MdMADS6 Recruits Histone Deacetylase MdHDA19 to Repress the Expression of the Carotenoid Synthesis-Related Gene MdCCD1 during Fruit Ripening
Abstract
:1. Introduction
2. Results
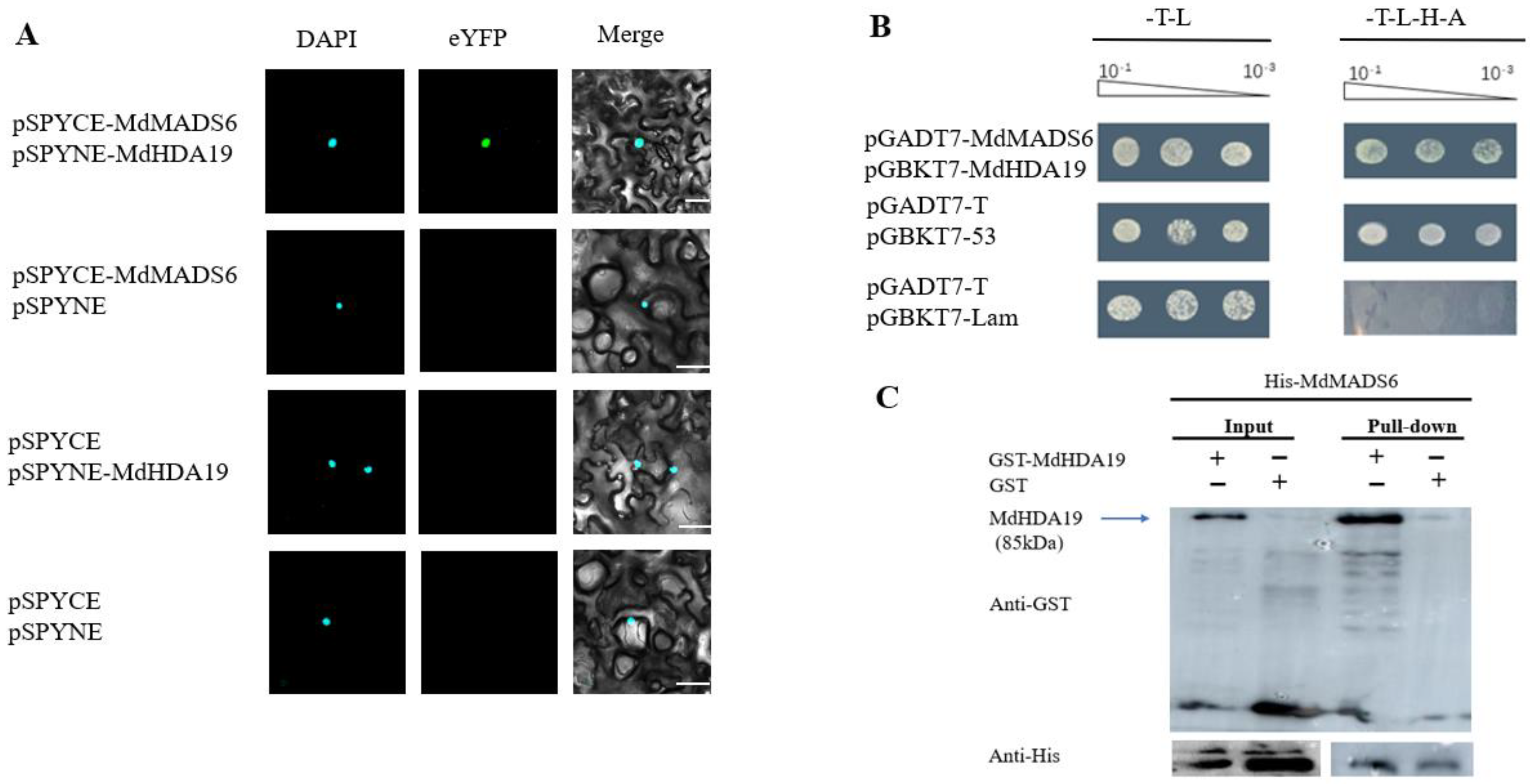
2.1. MdHDA19 Interacts with MdMADS6
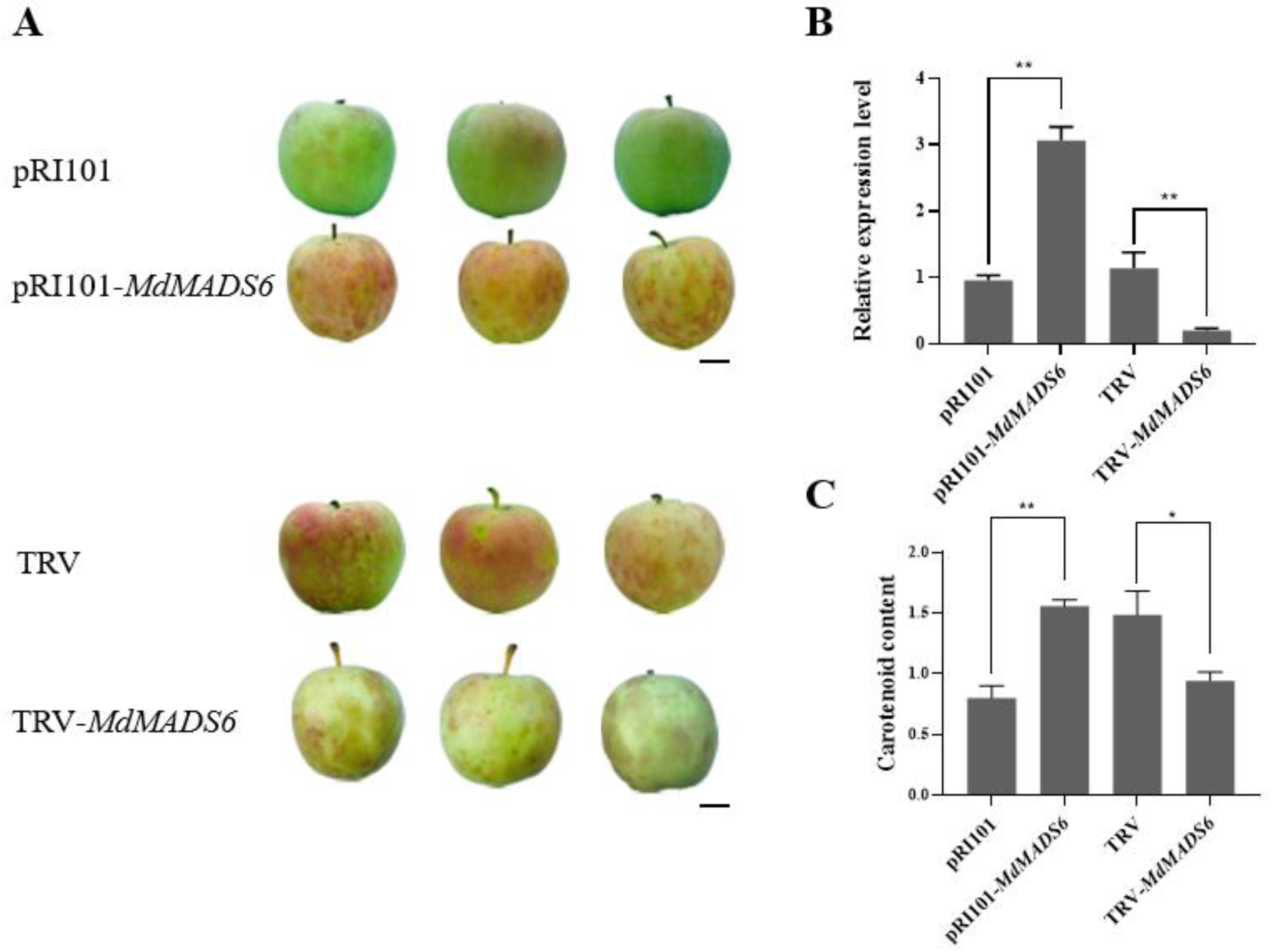
2.2. MdMADS6 Promotes Apple Fruit Ripening
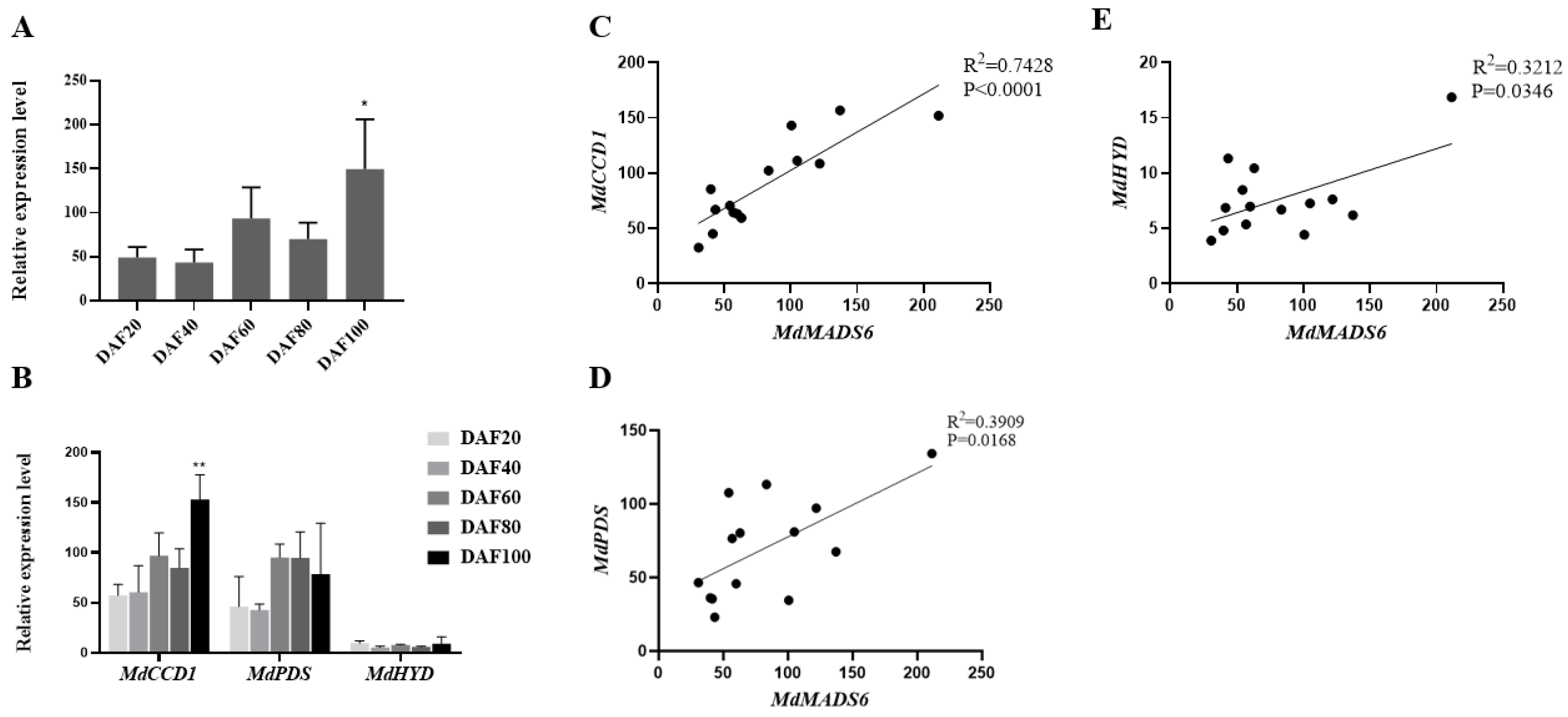
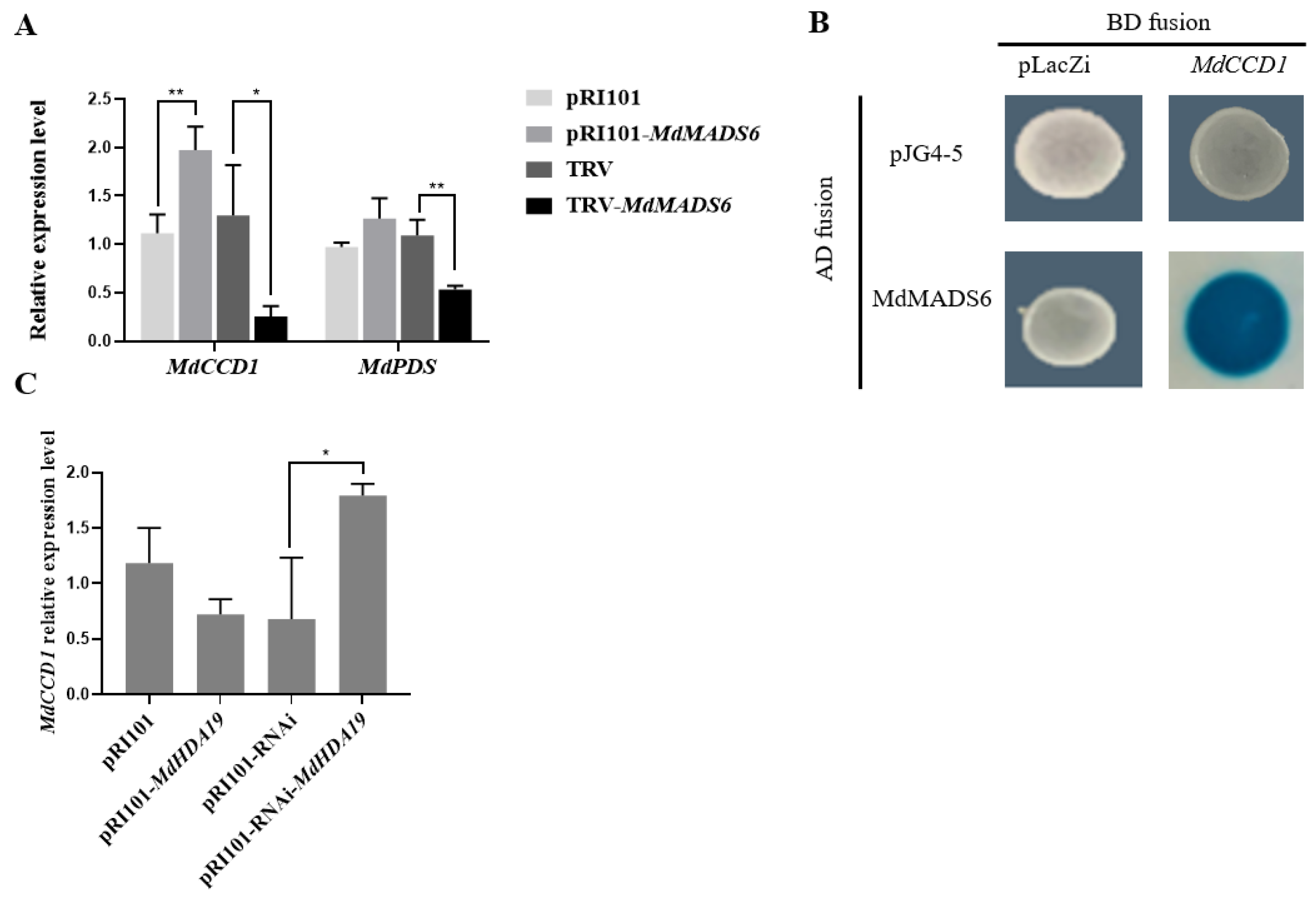
2.3. MdMADS6 Binds to the Promoter of the Carotenoid Biosynthesis Gene MdCCD1
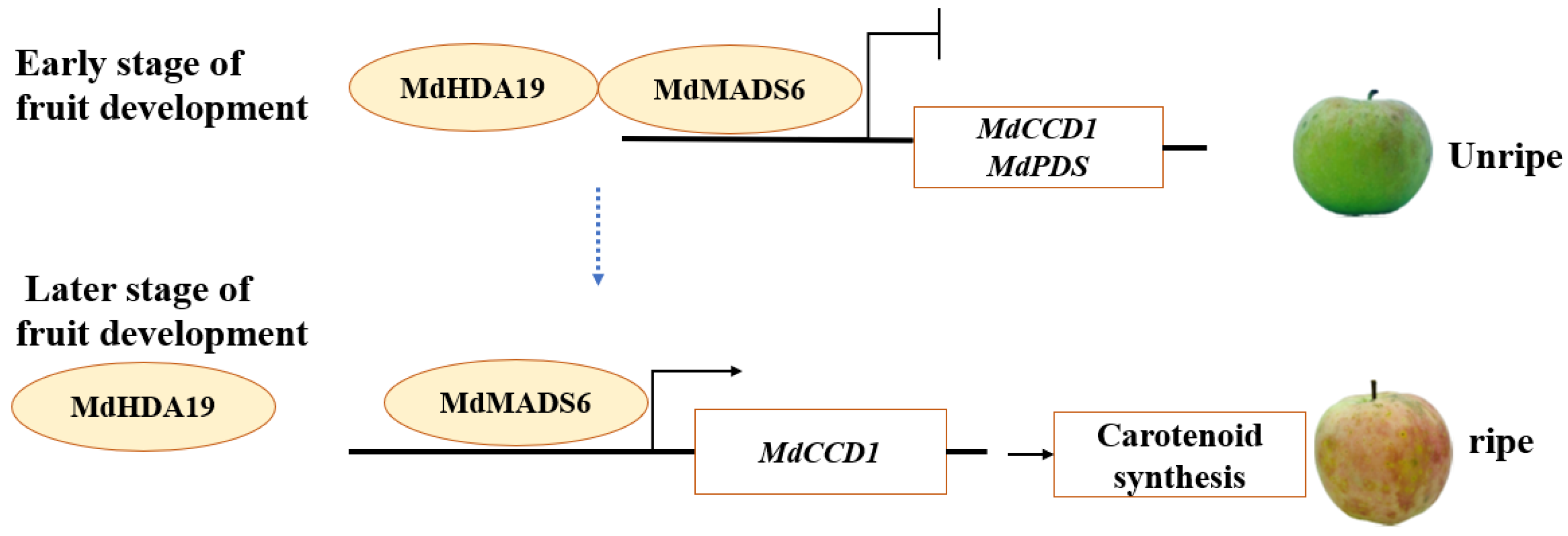
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. DNA Extraction, Total RNA Isolation and cDNA Synthesis
4.3. Gene Cloning
4.4. Protein Purification
4.5. Yeast Two-Hybrid Assay
4.6. BiFC Assay
4.7. Agrobacterium-Mediated Transient Transformation and Treatments
4.8. Yeast One-Hybrid Assay
4.9. Real-Time Fluorescence Quantification
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tucker, G.; Yin, X.; Zhang, A. Ethylene and fruit softening. Food Qual. Saf. 2017, 1, 253–267. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yang, S.; Zhao, M.; Luo, M.; Yu, C.-W.; Chen, C.-Y.; Tai, R.; Wu, K. Transcriptional repression by histone deacetylases in plants. Mol. Plant 2014, 7, 764–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.E.; Hu, Z.; Zhu, M.; Li, F.; Zhu, Z.; Lu, Y.; Chen, G. The tomato histone deacetylase SlHDA1 contributes to the repression of fruit ripening and carotenoid accumulation. Sci. Rep. 2017, 7, 7930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.E.; Hu, Z.; Yu, X.; Li, A.; Li, F.; Wang, Y.; Tian, S.; Chen, G. A histone deacetylase gene, SlHDA3, acts as a negative regulator of fruit ripening and carotenoid accumulation. Plant Cell Rep. 2018, 37, 125–135. [Google Scholar] [CrossRef]
- Vrebalov, J.; Ruezinsky, D.; Padmanabhan, V.; White, R.; Medrano, D.; Drake, R.; Schuch, W.; Giovannoni, J. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 2002, 296, 343–346. [Google Scholar] [CrossRef]
- Li, S.; Xu, H.; Ju, Z.; Cao, D.; Zhu, H.; Fu, D.; Grierson, D.; Qin, G.; Luo, Y.; Zhu, B. The RIN-MC fusion of MADS-box transcription factors has transcriptional activity and modulates expression of many ripening genes. Plant Physiol. 2018, 176, 891–909. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhang, L.; Ma, R. Functional characterization and mapping of two MADS box genes from peach (Prunus persica). Chin. Sci. Bull. 2008, 53, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Xi, W.; Shen, L.; Tan, C.; Yu, H. Regulation of floral patterning by flowering time genes. Dev. Cell 2009, 16, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.; Hu, Z.; Deng, L.; Wang, Y.; Zhu, M.; Zhang, J.; Chen, G. A tomato MADS-box transcription factor, SlMADS1, acts as a negative regulator of fruit ripening. Plant Physiol. 2013, 163, 1026–1036. [Google Scholar] [CrossRef] [Green Version]
- Boss, P.K.; Vivier, M.; Matsumoto, S.; Dry, I.B.; Thomas, M.R. A cDNA from grapevine (Vitis vinifera L.), which shows homology to AGAMOUS and SHATTERPROOF, is not only expressed in flowers but also throughout berry development. Plant Mol. Biol. 2001, 45, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.B.; Ryder, C.D.; Cevik, V.; Hammond, J.P.; Popovich, A.; King, G.J.; Vrebalov, J.; Giovannoni, J.J.; Manning, K. A SEPALLATA gene is involved in the development and ripening of strawberry (Fragaria × ananassa Duch.) fruit, a non-climacteric tissue. J. Exp. Bot. 2011, 62, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Poole, M.; Jones, M.O.; Kämäräinen-Karppinen, T.; Koskimäki, J.J.; Hohtola, A.; Häggman, H.; Fraser, P.D.; Manning, K.; King, G.J.; et al. A Squamosa MADS box gene involved in the regulation of anthocyanin accumulation in bilberry fruits. Plant Physiol. 2010, 153, 1619–1629. [Google Scholar] [CrossRef] [Green Version]
- Itkin, M.; Seybold, H.; Breitel, D.; Rogachev, I.; Meir, S.; Aharoni, A. Tomato Agamous-like 1 is a component of the fruit ripening regulatory network. Plant J. 2009, 60, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, Y.; Zhu, K.; Yang, W.; Ye, J.; Chai, L.; Xu, Q.; Deng, X. The citrus transcription factor CsMADS6 modulates carotenoid metabolism by directly regulating carotenogenic genes. Plant Physiol. 2018, 176, 2657–2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.C.; Kuang, J.F.; Chen, J.Y.; Liu, X.C.; Xiao, Y.Y.; Fu, C.C.; Wang, J.-N.; Wu, K.-Q.; Lu, W.J. Banana transcription factor MaERF11 recruits histone deacetylase MaHDA1 and represses the expression of MaACO1 and expansins during fruit ripening. Plant Physiol. 2016, 171, 1070–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Lu, J.; Zhang, J.; Wu, P.-Y.; Yang, S.; Wu, K. Identification and characterization of histone deacetylases in tomato (Solanum lycopersicum). Front. Plant Sci. 2015, 5, 760. [Google Scholar] [CrossRef] [Green Version]
- Kuang, J.; Chen, J.; Luo, M.; Wu, K.-Q.; Sun, W.; Jiang, Y.-M.; Lu, W.-J. Histone deacetylase HD2 interacts with ERF1 and is involved in longan fruit senescence. J. Exp. Bot. 2012, 63, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.N.; Han, Z.Y.; Wang, T.; Li, H.; Li, Q.; Wang, S.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X.; et al. Ethylene response factor MdERF4 and histone deacetylase MdHDA19 suppress apple fruit ripening through histone deacetylation of ripening-related genes. Plant Physiol. 2022, 2022, kiac016. [Google Scholar] [CrossRef]
- Berger, S.L. The complex language of chromatin regulation during transcription. Nature 2007, 447, 407–412. [Google Scholar] [CrossRef]
- Yinglin, J.; Mingyang, X.U.; Wang, A. Recent advances in the regulation of climacteric fruit ripening. Front. Agric. Sci. Eng. 2021, 8, 314–334. [Google Scholar]
- Guo, J.E.; Hu, Z.; Li, F.; Zhang, L.; Yu, X.; Tang, B.; Chen, G. Silencing of histone deacetylase SlHDT3 delays fruit ripening and suppresses carotenoid accumulation in tomato. Plant Sci. 2017, 265, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Luo, M. Regulation of flowering time by the histone deacetylase HDA5 in Arabidopsis. Plant J. 2015, 82, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Bemer, M.; Karlova, R.; Ballester, A.R.; Tikunov, Y.M.; Bovy, A.G.; Wolters-Arts, M.; de Barros Rossetto, P.; Angenent, G.C.; de Maagd, R.A. The Tomato FRUITFULL Homologs TDR4/FUL1 and MBP7/FUL2 Regulate Ethylene-Independent Aspects of Fruit Ripening. Plant Cell. 2012, 24, 4437–4451. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, M.; Shima, Y.; Nakagawa, H.; Kitagawa, M.; Kimbara, J.; Nakano, T.; Kasumi, T.; Ito, Y. Transcriptional Regulation of Fruit Ripening by Tomato FRUITFULL Homologs and Associated MADS Box Proteins. Plant Cell. 2014, 26, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Hu, Y.; Lv, Y.; Rose, J.K.; Sun, Y.; Shen, F.; Wang, Y.; Zhang, X.; Xu, X.; Wu, T.; et al. Natural variation underlies differences in Ethylene Response Factor17 activity in fruit peel degreening. Plant Physiol. 2018, 176, 2292–2304. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Han, Z.; Sun, Y.; Wang, S.; Wang, T.; Wang, Y.; Xu, K.; Zhang, X.; Xu, X.; Han, Z.; et al. ERF4 affects fruit firmness through TPL4 by reducing ethylene production. Plant J. 2020, 103, 937–950. [Google Scholar] [CrossRef]
- Li, X.; Shen, F.; Xu, X.; Zheng, Q.; Wang, Y.; Wu, T.; Li, W.; Qiu, C.; Xu, X.; Han, Z.; et al. An HD-ZIP transcription factor, MxHB13, integrates auxin—regulated and juvenility—Determined control of adventitious rooting in Malus xiaojinensis. Plant J. 2021, 107, 1663–1680. [Google Scholar] [CrossRef]
- Mao, Y.B.; Liu, Y.Q.; Chen, D.Y.; Chen, F.Y.; Fang, X.; Hong, G.J.; Wang, L.J.; Wang, J.W.; Chen, X.Y. Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat. Commun. 2017, 8, 13925. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wang, T.; Xu, C.; Li, M.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X.; Han, Z.; Wu, T. MdMADS6 Recruits Histone Deacetylase MdHDA19 to Repress the Expression of the Carotenoid Synthesis-Related Gene MdCCD1 during Fruit Ripening. Plants 2022, 11, 668. https://doi.org/10.3390/plants11050668
Li Q, Wang T, Xu C, Li M, Tian J, Wang Y, Zhang X, Xu X, Han Z, Wu T. MdMADS6 Recruits Histone Deacetylase MdHDA19 to Repress the Expression of the Carotenoid Synthesis-Related Gene MdCCD1 during Fruit Ripening. Plants. 2022; 11(5):668. https://doi.org/10.3390/plants11050668
Chicago/Turabian StyleLi, Qiqi, Ting Wang, Chen Xu, Meishuo Li, Ji Tian, Yi Wang, Xinzhong Zhang, Xuefeng Xu, Zhenhai Han, and Ting Wu. 2022. "MdMADS6 Recruits Histone Deacetylase MdHDA19 to Repress the Expression of the Carotenoid Synthesis-Related Gene MdCCD1 during Fruit Ripening" Plants 11, no. 5: 668. https://doi.org/10.3390/plants11050668
APA StyleLi, Q., Wang, T., Xu, C., Li, M., Tian, J., Wang, Y., Zhang, X., Xu, X., Han, Z., & Wu, T. (2022). MdMADS6 Recruits Histone Deacetylase MdHDA19 to Repress the Expression of the Carotenoid Synthesis-Related Gene MdCCD1 during Fruit Ripening. Plants, 11(5), 668. https://doi.org/10.3390/plants11050668







