reproduction despite Sodium Accumulation in Its Floral Organs
Abstract
1. Introduction
2. Results
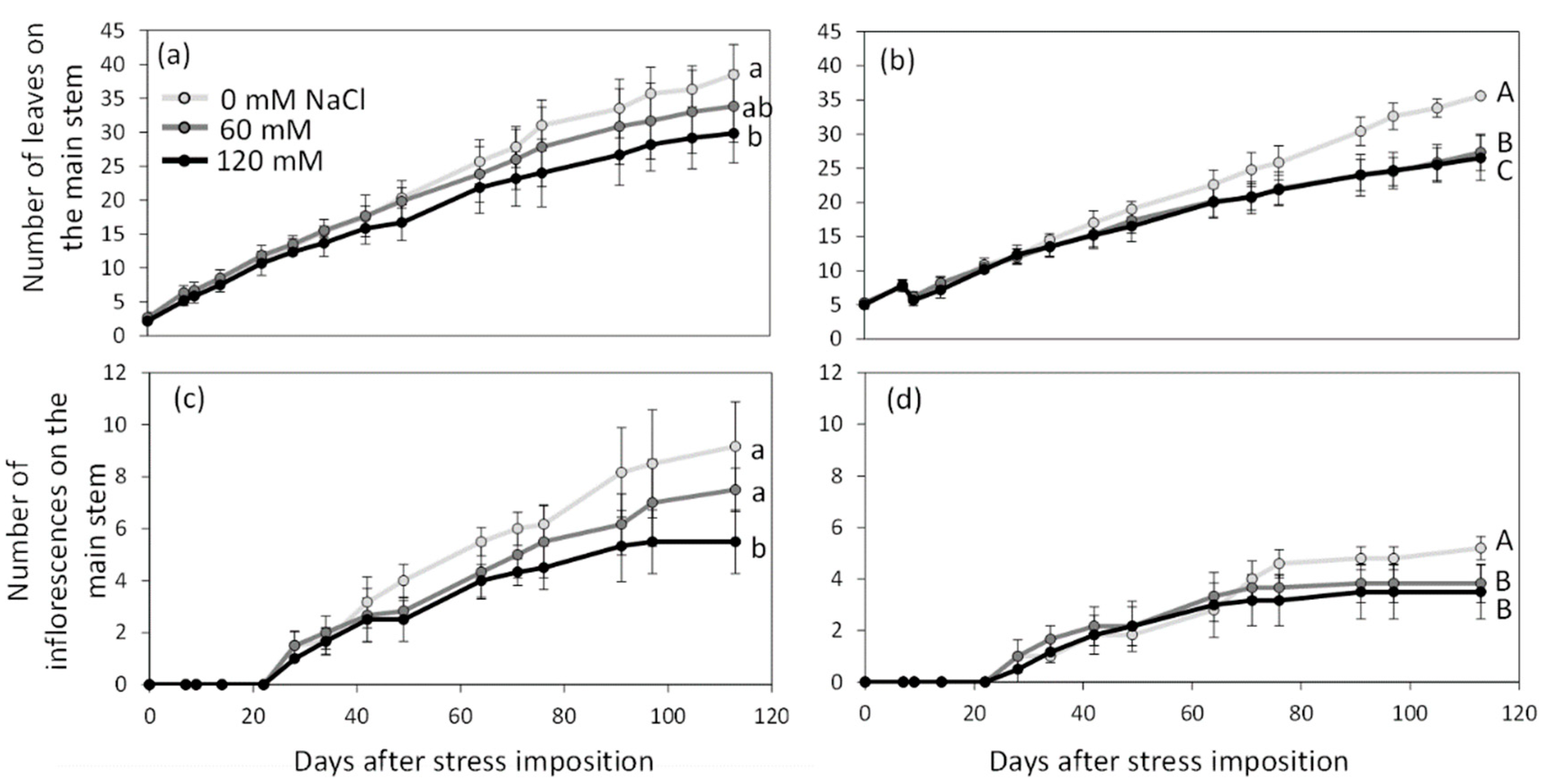
2.1. Impact of Salinity on Reproductive Growth
2.2. Impact of Salinity on Flower Morphology and Fertility
2.3. Impact of Salinity on Fruit Production and Quality
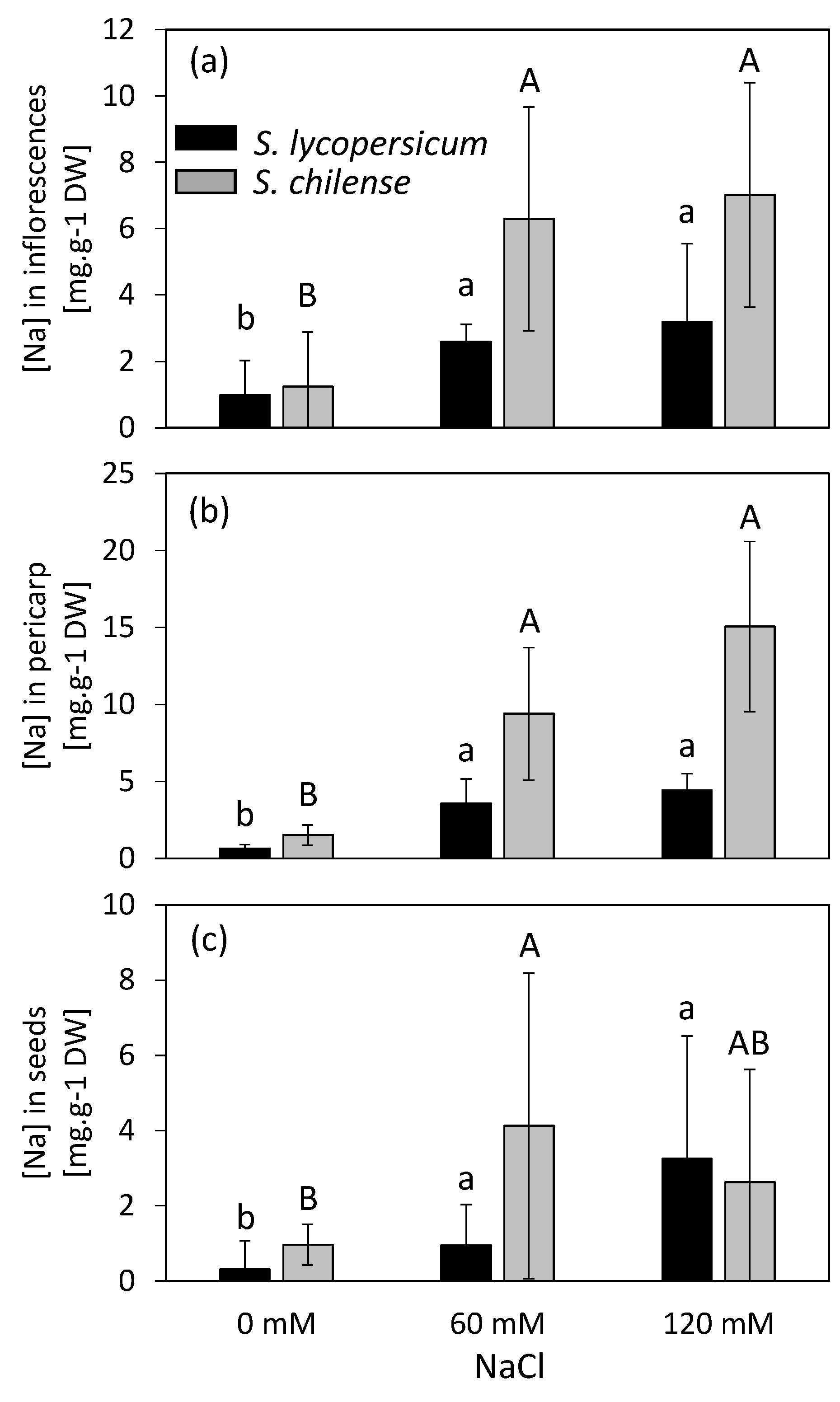
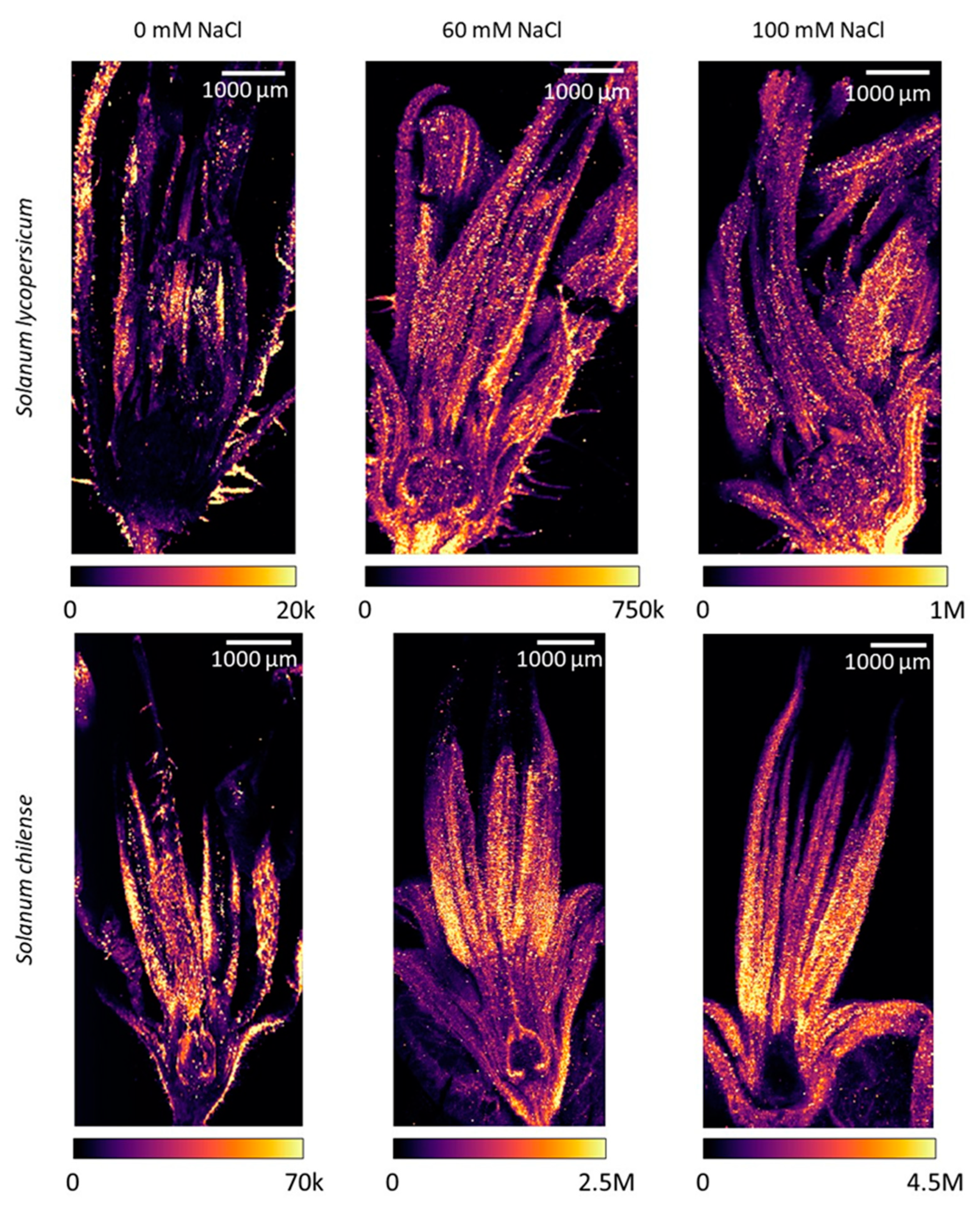
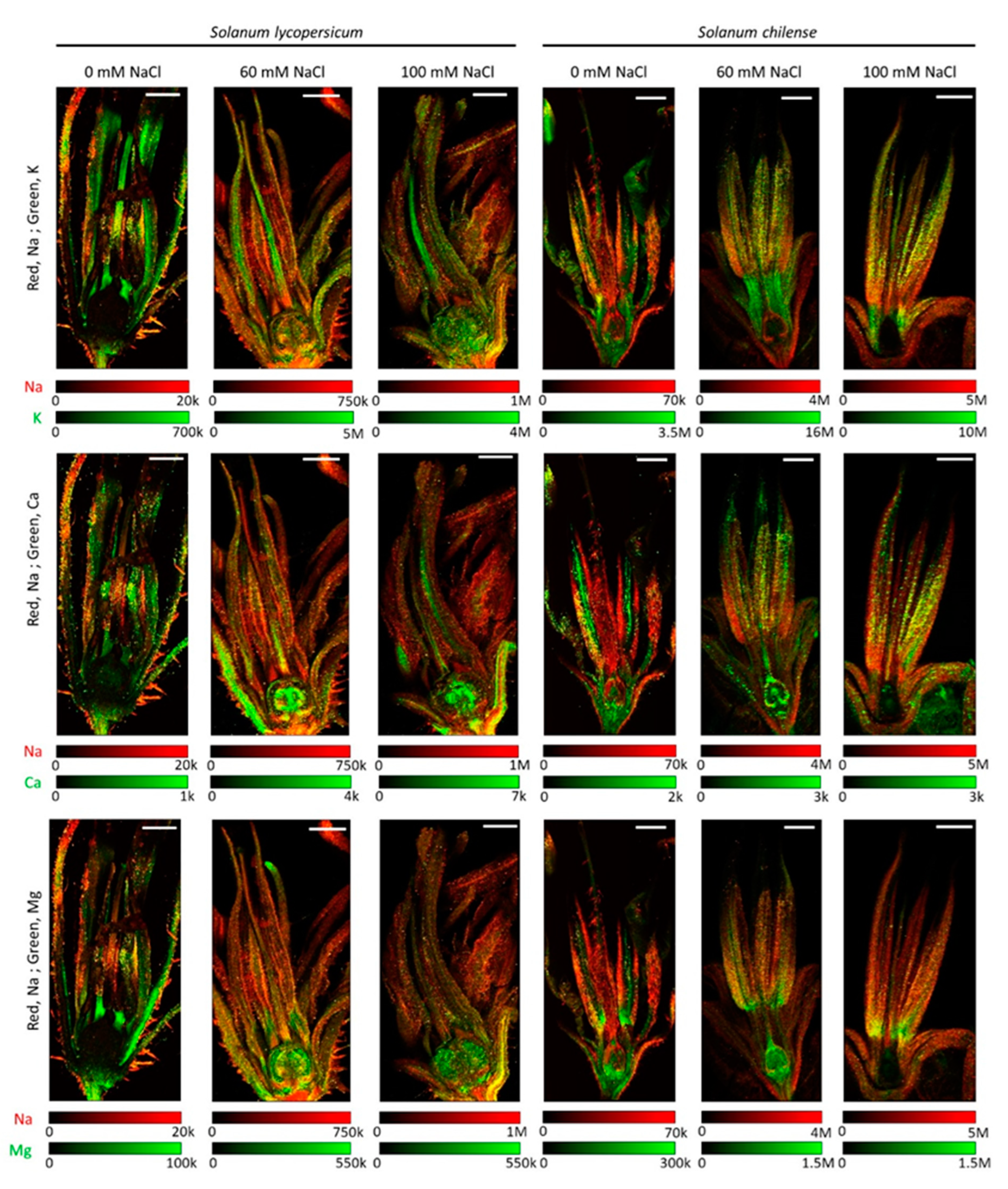
2.4. Impact of Salinity on Mineral Concentration and Distribution in Reproductive Organs
2.4.1. Inflorescences and Flowers
2.4.2. Fruits and Seeds
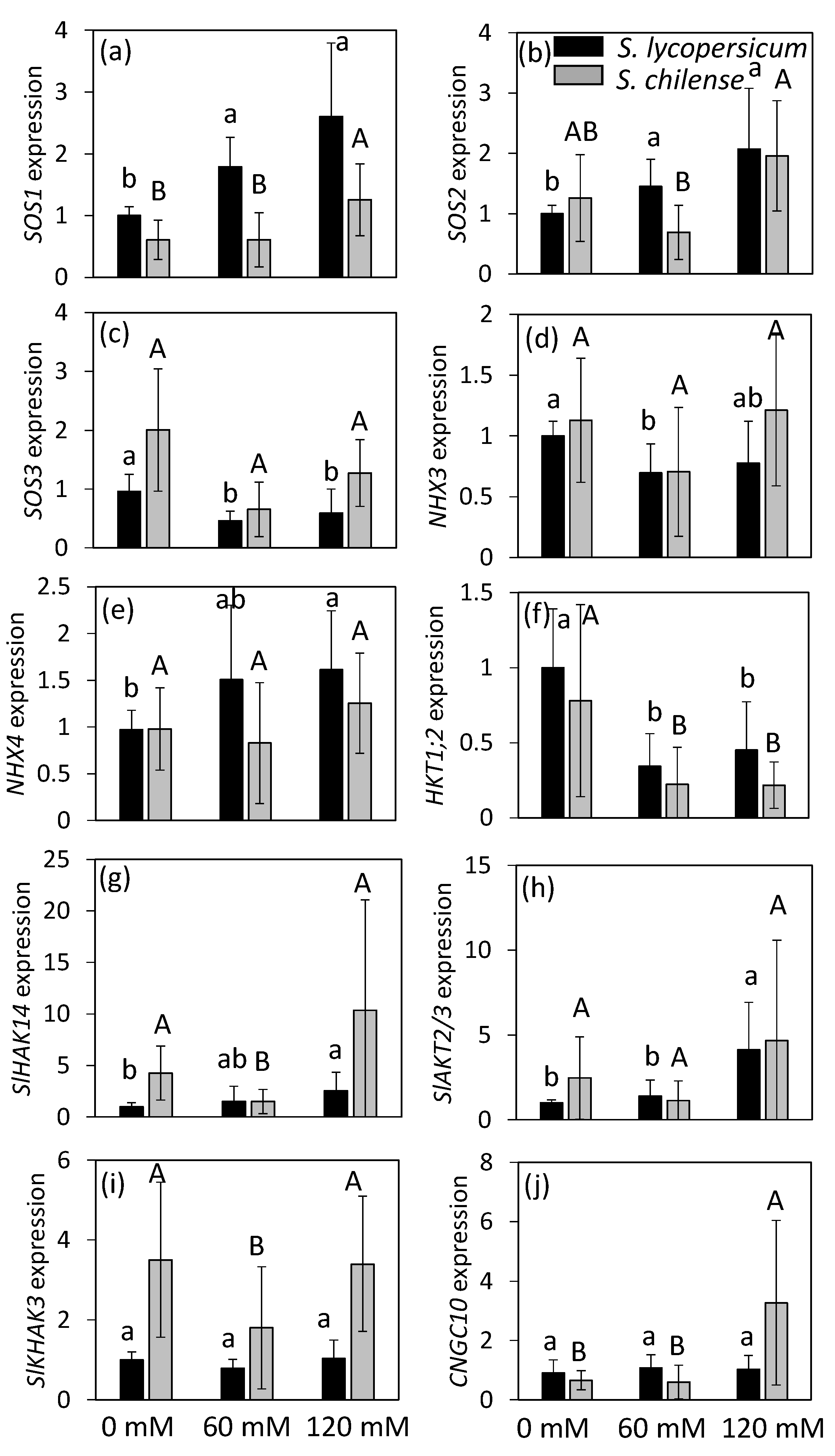
2.5. Impact of Salinity on the Expression of Mineral Transporters in Flowers
2.6. Correlations among Flower Morphology, Mineral Concentrations, and Gene Expression
3. Discussion
3.1. Salinity Affects Reproductive Structures in Both Species
3.2. Salinity Affects Mineral Accumulation and Distribution Which May Affect Fertility
3.3. Mineral Transporters Are Involved in Na Accumulation and Partitioning in the Reproductive Structures
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Growth
4.3. Flower Fertility
4.4. Fruit Parameters
4.5. Mineral Elements Concentrations and Element Distribution
4.6. Transporters Expression Analysis by qRT-PCR
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOStats Tomato Yields and Cultivated Area of Tomato in the World. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 28 January 2022).
- Gharbi, E.; Martínez, J.-P.; Benahmed, H.; Fauconnier, M.-L.; Lutts, S.; Quinet, M. Salicylic Acid Differently Impacts Ethylene and Polyamine Synthesis in the Glycophyte Solanum lycopersicum and the Wild-Related Halophyte Solanum chilense Exposed to Mild Salt Stress. Physiol. Plant. 2016, 158, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Prasad, P.V.V.; Reddy, K.R. Impacts of Changing Climate and Climate Variability on Seed Production and Seed Industry. In Advances in Agronomy; Elsevier: Rome, Italy, 2013; Volume 118, pp. 49–110. ISBN 978-0-12-405942-9. [Google Scholar]
- Dudley, L. Salinity in the Soil Environment. In Handbook of Plant and Crop Stress; Marcel Dekker, Inc.: New York, NY, USA, 1994; pp. 13–30. [Google Scholar]
- Campos, C.A.B.; Fernandes, P.D.; Gheyi, H.R.; Blanco, F.F.; Gonçalves, C.B.; Campos, S.A.F. Yield and Fruit Quality of Industrial Tomato under Saline Irrigation. Sci. Agric. 2006, 63, 146–152. [Google Scholar] [CrossRef]
- Gerszberg, A.; Hnatuszko-Konka, K. Tomato Tolerance to Abiotic Stress: A Review of Most Often Engineered Target Sequences. Plant Growth Regul. 2017, 83, 175–198. [Google Scholar] [CrossRef]
- Bai, Y.; Lindhout, P. Domestication and Breeding of Tomatoes: What Have We Gained and What Can We Gain in the Future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef]
- Diouf, I.A.; Derivot, L.; Bitton, F.; Pascual, L.; Causse, M. Water Deficit and Salinity Stress Reveal Many Specific QTL for Plant Growth and Fruit Quality Traits in Tomato. Front. Plant Sci. 2018, 9, 279. [Google Scholar] [CrossRef]
- Rick, C.M.; Chetelat, R.T. Utilization of Related Wild Species for Tomato Improvement. Acta Hortic. 1995, 412, 21–38. [Google Scholar] [CrossRef]
- Miller, J.C.; Tanksley, S.D. RFLP Analysis of Phylogenetic Relationships and Genetic Variation in the Genus Lycopersicon. Theor. Appl. Genet. 1990, 80, 437–448. [Google Scholar] [CrossRef]
- Bretó, M.P.; Asins, M.J.; Carbonell, E.A. Genetic Variability in Lycopersicon Species and Their Genetic Relationships. Theor. Appl. Genet. 1993, 86, 113–120. [Google Scholar] [CrossRef]
- Asamizu, E.; Ezura, H. Inclusion of Tomato in the Genus Solanum as “Solanum lycopersicum” Is Evident from Phylogenetic Studies. J. Jpn. Soc. Hortic. Sci. 2009, 78, 3–5. [Google Scholar] [CrossRef]
- Peralta, I.E.; Spooner, D.M.; Knapp, S. Taxonomy of Wild Tomatoes and Their Relatives (Solanum Sect. Lycopersicoides, Sect. Juglandifolia, Sect. Lycopersicon; Solanaceae). Syst. Bot. Monogr. 2008, 84, 186. [Google Scholar]
- Dwivedi, S.L.; Scheben, A.; Edwards, D.; Spillane, C.; Ortiz, R. Assessing and Exploiting Functional Diversity in Germplasm Pools to Enhance Abiotic Stress Adaptation and Yield in Cereals and Food Legumes. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Houston, J. Variability of Precipitation in the Atacama Desert: Its Causes and Hydrological Impact. Int. J. Climatol. 2006, 26, 2181–2198. [Google Scholar] [CrossRef]
- Chetelat, R.T.; Pertuzé, R.A.; Faúndez, L.; Graham, E.B.; Jones, C.M. Distribution, Ecology and Reproductive Biology of Wild Tomatoes and Related Nightshades from the Atacama Desert Region of Northern Chile. Euphytica 2009, 167, 77–93. [Google Scholar] [CrossRef]
- Nakazato, T.; Warren, D.L.; Moyle, L.C. Ecological and Geographic Modes of Species Divergence in Wild Tomatoes. Am. J. Bot. 2010, 97, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Böndel, K.B.; Nosenko, T.; Stephan, W. Signatures of Natural Selection in Abiotic Stress-Responsive Genes of Solanum chilense. R. Soc. Open Sci. 2018, 5, 171198. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Scott, J.W.; Schuster, D.J.; Maxwell, D.P. Molecular Mapping of Ty-4, a New Tomato Yellow Leaf Curl Virus Resistance Locus on Chromosome 3 of Tomato. J. Am. Soc. Hortic. Sci. 2009, 134, 281–288. [Google Scholar] [CrossRef]
- Stamova, B.S.; Chetelat, R.T. Inheritance and Genetic Mapping of Cucumber Mosaic Virus Resistance Introgressed from Lycopersicon chilense into Tomato. Theor. Appl. Genet. 2000, 101, 527–537. [Google Scholar] [CrossRef]
- Martínez, J.-P.; Antúnez, A.; Pertuzé, R.; Acosta, M.D.P.; Palma, X.; Fuentes, L.; Ayala, A.; Araya, H.; Lutts, S. Effects of Saline Water on Water Status, Yield and Fruit Quality of Wild (Solanum chilense) and Domesticated (Solanum lycopersicum Var. Cerasiforme) Tomatoes. Exp. Agric. 2012, 48, 573–586. [Google Scholar] [CrossRef]
- Bolarín, M.C.; Pérez-Alfocea, F.; Cano, E.A.; Estañ, M.T.; Caro, M. Growth, Fruit Yield, and Ion Concentration in Tomato Genotypes after Pre- and Post-Emergence Salt Treatments. J. Am. Soc. Hortic. Sci. 1993, 118, 655–660. [Google Scholar] [CrossRef]
- Tapia, G.; Méndez, J.; Inostroza, L. Different Combinations of Morpho-Physiological Traits Are Responsible for Tolerance to Drought in Wild Tomatoes Solanum chilense and Solanum peruvianum. Plant Biol. 2016, 18, 406–416. [Google Scholar] [CrossRef]
- Samineni, S.; Siddique, K.H.M.; Gaur, P.M.; Colmer, T.D. Salt Sensitivity of the Vegetative and Reproductive Stages in Chickpea (Cicer arietinum L.): Podding Is a Particularly Sensitive Stage. Environ. Exp. Bot. 2011, 71, 260–268. [Google Scholar] [CrossRef]
- Romero-Aranda, M.R.; González-Fernández, P.; Pérez-Tienda, J.R.; López-Diaz, M.R.; Espinosa, J.; Granum, E.; Traverso, J.Á.; Pineda, B.; Garcia-Sogo, B.; Moreno, V.; et al. Na+ Transporter HKT1;2 Reduces Flower Na+ Content and Considerably Mitigates the Decline in Tomato Fruit Yields under Saline Conditions. Plant Physiol. Biochem. 2020, 154, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.P.; Fuentes, R.; Farías, K.; Lizana, C.; Alfaro, J.F.; Fuentes, L.; Calabrese, N.; Bigot, S.; Quinet, M.; Lutts, S. Effects of Salt Stress on Fruit Antioxidant Capacity of Wild (Solanum chilense) and Domesticated (Solanum lycopersicum Var. Cerasiforme) Tomatoes. Agronomy 2020, 10, 1481. [Google Scholar] [CrossRef]
- Delesalle, V.A.; Mazer, S.J. Nutrient Levels and Salinity Affect Gender and Floral Traits in the Autogamous Spergularia marina. Int. J. Plant Sci. 1996, 157, 621–631. [Google Scholar] [CrossRef]
- White, A.C.; Colmer, T.D.; Cawthray, G.R.; Hanley, M.E. Variable Response of Three Trifolium repens Ecotypes to Soil Flooding by Seawater. Ann. Bot. 2014, 114, 347–355. [Google Scholar] [CrossRef]
- Ghanem, M.E.; van Elteren, J.; Albacete, A.; Quinet, M.; Martínez-Andújar, C.; Kinet, J.-M.; Pérez-Alfocea, F.; Lutts, S. Impact of Salinity on Early Reproductive Physiology of Tomato (Solanum lycopersicum) in Relation to a Heterogeneous Distribution of Toxic Ions in Flower Organs. Funct. Plant Biol. 2009, 36, 125. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Chen, H.; Zhou, B. Genome-Wide Transcriptome Analysis Reveals the Molecular Mechanism of High Temperature-Induced Floral Abortion in Litchi chinensis. BMC Genom. 2019, 20, 127. [Google Scholar] [CrossRef]
- Shabala, S. Learning from Halophytes: Physiological Basis and Strategies to Improve Abiotic Stress Tolerance in Crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- Wu, H. Plant Salt Tolerance and Na+ Sensing and Transport. Crop J. 2018, 6, 215–225. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, Y.; Zhu, G.; Li, Y.; Niu, Q.; Yao, J.; Hua, K.; Bai, J.; Zhu, Y.; Shi, H.; et al. Loss of Salt Tolerance during Tomato Domestication Conferred by Variation in a Na+/K+ Transporter. EMBO J. 2020, 39, e103256. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Kumar, A.; Benazir, I.; Kumar, G. Reassessing the Role of Ion Homeostasis for Improving Salinity Tolerance in Crop Plants. Physiol. Plant. 2020, 174, 502–519. [Google Scholar] [CrossRef]
- Asins, M.J.; Raga, V.; Roca, D.; Belver, A.; Carbonell, E.A. Genetic Dissection of Tomato Rootstock Effects on Scion Traits under Moderate Salinity. Theor. Appl. Genet. 2015, 128, 667–679. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, T.; Bassil, E.; Zhang, S.; Blumwald, E. Vacuolar Na+/H+ NHX-Type Antiporters Are Required for Cellular K+ Homeostasis, Microtubule Organization and Directional Root Growth. Plants 2014, 3, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Bassil, E.; Zhang, S.; Gong, H.; Tajima, H.; Blumwald, E. Cation Specificity of Vacuolar NHX-Type Cation/H+ Antiporters. Plant Physiol. 2019, 179, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.; Fon, M.; Ródenas, R.; Nieves-Cordones, M.; Alemán, F.; Rivero, R.M.; Martínez, V. A Low K+ Signal Is Required for Functional High-Affinity K+ Uptake through HAK5 Transporters. Physiol. Plant. 2014, 152, 558–570. [Google Scholar] [CrossRef]
- Deeken, R.; Geiger, D.; Fromm, J.; Koroleva, O.; Ache, P.; Langenfeld-Heyser, R.; Sauer, N.; May, S.; Hedrich, R. Loss of the AKT2/3 Potassium Channel Affects Sugar Loading into the Phloem of Arabidopsis. Planta 2002, 216, 334–344. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. The Role of Monovalent Cation Transporters in Plant Responses to Salinity. J. Exp. Bot. 2006, 57, 1137–1147. [Google Scholar] [CrossRef]
- Quinet, M.; Kinet, J.-M. Transition to Flowering and Morphogenesis of Reproductive Structures in Tomato. Int. J. Plant Dev. Biol. 2007, 1, 64–74. [Google Scholar]
- Soyk, S.; Müller, N.A.; Park, S.J.; Schmalenbach, I.; Jiang, K.; Hayama, R.; Zhang, L.; Van Eck, J.; Jiménez-Gómez, J.M.; Lippman, Z.B. Variation in the Flowering Gene SELF PRUNING 5G Promotes Day-Neutrality and Early Yield in Tomato. Nat. Genet. 2017, 49, 162–168. [Google Scholar] [CrossRef]
- Welty, N.; Radovich, C.; Meulia, T.; van der Knaap, E. Inflorescence Development in Two Tomato Species. Can. J. Bot. 2007, 85, 111–118. [Google Scholar] [CrossRef][Green Version]
- Aref, F.; Rad, H.E. Physiological Characterization of Rice under Salinity Stress during Vegetative and Reproductive Stages. Indian J. Sci. Technol. 2012, 5, 10. [Google Scholar] [CrossRef]
- Lozano, R.; Angosto, T.; Gómez, P.; Payán, C.; Capel, J.; Huijser, P.; Salinas, J.; Martínez-Zapater, J.M. Tomato Flower Abnormalities Induced by Low Temperatures Are Associated with Changes of Expression of MADS-Box Genes. Plant Physiol. 1998, 117, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Giorno, F.; Wolters-Arts, M.; Mariani, C.; Rieu, I. Ensuring Reproduction at High Temperatures: The Heat Stress Response during Anther and Pollen Development. Plants 2013, 2, 489–506. [Google Scholar] [CrossRef]
- Stanton, M.L.; Preston, R.E. Ecological Consequences and Phenotypic Correlates of Petal Size Variation in Wild Radish, Raphanus sativus (Brassicaceae). Am. J. Bot. 1988, 75, 528–539. [Google Scholar] [CrossRef]
- Descamps, C.; Boubnan, N.; Jacquemart, A.-L.; Quinet, M. Growing and Flowering in a Changing Climate: Effects of Higher Temperatures and Drought Stress on the Bee-Pollinated Species Impatiens glandulifera Royle. Plants 2021, 10, 988. [Google Scholar] [CrossRef]
- Descamps, C.; Quinet, M.; Baijot, A.; Jacquemart, A.-L. Temperature and Water Stress Affect Plant-Pollinator Interactions in Borago officinalis (Boraginaceae). Ecol. Evol. 2018, 8, 3443–3456. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, M.; Xu, W.; Wang, Y.; Huang, K.; Zhang, C.; Wen, J. Understanding the Molecular Mechanism of Anther Development under Abiotic Stresses. Plant Mol. Biol. 2021, 105, 1–10. [Google Scholar] [CrossRef]
- Xu, J.; Wolters-Arts, M.; Mariani, C.; Huber, H.; Rieu, I. Heat Stress Affects Vegetative and Reproductive Performance and Trait Correlations in Tomato (Solanum lycopersicum). Euphytica 2017, 213, 156. [Google Scholar] [CrossRef]
- Pan, C.; Yang, D.; Zhao, X.; Jiao, C.; Yan, Y.; Lamin-Samu, A.T.; Wang, Q.; Xu, X.; Fei, Z.; Lu, G. Tomato Stigma Exsertion Induced by High Temperature Is Associated with the Jasmonate Signalling Pathway. Plant Cell Environ. 2019, 42, 1205–1221. [Google Scholar] [CrossRef]
- Baby, T.; Collins, C.; Tyerman, S.D.; Gilliham, M. Salinity Negatively Affects Pollen Tube Growth and Fruit Set in Grapevines and Is Not Mitigated by Silicon. Am. J. Enol. Vitic. 2016, 67, 218–228. [Google Scholar] [CrossRef]
- Herrera, C.M. Post-Floral Perianth Functionality: Contribution of Persistent Sepals to Seed Development in Helleborus foetidus (Ranunculaceae). Am. J. Bot. 2005, 92, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, S.E.; Smillie, R.M.; Davies, W.J. Photosynthetic Activities of Vegetative and Fruiting Tissues of Tomato. J. Exp. Bot. 1998, 49, 1173–1181. [Google Scholar] [CrossRef]
- Lytovchenko, A.; Eickmeier, I.; Pons, C.; Osorio, S.; Szecowka, M.; Lehmberg, K.; Arrivault, S.; Tohge, T.; Pineda, B.; Anton, M.T.; et al. Tomato Fruit Photosynthesis Is Seemingly Unimportant in Primary Metabolism and Ripening but Plays a Considerable Role in Seed Development. Plant Physiol. 2011, 157, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- El-Mogy, M.M.; Garchery, C.; Stevens, R. Irrigation with Salt Water Affects Growth, Yield, Fruit Quality, Storability and Marker-Gene Expression in Cherry Tomato. Acta Agric. Scand. Sect. B Soil Plant Sci. 2018, 68, 727–737. [Google Scholar] [CrossRef]
- Karapanos, I.C.; Mahmood, S.; Thanopoulos, C. Fruit Set in Solanaceous Vegetable Crops as Affected by Floral and Environmental Factors. Eur. J. Plant Sci. Biotechnol. 2008, 1, 88–105. [Google Scholar]
- Khatun, S.; Flowers, T.J. Effects of Salinity on Seed Set in Rice. Plant Cell Environ. 1995, 18, 61–67. [Google Scholar] [CrossRef]
- An, D.; Chen, J.-G.; Gao, Y.-Q.; Li, X.; Chao, Z.-F.; Chen, Z.-R.; Li, Q.-Q.; Han, M.-L.; Wang, Y.-L.; Wang, Y.-F.; et al. AtHKT1 Drives Adaptation of Arabidopsis thaliana to Salinity by Reducing Floral Sodium Content. PLoS Genet. 2017, 13, e1007086. [Google Scholar] [CrossRef]
- Vromman, D.; Lutts, S.; Lefèvre, I.; Somer, L.; De Vreese, O.; Šlejkovec, Z.; Quinet, M. Effects of Simultaneous Arsenic and Iron Toxicities on Rice (Oryza sativa L.) Development, Yield-Related Parameters and As and Fe Accumulation in Relation to As Speciation in the Grains. Plant Soil 2013, 371, 199–217. [Google Scholar] [CrossRef]
- Han, R.; Quinet, M.; André, E.; van Elteren, J.T.; Destrebecq, F.; Vogel-Mikuš, K.; Cui, G.; Debeljak, M.; Lefèvre, I.; Lutts, S. Accumulation and Distribution of Zn in the Shoots and Reproductive Structures of the Halophyte Plant Species Kosteletzkya virginica as a Function of Salinity. Planta 2013, 238, 441–457. [Google Scholar] [CrossRef]
- Gharbi, E.; Martínez, J.-P.; Benahmed, H.; Lepoint, G.; Vanpee, B.; Quinet, M.; Lutts, S. Inhibition of Ethylene Synthesis Reduces Salt-Tolerance in Tomato Wild Relative Species Solanum chilense. J. Plant Physiol. 2017, 210, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Bacha, H.; Tekaya, M.; Drine, S.; Guasmi, F.; Touil, L.; Enneb, H.; Triki, T.; Cheour, F.; Ferchichi, A. Impact of Salt Stress on Morpho-Physiological and Biochemical Parameters of Solanum lycopersicum Cv. Microtom Leaves. S. Afr. J. Bot. 2017, 108, 364–369. [Google Scholar] [CrossRef]
- Müller, F.; Xu, J.; Kristensen, L.; Wolters-Arts, M.; de Groot, P.F.M.; Jansma, S.Y.; Mariani, C.; Park, S.; Rieu, I. High-Temperature-Induced Defects in Tomato (Solanum lycopersicum) Anther and Pollen Development Are Associated with Reduced Expression of B-Class Floral Patterning Genes. PLoS ONE 2016, 11, e0167614. [Google Scholar] [CrossRef]
- Bassil, E.; Tajima, H.; Liang, Y.-C.; Ohto, M.; Ushijima, K.; Nakano, R.; Esumi, T.; Coku, A.; Belmonte, M.; Blumwald, E. The Arabidopsis Na+/H+ Antiporters NHX1 and NHX2 Control Vacuolar PH and K+ Homeostasis to Regulate Growth, Flower Development, and Reproduction. Plant Cell 2011, 23, 3482–3497. [Google Scholar] [CrossRef]
- Gharbi, E.; Martínez, J.-P.; Benahmed, H.; Hichri, I.; Dobrev, P.I.; Motyka, V.; Quinet, M.; Lutts, S. Phytohormone Profiling in Relation to Osmotic Adjustment in NaCl-Treated Plants of the Halophyte Tomato Wild Relative Species Solanum chilense Comparatively to the Cultivated Glycophyte Solanum lycopersicum. Plant Sci. 2017, 258, 77–89. [Google Scholar] [CrossRef]
- Wakeel, A.; Farooq, M.; Qadir, M.; Schubert, S. Potassium Substitution by Sodium in Plants. Crit. Rev. Plant Sci. 2011, 30, 401–413. [Google Scholar] [CrossRef]
- Hu, W.; Coomer, T.D.; Loka, D.A.; Oosterhuis, D.M.; Zhou, Z. Potassium Deficiency Affects the Carbon-Nitrogen Balance in Cotton Leaves. Plant Physiol. Biochem. 2017, 115, 408–417. [Google Scholar] [CrossRef]
- Rehman, S.; Yun, S.J. Developmental Regulation of K Accumulation in Pollen, Anthers, and Papillae: Are Anther Dehiscence, Papillae Hydration, and Pollen Swelling Leading to Pollination and Fertilization in Barley (Hordeum vulgare L.) Regulated by Changes in K Concentration? J. Exp. Bot. 2006, 57, 1315–1321. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Miller, A.J.; Luo, B.; Wang, M.; Zhu, Z.; Ouwerkerk, P.B.F. OsCHX14 Is Involved in the K+ Homeostasis in Rice (Oryza sativa) Flowers. Plant Cell Physiol. 2016, 57, 1530–1543. [Google Scholar] [CrossRef]
- Mel, V.C.; Bado, V.B.; Ndiaye, S.; Djaman, K.; Aissata Bama Nati, D.; Manneh, B.; Futakuchi, K. Predicting Rice Yield under Salinity Stress Using K/Na Ratio Variable in Plant Tissue. Commun. Soil Sci. Plant Anal. 2019, 50, 1321–1329. [Google Scholar] [CrossRef]
- Albaladejo, I.; Meco, V.; Plasencia, F.; Flores, F.B.; Bolarin, M.C.; Egea, I. Unravelling the Strategies Used by the Wild Tomato Species Solanum pennellii to Confront Salt Stress: From Leaf Anatomical Adaptations to Molecular Responses. Environ. Exp. Bot. 2017, 135, 1–12. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Roles and Transport of Sodium and Potassium in Plants. In The Alkali Metal Ions: Their Role for Life; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 16, pp. 291–324. ISBN 978-3-319-21755-0. [Google Scholar]
- Li, J.; Huang, Y.; Tan, H.; Yang, X.; Tian, L.; Luan, S.; Chen, L.; Li, D. An Endoplasmic Reticulum Magnesium Transporter Is Essential for Pollen Development in Arabidopsis. Plant Sci. 2015, 231, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kirkby, E.A. Role of Magnesium in Carbon Partitioning and Alleviating Photooxidative Damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Steinhorst, L.; Kudla, J. Calcium—A Central Regulator of Pollen Germination and Tube Growth. Biochim. Biophys. Acta BBA Mol. Cell Res. 2013, 1833, 1573–1581. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit Calcium: Transport and Physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef]
- Ishitani, M.; Liu, J.; Halfter, U.; Kim, C.-S.; Shi, W.; Zhu, J.-K. SOS3 Function in Plant Salt Tolerance Requires N-Myristoylation and Calcium Binding. Plant Cell 2000, 12, 1667–1677. [Google Scholar] [CrossRef]
- Belver, A.; Olías, R.; Huertas, R.; Rodríguez-Rosales, M.P. Involvement of SlSOS2 in Tomato Salt Tolerance. Bioengineered 2012, 3, 298–302. [Google Scholar] [CrossRef]
- Li, D.; Ma, N.-N.; Wang, J.-R.; Yang, D.-Y.; Zhao, S.-J.; Meng, Q.-W. Overexpression of Tomato Enhancer of SOS3-1 (LeENH1) in Tobacco Enhanced Salinity Tolerance by Excluding Na+ from the Cytosol. Plant Physiol. Biochem. 2013, 70, 150–158. [Google Scholar] [CrossRef]
- Ji, H.; Pardo, J.M.; Batelli, G.; Van Oosten, M.J.; Bressan, R.A.; Li, X. The Salt Overly Sensitive (SOS) Pathway: Established and Emerging Roles. Mol. Plant 2013, 6, 275–286. [Google Scholar] [CrossRef]
- Álvarez-Aragón, R.; Haro, R.; Benito, B.; Rodríguez-Navarro, A. Salt Intolerance in Arabidopsis: Shoot and Root Sodium Toxicity, and Inhibition by Sodium-plus-Potassium Overaccumulation. Planta 2016, 243, 97–114. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ Homeostasis in Plants: Towards Improved Salt Stress Tolerance in Crop Plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef]
- Shi, H.; Lee, B.; Wu, S.-J.; Zhu, J.-K. Overexpression of a Plasma Membrane Na+/H+ Antiporter Gene Improves Salt Tolerance in Arabidopsis thaliana. Nat. Biotechnol. 2003, 21, 81–85. [Google Scholar] [CrossRef]
- Huertas, R.; Olías, R.; Eljakaoui, Z.; Gálvez, F.J.; Li, J.; De Morales, P.A.; Belver, A.; Rodríguez-Rosales, M.P. Overexpression of SlSOS2 (SlCIPK24) Confers Salt Tolerance to Transgenic Tomato: SlSOS2 and Tomato Salt Tolerance. Plant Cell Environ. 2012, 35, 1467–1482. [Google Scholar] [CrossRef]
- Batelli, G.; Verslues, P.E.; Agius, F.; Qiu, Q.; Fujii, H.; Pan, S.; Schumaker, K.S.; Grillo, S.; Zhu, J.-K. SOS2 Promotes Salt Tolerance in Part by Interacting with the Vacuolar H+-ATPase and Upregulating Its Transport Activity. Mol. Cell. Biol. 2007, 27, 7781–7790. [Google Scholar] [CrossRef] [PubMed]
- Ariga, H.; Katori, T.; Yoshihara, R.; Hase, Y.; Nozawa, S.; Narumi, I.; Iuchi, S.; Kobayashi, M.; Tezuka, K.; Sakata, Y.; et al. Arabidopsis Sos1 Mutant in a Salt-Tolerant Accession Revealed an Importance of Salt Acclimation Ability in Plant Salt Tolerance. Plant Signal. Behav. 2013, 8, e24779. [Google Scholar] [CrossRef] [PubMed]
- Leidi, E.O.; Barragán, V.; Rubio, L.; El-Hamdaoui, A.; Ruiz, M.T.; Cubero, B.; Fernández, J.A.; Bressan, R.A.; Hasegawa, P.M.; Quintero, F.J.; et al. The AtNHX1 Exchanger Mediates Potassium Compartmentation in Vacuoles of Transgenic Tomato. Plant J. 2010, 61, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, F.J.; Baghour, M.; Hao, G.; Cagnac, O.; Rodríguez-Rosales, M.P.; Venema, K. Expression of LeNHX Isoforms in Response to Salt Stress in Salt Sensitive and Salt Tolerant Tomato Species. Plant Physiol. Biochem. 2012, 51, 109–115. [Google Scholar] [CrossRef]
- Villalta, I.; Reina-Sánchez, A.; Bolarín, M.C.; Cuartero, J.; Belver, A.; Venema, K.; Carbonell, E.A.; Asins, M.J. Genetic Analysis of Na+ and K+ Concentrations in Leaf and Stem as Physiological Components of Salt Tolerance in Tomato. Theor. Appl. Genet. 2008, 116, 869–880. [Google Scholar] [CrossRef]
- Almeida, P.; de Boer, G.-J.; de Boer, A.H. Differences in Shoot Na+ Accumulation between Two Tomato Species Are Due to Differences in Ion Affinity of HKT1;2. J. Plant Physiol. 2014, 171, 438–447. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamaji, N.; Costa, A.; Okuma, E.; Kobayashi, N.I.; Kashiwagi, T.; Katsuhara, M.; Wang, C.; Tanoi, K.; Murata, Y.; et al. OsHKT1;4-Mediated Na+ Transport in Stems Contributes to Na+ Exclusion from Leaf Blades of Rice at the Reproductive Growth Stage upon Salt Stress. BMC Plant Biol. 2016, 16, 22. [Google Scholar] [CrossRef]
- Deeken, R.; Sanders, C.; Ache, P.; Hedrich, R. Developmental and Light-Dependent Regulation of a Phloem-Localised K+ Channel of Arabidopsis thaliana: Regulation of Akt2/3-MRNA Expression. Plant J. 2000, 23, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Zouine, M.; Maza, E.; Djari, A.; Lauvernier, M.; Frasse, P.; Smouni, A.; Pirrello, J.; Bouzayen, M. TomExpress, a Unified Tomato RNA-Seq Platform for Visualization of Expression Data, Clustering and Correlation Networks. Plant J. 2017, 92, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Liu, J.; Cui, M.; Huang, Y.; Tian, Y.; Chen, A.; Xu, G. The Potassium Transporter SlHAK10 Is Involved in Mycorrhizal Potassium Uptake. Plant Physiol. 2019, 180, 465–479. [Google Scholar] [CrossRef]
- Jin, Y.; Jing, W.; Zhang, Q.; Zhang, W. Cyclic Nucleotide Gated Channel 10 Negatively Regulates Salt Tolerance by Mediating Na+ Transport in Arabidopsis. J. Plant Res. 2015, 128, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Dafni, A.; Maués, M.M. A Rapid and Simple Procedure to Determine Stigma Receptivity. Sex. Plant Reprod. 1998, 11, 177–180. [Google Scholar] [CrossRef]
- Alexander, M.P. Differential Staining of Aborted and Nonaborted Pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Ayenan, M.A.T.; Danquah, A.; Ampomah-Dwamena, C.; Hanson, P.; Asante, I.K.; Danquah, E.Y. Optimizing Pollencounter for High Throughput Phenotyping of Pollen Quality in Tomatoes. MethodsX 2020, 7, 100977. [Google Scholar] [CrossRef]
- van Acker, T.; Van Malderen, S.J.M.; Van Helden, T.; Stremtan, C.; Šala, M.; van Elteren, J.T.; Vanhaecke, F. Analytical Figures of Merit of a Low-Dispersion Aerosol Transport System for High-Throughput LA-ICP-MS Analysis. J. Anal. At. Spectrom. 2021, 36, 1201–1209. [Google Scholar] [CrossRef]
- van Elteren, J.T.; Šelih, V.S.; Šala, M. Insights into the Selection of 2D LA-ICP-MS (Multi)Elemental Mapping Conditions. J. Anal. At. Spectrom. 2019, 34, 1919–1931. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Olías, R.; Eljakaoui, Z.; Li, J.; De Morales, P.A.; Marín-Manzano, M.C.; Pardo, J.M.; Belver, A. The Plasma Membrane Na+/H+ Antiporter SOS1 Is Essential for Salt Tolerance in Tomato and Affects the Partitioning of Na+ between Plant Organs. Plant Cell Environ. 2009, 32, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Asins, M.J.; Villalta, I.; Aly, M.M.; Olías, R.; Álvarez De Morales, P.; Huertas, R.; Li, J.; Jaime-Pérez, N.; Haro, R.; Raga, V.; et al. Two Closely Linked Tomato HKT Coding Genes Are Positional Candidates for the Major Tomato QTL Involved in Na+/K+ Homeostasis: HKT Genes Likely to Underlie a Major Tomato QTL. Plant Cell Environ. 2013, 36, 1171–1191. [Google Scholar] [CrossRef] [PubMed]
- Jaime-Pérez, N.; Pineda, B.; García-Sogo, B.; Atares, A.; Athman, A.; Byrt, C.S.; Olías, R.; Asins, M.J.; Gilliham, M.; Moreno, V.; et al. The Sodium Transporter Encoded by the HKT1;2 Gene Modulates Sodium/Potassium Homeostasis in Tomato Shoots under Salinity. Plant Cell Environ. 2017, 40, 658–671. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an Enhanced Web Interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef]
- Leelatanawit, R.; Saetung, T.; Phuengwas, S.; Karoonuthaisiri, N.; Devahastin, S. Selection of Reference Genes for Quantitative Real-Time PCR in Postharvest Tomatoes (Lycopersicon esculentum) Treated by Continuous Low-Voltage Direct Current Electricity to Increase Secondary Metabolites. Int. J. Food Sci. Technol. 2017, 52, 1942–1950. [Google Scholar] [CrossRef]

| Flowering Parameters | S. lycopersicum | S. chilense | ||||
|---|---|---|---|---|---|---|
| 0 mM NaCl | 60 mM NaCl | 120 mM NaCl | 0 mM NaCl | 60 mM NaCl | 120 mM NaCl | |
| FT initial segment 1 | 11.2 ± 1.3 a | 10.8 ± 1.2 a | 11.0 ± 0.9 a | 12 ± 1.1 A | 10.0 ± 1.5 A | 11.8 ± 1.7 A |
| FT sympodial segment 1 | 2.7 ± 0.3 a | 3.0 ± 0.3 a | 3.0 ± 0.4 a | 3.8 ± 1.2 A | 3.1 ± 0.5 A | 4.2 ± 2.4 A |
| inflorescences per plant | 96.0 ± 61.9 a | 28.8 ± 5.3 a | 10.0 ± 1.4 b | 160.3 ± 58.9 A | 53.5 ± 17.9 A | 7.8 ± 4.9 B |
| floral buds per inflorescence | 8.32 ± 2.29 a | 6.56 ± 1.19 b | 6.14 ± 1.39 b | 12.5 ± 7.18 A | 11.38 ± 9.40 A | 10.40 ± 5.58 A |
| open flowers per inflorescence (%) | 74.6 ± 19.4 a | 55.6 ± 25.5 b | 50.7 ± 27.1 b | 71.3 ± 29.6 A | 54.5 ± 34.0 A | 47.5 ± 30.8 A |
| Flower Parameters | S. lycopersicum | S. chilense | ||||
|---|---|---|---|---|---|---|
| 0 mM NaCl | 60 mM NaCl | 120 mM NaCl | 0 mM NaCl | 60 mM NaCl | 120 mM NaCl | |
| Sepal length (cm) | 1.18 ± 0.23 a | 0.89 ± 0.16 b | 0.91 ± 0.15 b | 0.62 ± 0.1 A | 0.61 ± 0.08 A | 0.63 ± 0.15 A |
| Petal length (cm) | 1.36 ± 0.18 a | 1.24 ± 0.23 a | 1.34 ± 0.17 a | 1.12 ± 0.2 A | 1.25 ± 0.22 A | 1.18 ± 0.17 A |
| Stamen length (cm) | 0.84 ± 0.09 a | 0.8 ± 0.08 a | 0.85 ± 0.07 a | 0.80 ± 0.05 AB | 0.82 ± 0.08 A | 0.74 ± 0.06 B |
| Style + ovary length (cm) | 0.94 ± 0.07 a | 0.86 ± 0.1 a | 0.94 ± 0.09 a | 1.18 ± 0.11 A | 1.11 ± 0.12 A | 1.08 ± 0.09 A |
| Style exsertion (cm) | ND | ND | ND | 0.38 ± 0.12 A | 0.29 ± 0.15 A | 0.33 ± 0.09 A |
| Stigma receptivity (%) | 88.6 ± 26.4 a | 81 ± 29.5 a | 84.4 ± 30.1 a | 96.4 ± 13.4 A | 100 ± 0 A | 100 ± 0 A |
| Pollen viability (%) | 84.7 ± 13.5 a | 82.5 ± 21.2 a | 81.6 ± 14.2 a | 58.3 ± 26.1 B | 68.9 ± 25 A | 63 ± 14.3 AB |
| Pollen grains per anther (×1000) | 19.2 ± 14.2 a | 13.9 ± 15.2 a | 16.3 ± 10.2 a | 68.0 ± 35.1 A | 48.5 ± 23.8 AB | 37.4 ± 19.3 B |
| Fruit Parameters | S. lycopersicum | S. chilense | ||||
|---|---|---|---|---|---|---|
| 0 mM NaCl | 60 mM NaCl | 120 mM NaCl | 0 mM NaCl | 60 mM NaCl | 120 mM NaCl | |
| Fruit set (%) | 47.9 ± 15.1 a | 43.5 ± 22.5 a | 38.9 ± 21.1 a | 51.7 ± 40.6 A | 60 ± 37.7 A | 44.3 ± 33.2 A |
| FW (g) | 47.7 ± 10.3 a | 22.5 ± 6 b | 14.5 ± 5.3 c | 0.65 ± 0.2 A | 0.79 ± 0.26 A | 0.84 ± 0.21 A |
| DW (g) | 3.42 ± 1.98 a | 2.01 ± 0.56 b | 1.44 ± 0.62 b | 0.12 ± 0.03 A | 0.10 ± 0.02 A | 0.10 ± 0.01 A |
| WC (%) | 91.91 ± 3.77 a | 89.67 ± 0.42 b | 88.19 ± 0.9 c | 80.72 ± 2.87 C | 82.49 ± 7.17 B | 87.49 ± 1.6 A |
| Circumference (cm) | 14.4 ± 0.92 a | 11.62 ± 0.74 b | 10.1 ± 1.13 c | 3.15 ± 0.24 A | 3.48 ± 0.73 A | 3.57 ± 0.62 A |
| Number of seeds/fruit | 91.17 ± 46.02 a | 72.77 ± 33.19 ab | 50.08 ± 16.04 b | 21.22 ± 4.47 A | 22.00 ± 5.28 A | 24.88 ± 10.21 A |
| Number of seeds/fruit FW (g) | 2.11 ± 0.73 b | 3.31 ± 0.91 a | 3.76 ± 2.1 a | 34.37 ± 13.44 A | 30.72 ± 11.83 A | 25.93 ± 7.18 A |
| Sugar concentration (°Brix) | 5.54 ± 0.52 c | 7.95 ± 0.44 b | 9.2 ± 0.95 a | 18.4 ± 3.2 A | 11.95 ± 4.12 B | 10.15 ± 2.35 B |
| pH | 4.52 ± 0.09 a | 4.4 ± 0.11 b | 4.31 ± 0.11 b | 4.67 ± 0.26 A | 4.07 ± 0.49 B | 3.8 ± 0.26 B |
| Mineral | S. lycopersicum | S. chilense | ||||
|---|---|---|---|---|---|---|
| 0 mM NaCl | 60 mM NaCl | 100 mM NaCl | 0 mM NaCl | 60 mM NaCl | 100 mM NaCl | |
| vegetative/reproductive floral organs | ||||||
| Na | 0.36 ± 0.12 | 0.65 ± 0.11 | 1 ± 0.26 | 0.18 ± 0.07 | 0.43 ± 0.04 | 0.31 ± 0.02 |
| K | 0.45 ± 0.1 | 0.43 ± 0.05 | 0.5 ± 0.03 | 0.43 ± 0.19 | 0.22 ± 0.09 | 0.14 ± 0.02 |
| Ca | 0.54 ± 0.07 | 0.42 ± 0.05 | 0.69 ± 0.24 | 0.34 ± 0.29 | 0.29 ± 0.12 | 0.26 ± 0.08 |
| Mg | 0.86 ± 0.16 | 0.34 ± 0.1 | 0.77 ± 0.19 | 0.67 ± 0.46 | 0.23 ± 0.02 | 0.3 ± 0.08 |
| male/female floral organs | ||||||
| Na | 0.82 ± 0.05 | 0.55 ± 0.16 | 0.5 ± 0.07 | 1.24 ± 0.68 | 0.59 ± 0.27 | 0.7 ± 0.49 |
| K | 0.63 ± 0.03 | 0.41 ± 0 | 0.34 ± 0.2 | 0.83 ± 0.06 | 0.6 ± 0.16 | 0.68 ± 0.54 |
| Ca | 0.64 ± 0.24 | 0.26 ± 0.02 | 0.3 ± 0.14 | 1.32 ± 0.07 | 0.35 ± 0.11 | 0.68 ± 0.29 |
| Mg | 0.49 ± 0.05 | 0.35 ± 0.12 | 0.3 ± 0.09 | 0.62 ± 0.2 | 0.38 ± 0.04 | 0.52 ± 0.37 |
| Mineral | S. lycopersicum | S. chilense | ||||
|---|---|---|---|---|---|---|
| 0 mM NaCl | 60 mM NaCl | 120 mM NaCl | 0 mM NaCl | 60 mM NaCl | 120 mM NaCl | |
| Inflorescences | ||||||
| K (mg g−1 DW) | 27.63 ± 2.62 a | 26.09 ± 6 a | 23.97 ± 4.36 a | 30.76 ± 5.06 A | 31.97 ± 4.81 A | 26.47 ± 5.21 A |
| K/Na | 43.01 ± 23.32 a | 10.34 ± 2.87 b | 10.15 ± 5.7 b | 10.66 ± 17.26 A | 4.74 ± 4.43 B | 1.26 ± 1.14 B |
| Ca (mg g−1 DW) | 1.03 ± 0.87 a | 1.22 ± 0.82 a | 1.09 ± 1.02 a | 0.18 ± 0.14 A | 0.08 ± 0.08 A | 0.29 ± 0.01 A |
| Mg (mg g−1 DW) | 4.92 ± 1.22 a | 5.62 ± 1.96 a | 3.64 ± 0.62 b | 3.45 ± 1.04 A | 2.94 ± 0.42 A | 3.04 ± 0.94 A |
| Pericarp | ||||||
| K (mg g−1 DW) | 38.98 ± 6.36 a | 32.14 ± 9.24 b | 26.20 ± 5.88 b | 39.22 ± 4.66 A | 27.75 ± 4.47 B | 27.99 ± 6.74 B |
| Ca (mg g−1 DW) | 0.73 ± 0.23 a | 0.49 ± 0.23 b | 0.64 ± 0.24 ab | 1.49 ± 0.56 A | 1.27 ± 0.23 A | 1.54 ± 0.56 A |
| Mg (mg g−1 DW) | 1.36 ± 0.26 a | 1.09 ± 0.43 b | 1.08 ± 0.12 b | 2.10 ± 0.33 A | 2.14 ± 0.58 A | 2.46 ± 0.40 A |
| seeds | ||||||
| K (mg g−1 DW) | 8.09 ± 5.09 a | 9.79 ± 7.28 a | 14.42 ± 9.2 a | 20.43 ± 7.6 A | 9.85 ± 5.68 B | 8.71 ± 7.21 B |
| Ca (mg g−1 DW) | 0.77 ± 0.53 a | 0.71 ± 0.54 a | 0.52 ± 0.13 a | 0.89 ± 0.23 A | 0.85 ± 0.24 A | 0.85 ± 0.39 A |
| Mg (mg g−1 DW) | 3.60 ± 0.93 a | 3.57 ± 0.92 a | 2.40 ± 0.75 a | 2.74 ± 0.27 A | 2.38 ± 0.26 B | 2.69 ± 0.37 AB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigot, S.; Pongrac, P.; Šala, M.; van Elteren, J.T.; Martínez, J.-P.; Lutts, S.; Quinet, M. The Halophyte Species Solanum chilense Dun. Maintains Its Reproduction despite Sodium Accumulation in Its Floral Organs. Plants 2022, 11, 672. https://doi.org/10.3390/plants11050672
Bigot S, Pongrac P, Šala M, van Elteren JT, Martínez J-P, Lutts S, Quinet M. The Halophyte Species Solanum chilense Dun. Maintains Its Reproduction despite Sodium Accumulation in Its Floral Organs. Plants. 2022; 11(5):672. https://doi.org/10.3390/plants11050672
Chicago/Turabian StyleBigot, Servane, Paula Pongrac, Martin Šala, Johannes T. van Elteren, Juan-Pablo Martínez, Stanley Lutts, and Muriel Quinet. 2022. "The Halophyte Species Solanum chilense Dun. Maintains Its Reproduction despite Sodium Accumulation in Its Floral Organs" Plants 11, no. 5: 672. https://doi.org/10.3390/plants11050672
APA StyleBigot, S., Pongrac, P., Šala, M., van Elteren, J. T., Martínez, J.-P., Lutts, S., & Quinet, M. (2022). The Halophyte Species Solanum chilense Dun. Maintains Its Reproduction despite Sodium Accumulation in Its Floral Organs. Plants, 11(5), 672. https://doi.org/10.3390/plants11050672







