Throttling Growth Speed: Evaluation of aux1-7 Root Growth Profile by Combining D-Root system and Root Penetration Assay
Abstract
:1. Introduction
2. Results
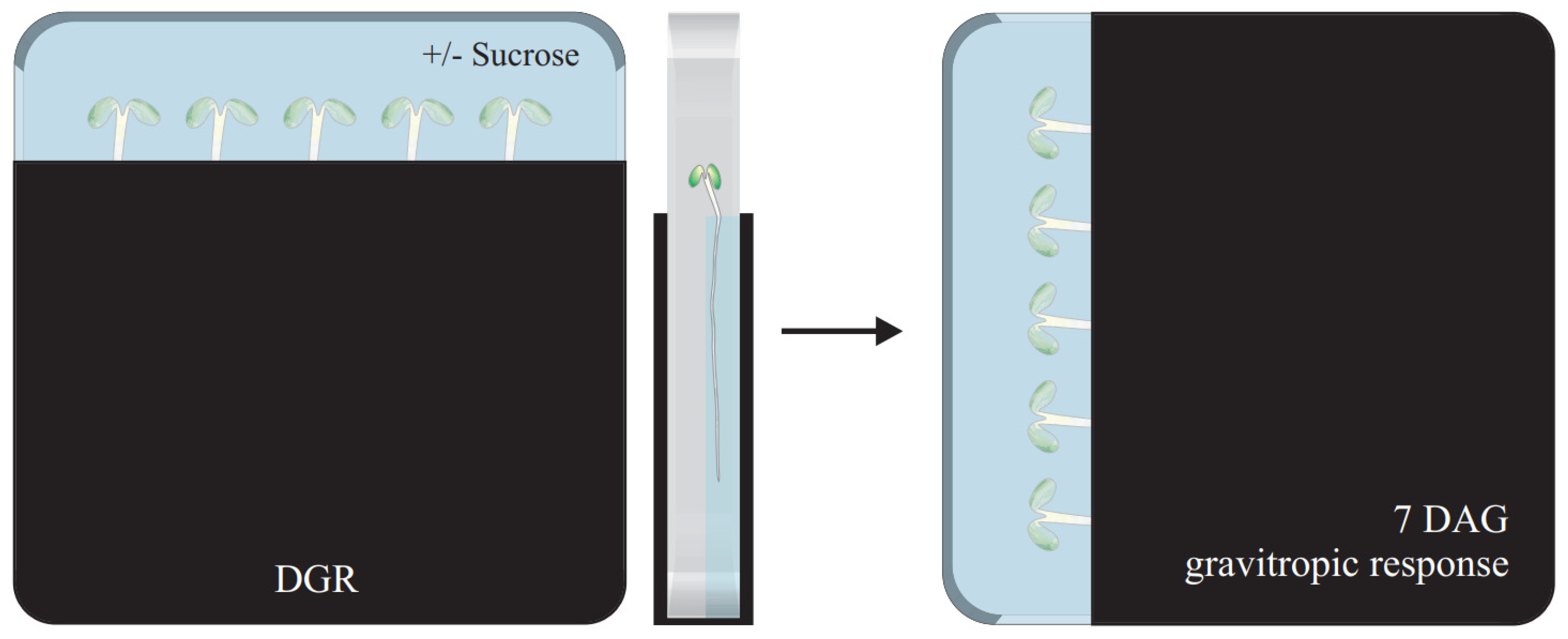
2.1. Introducing the Combination of D-Rootsystem and Root Penetration Assay to Study Directional Root Growth Adaptation
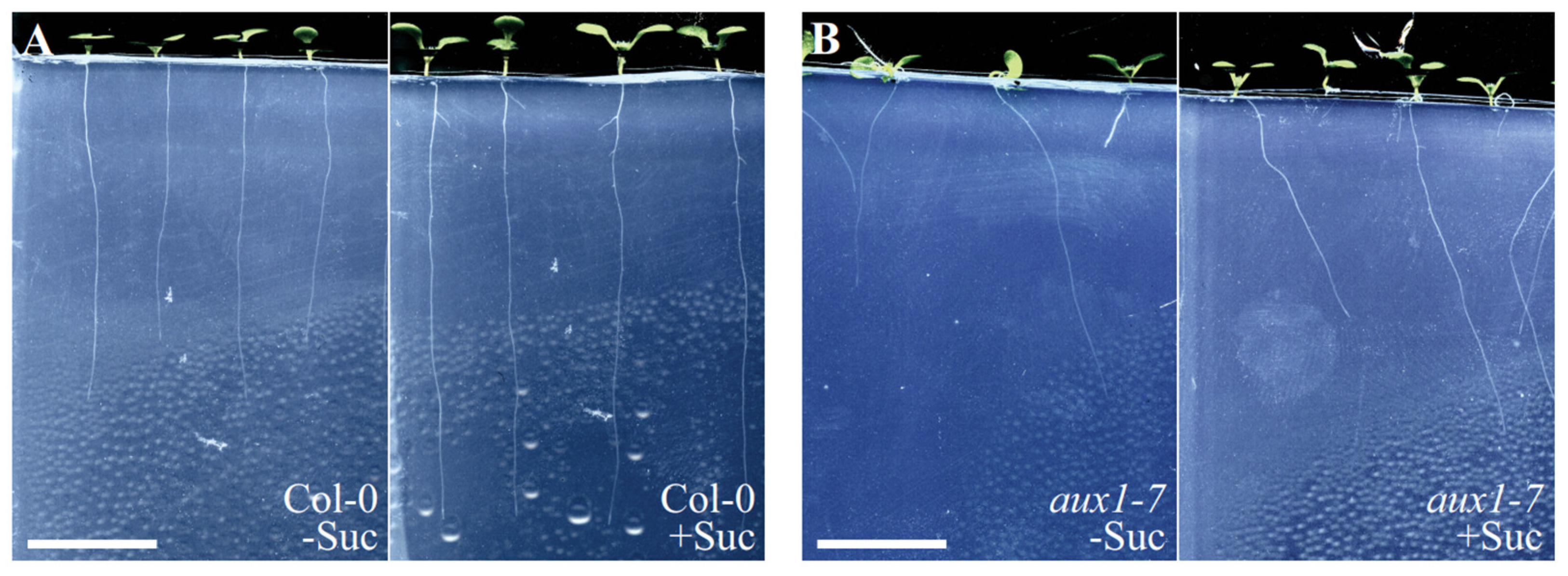
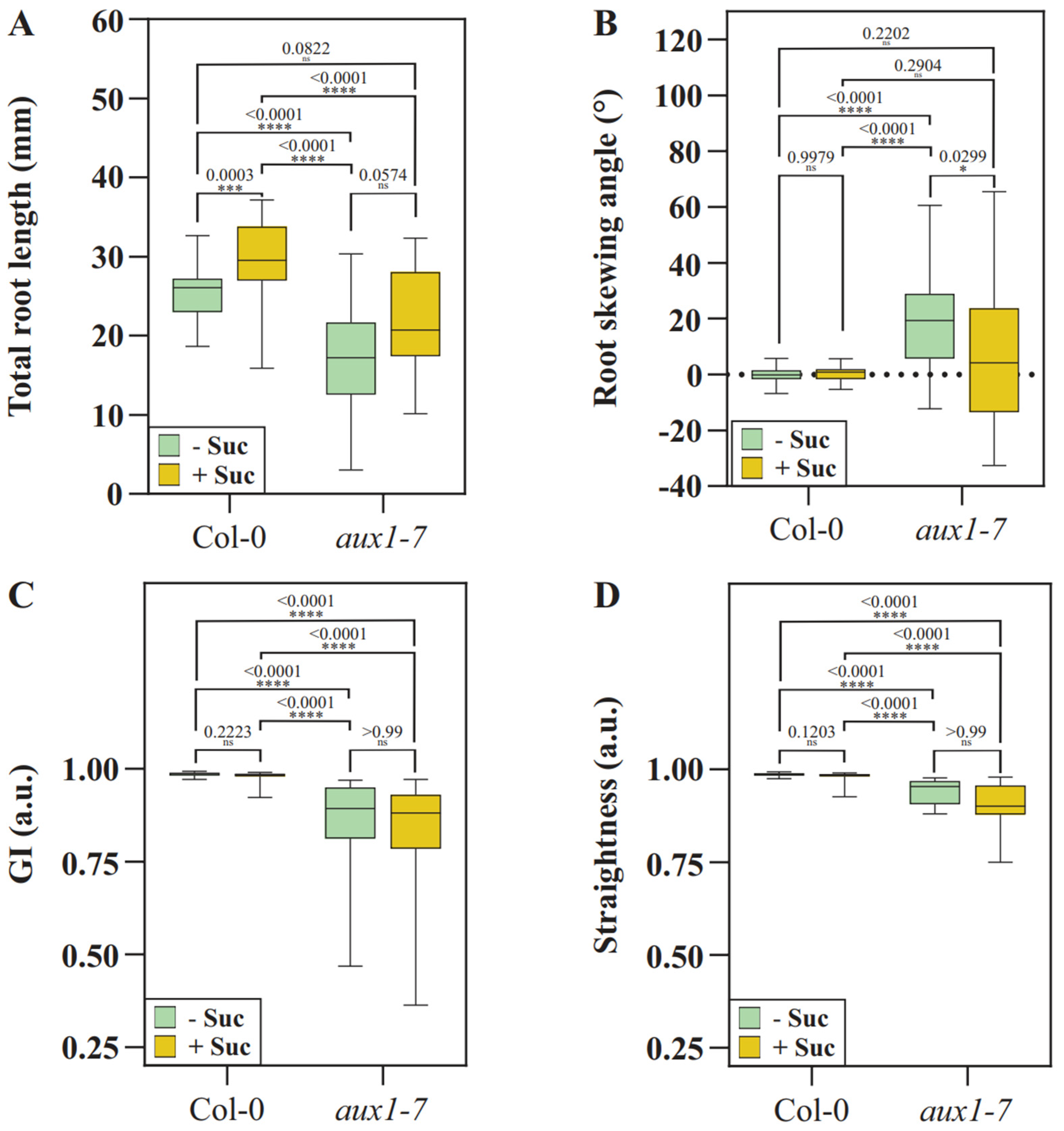
2.2. Loss of AUX1 Results in Reduced Growth Medium Penetration Efficiency
2.3. Loss of AUX1 Expectedly Results in an Uncoordinated Root Growth Pattern When Grown in Medium
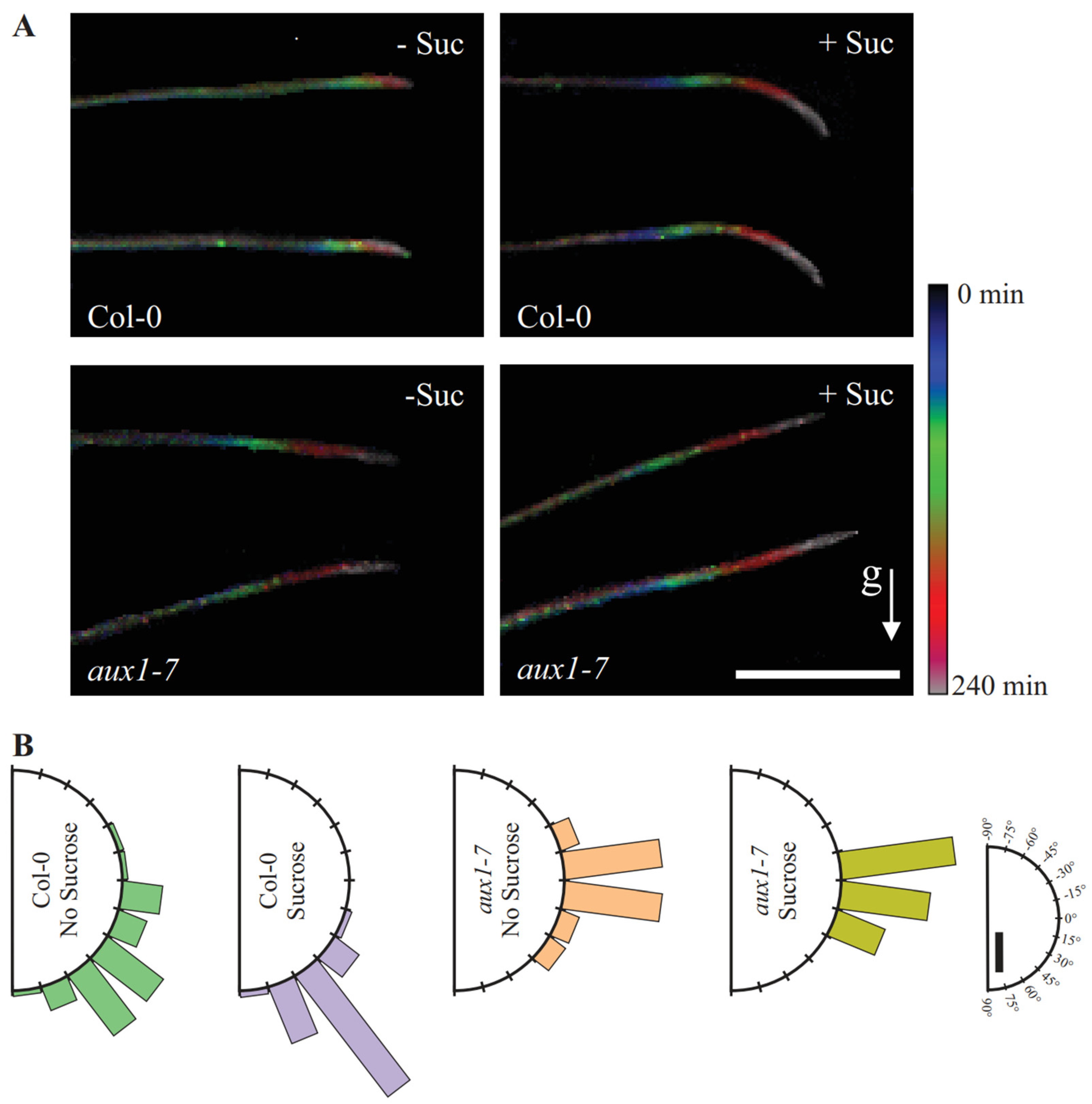
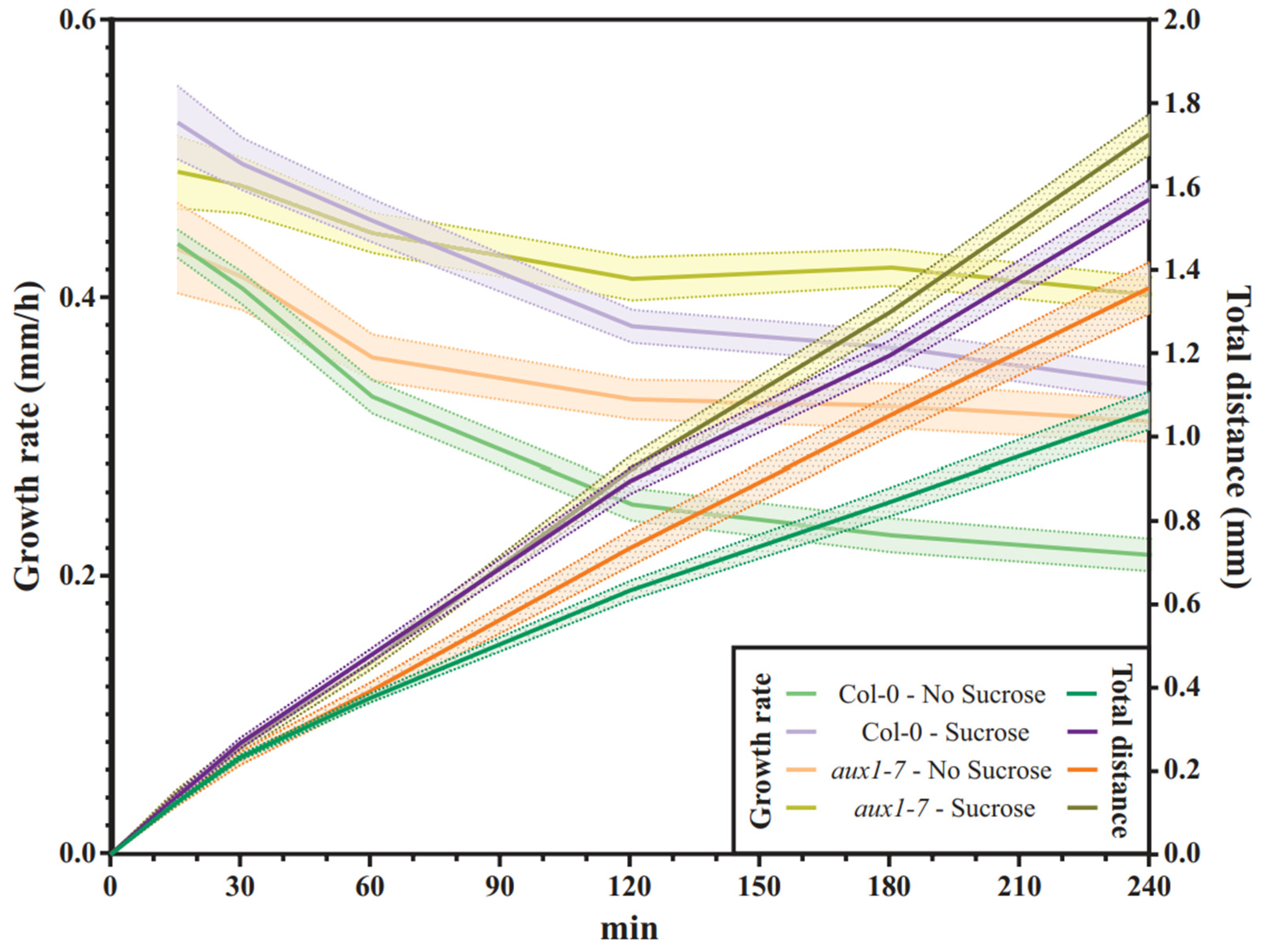
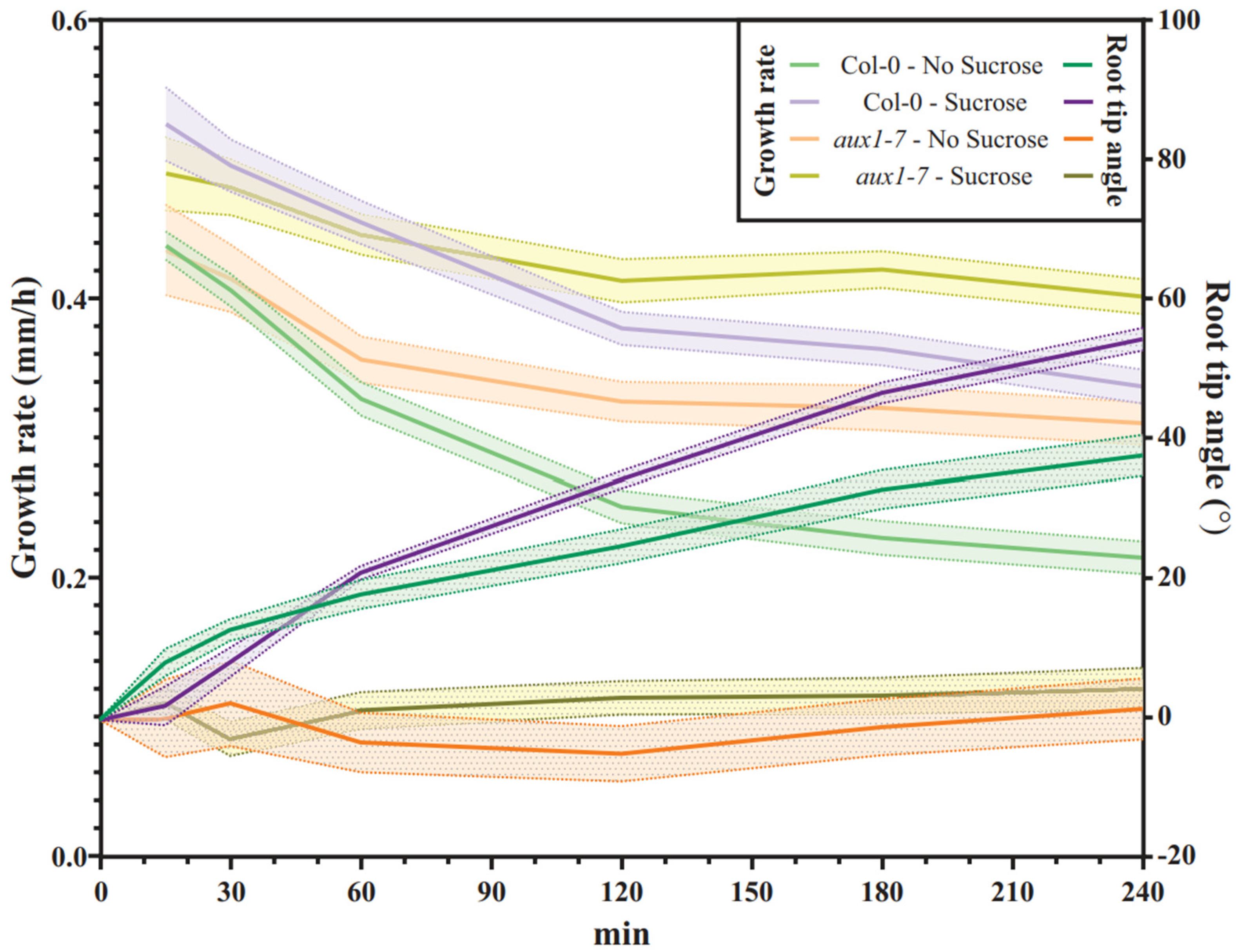
2.4. Col-0 Roots Throttle Elongation Speed during Gravitropic Response, but Not aux1-7
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Root Parameter and Bending Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swarup, R.; Bennett, M.J. Root Gravitropism. In Annual Plant Reviews: Root Development; Blackwell Publishing: Oxford, UK, 2009. [Google Scholar] [CrossRef]
- Retzer, K.; Korbei, B.; Luschnig, C. Auxin and Tropisms. In Auxin and Its Role in Plant Development; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Kiss, J.Z. Plant responses to gravity. Semin. Cell Dev. Biol. 2019, 92, 122–125. [Google Scholar] [CrossRef]
- Lacek, J.; García-González, J.; Weckwerth, W.; Retzer, K. Lessons Learned from the Studies of Roots Shaded from Direct Root Illumination. Int. J. Mol. Sci. 2021, 22, 12784. [Google Scholar] [CrossRef] [PubMed]
- Kolb, E.; Legué, V.; Bogeat-Triboulot, M.-B. Physical root–soil interactions. Phys. Biol. 2017, 14, 065004. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.; Lehner, K.; McCaskey, E.; Nirmal, N.; Ozkan-Aydin, Y.; Murray-Cooper, M.; Jain, R.; Hawkes, E.W.; Ronald, P.C.; Goldman, D.I.; et al. Mechanism and function of root circumnutation. Proc. Natl. Acad. Sci. USA 2021, 118, e2018940118. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Takatani, S.; Motose, H.; Iida, H.; Takahashi, T. The root growth reduction in response to mechanical stress involves ethylene-mediated microtubule reorganization and transmembrane receptor-mediated signal transduction in Arabidopsis. Plant Cell Rep. 2021, 40, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.G.R.; Jervis, G.; Xu, J.; Topping, J.F.; Lindsey, K. Root growth responses to mechanical impedance are regulated by a network of ROS, ethylene and auxin signalling in Arabidopsis. New Phytol. 2021, 231, 225–242. [Google Scholar] [CrossRef]
- Tojo, H.; Nakamura, A.; Ferjani, A.; Kazama, Y.; Abe, T.; Iida, H. A Method Enabling Comprehensive Isolation of Arabidopsis Mutants Exhibiting Unusual Root Mechanical Behavior. Front. Plant Sci. 2021, 12, 646404. [Google Scholar] [CrossRef]
- Potocka, I.; Szymanowska-Pułka, J. Morphological responses of plant roots to mechanical stress. Ann. Bot. 2018, 122, 711–723. [Google Scholar] [CrossRef]
- Del Dottore, E.; Mondini, A.; Sadeghi, A.; Mattoli, V.; Mazzolai, B. An efficient soil penetration strategy for explorative robots inspired by plant root circumnutation movements. Bioinspir. Biomim. 2017, 13, 015003. [Google Scholar] [CrossRef]
- Mishra, A.K.; Tramacere, F.; Guarino, R.; Pugno, N.M.; Mazzolai, B. A study on plant root apex morphology as a model for soft robots moving in soil. PLoS ONE 2018, 13, e0197411. [Google Scholar] [CrossRef] [Green Version]
- Tedone, F.; del Dottore, E.; Palladino, M.; Mazzolai, B.; Marcati, P. Optimal control of plant root tip dynamics in soil. Bioinspir. Biomim. 2020, 15, 056006. [Google Scholar] [CrossRef] [PubMed]
- Najrana, T.; Sanchez-Esteban, J. Mechanotransduction as an Adaptation to Gravity. Front. Pediatr. 2016, 4, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwin, C.; Darwin, F. The Power of Movement in Plants; John Murray: London, UK, 1880. [Google Scholar] [CrossRef]
- Singh, G.; Retzer, K.; Vosolsobě, S.; Napier, R. Advances in Understanding the Mechanism of Action of the Auxin Permease AUX1. Int. J. Mol. Sci. 2018, 19, 3391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halat, L.; Gyte, K.; Wasteneys, G. The Microtubule-Associated Protein CLASP Is Translationally Regulated in Light-Dependent Root Apical Meristem Growth. Plant Physiol. 2020, 184, 2154–2167. [Google Scholar] [CrossRef] [PubMed]
- Ötvös, K.; Marconi, M.; Vega, A.; O’Brien, J.; Johnson, A.; Abualia, R.; Antonielli, L.; Montesinos, J.C.; Zhang, Y.; Tan, S.; et al. Modulation of plant root growth by nitrogen source-defined regulation of polar auxin transport. EMBO J. 2021, 40, e106862. [Google Scholar] [CrossRef] [PubMed]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Petraášek, J.; Friml, J. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef] [Green Version]
- Retzer, K.; Lacek, J.; Skokan, R.; del Genio, C.I.; Vosolsobě, S.; Laňková, M.; Malínská, K.; Konstantinova, N.; Zažímalová, E.; Napier, R.M.; et al. Evolutionary Conserved Cysteines Function as cis-Acting Regulators of Arabidopsis PIN-FORMED 2 Distribution. Int. J. Mol. Sci. 2017, 18, 2274. [Google Scholar] [CrossRef] [Green Version]
- Lacek, J.; Retzer, K.; Luschnig, C.; Zazimalova, E. Polar Auxin Transport; Wiley Online Library, eLS: Hoboken, NJ, USA, 2017; pp. 1–11. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9780470015902.a0020116.pub2 (accessed on 10 January 2022).
- Retzer, K.; Akhmanova, M.; Konstantinova, N.; Malínská, K.; Leitner, J.; Petrasek, J.; Luschnig, C. Brassinosteroid signaling delimits root gravitropism via sorting of the Arabidopsis PIN2 auxin transporter. Nat. Commun. 2019, 10, 5516. [Google Scholar] [CrossRef]
- Fendrych, M.; Akhmanova, M.; Merrin, J.; Glanc, M.; Hagihara, S.; Takahashi, K.; Uchida, N.; Torii, K.U.; Friml, J. Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nat. Plants 2018, 4, 453–459. [Google Scholar] [CrossRef]
- Swarup, R.; Kramer, E.; Perry, P.; Knox, K.; Leyser, O.; Haseloff, J.; Beemster, G.; Bhalerao, R.; Bennett, M. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat. Cell Biol. 2005, 7, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Ueda, J.; Komaki, M.K.; Bell, C.J.; Shimura, Y. Requirement of the Auxin Polar Transport System in Early Stages of Arabidopsis Floral Bud Formation. Plant Cell 1991, 3, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, B.S.; Sharma, M.; Laxmi, A. Role of sugar and auxin crosstalk in plant growth and development. Physiol. Plant. 2021, 174, e13546. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Bhosale, R. Developmental Roles of AUX1/LAX Auxin Influx Carriers in Plants. Front. Plant Sci. 2019, 10, 1306. [Google Scholar] [CrossRef] [Green Version]
- Okada, K.; Shimura, Y. Reversible Root Tip Rotation in Arabidopsis Seedlings Induced by Obstacle-Touching Stimulus. Science 1990, 250, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Hammes, U.Z.; Murphy, A.S.; Schwechheimer, C. Auxin Transporters—A Biochemical View. Cold Spring Harb. Perspect. Biol. 2021, 14, a039875. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.M. A Retro-Perspective on Auxin Transport. Front. Plant Sci. 2021, 12, 756968. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Kargul, J.; Marchant, A.; Zadik, D.; Rahman, A.; Mills, R.; Yemm, A.; May, S.; Williams, L.; Millner, P.; et al. Structure-Function Analysis of the Presumptive Arabidopsis Auxin Permease AUX1. Plant Cell 2004, 16, 3069–3083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westermann, J.; Streubel, S.; Franck, C.M.; Lentz, R.; Dolan, L.; Boisson-Dernier, A. An Evolutionarily Conserved Receptor-like Kinases Signaling Module Controls Cell Wall Integrity during Tip Growth. Curr. Biol. 2019, 29, 3899–3908.e3. [Google Scholar] [CrossRef]
- Marchant, A.; Kargul, J.; May, S.; Muller, P.; Delbarre, A.; Perrot-Rechenmann, C.; Bennett, M. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1999, 18, 2066–2073. [Google Scholar] [CrossRef]
- Roy, R.; Bassham, D.C. Root growth movements: Waving and skewing. Plant Sci. 2014, 221–222, 42–47. [Google Scholar] [CrossRef]
- Yan, J.; Wang, B.; Zhou, Y.; Hao, S. Resistance from agar medium impacts the helical growth of Arabidopsis primary roots. J. Mech. Behav. Biomed. Mater. 2018, 85, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Robards, A.W.; Goss, M. Effects of Mechanical Impedance on Root Growth in Barley, Hordeum vulgare L.: II. Effects on Cell Development in Seminal Roots. J. Exp. Bot. 1977, 28, 1216–1227. [Google Scholar] [CrossRef]
- Silva-Navas, J.; Moreno-Risueno, M.A.; Manzano, C.; Pallero-Baena, M.; Navarro-Neila, S.; Téllez-Robledo, B.; Garcia-Mina, J.M.; Baigorri, R.; Gallego, F.J.; del Pozo, J.C. D-Root: A system for cultivating plants with the roots in darkness or under different light conditions. Plant J. 2015, 84, 244–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-González, J.; Lacek, J.; Retzer, K. Dissecting Hierarchies between Light, Sugar and Auxin Action Underpinning Root and Root Hair Growth. Plants 2021, 10, 111. [Google Scholar] [CrossRef]
- Wan, Y.; Yokawa, K.; Baluška, F. Arabidopsis Roots and Light: Complex Interactions. Mol. Plant 2019, 12, 1428–1430. [Google Scholar] [CrossRef]
- Miotto, Y.; da Costa, C.T.; Offringa, R.; Kleine-Vehn, J.; dos Santos Maraschin, F. Effects of Light Intensity on Root Development in a D-Root Growth System. Front. Plant Sci. 2021, 12, 778382. [Google Scholar] [CrossRef]
- Yan, J.; Wang, B.; Zhou, Y. A root penetration model of Arabidopsis thaliana in phytagel medium with different strength. J. Plant Res. 2017, 130, 941–950. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, L.; Li, L.; Fu, L.; Liu, Y.; Xiong, Y.; Sheen, J. Integration of nutrient, energy, light, and hormone signalling via TOR in plants. J. Exp. Bot. 2019, 70, 2227–2238. [Google Scholar] [CrossRef]
- Stevenson, C.C.; Harrington, G.N. The impact of supplemental carbon sources on Arabidopsis thaliana growth, chlorophyll content and anthocyanin accumulation. Plant Growth Regul. 2009, 59, 255–271. [Google Scholar] [CrossRef]
- Kircher, S.; Schopfer, P. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 11217–11221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-González, J.; Lacek, J.; Weckwerth, W.; Retzer, K. Exogenous carbon source supplementation counteracts root and hypocotyl growth limitations under increased cotyledon shading, with glucose and sucrose differentially modulating growth curves. Plant Signal. Behav. 2021, 16, 1969818. [Google Scholar] [CrossRef] [PubMed]
- Raya-González, J.; López-Bucio, J.S.; Prado-Rodríguez, J.C.; Ruiz-Herrera, L.F.; Guevara-García, Á.A.; López-Bucio, J. The MEDIATOR genes MED12 and MED13 control Arabidopsis root system configuration influencing sugar and auxin responses. Plant Mol. Biol. 2017, 95, 141–156. [Google Scholar] [CrossRef] [PubMed]
- De Pessemier, J.; Chardon, F.; Juraniec, M.; Delaplace, P.; Hermans, C. Natural variation of the root morphological response to nitrate supply in Arabidopsis thaliana. Mech. Dev. 2013, 130, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Omary, M.; Hu, Y.; Doron, O.; Hoermayer, L.; Chen, Q.; Megides, O.; Chekli, O.; Ding, Z.; Friml, J.; et al. Cell kinetics of auxin transport and activity in Arabidopsis root growth and skewing. Nat. Commun. 2021, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, B.; Farris, B.; Clark, G.; Roux, S.J. Modulation of Root Skewing in Arabidopsis by Apyrases and Extracellular ATP. Plant Cell Physiol. 2015, 56, 2197–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, B.S.; Singh, M.; Aggrawal, P.; Laxmi, A. Glucose and Auxin Signaling Interaction in Controlling Arabidopsis thaliana Seedlings Root Growth and Development. PLoS ONE 2009, 4, e4502. [Google Scholar] [CrossRef]
- Singh, M.; Gupta, A.; Laxmi, A. Striking the Right Chord: Signaling Enigma during Root Gravitropism. Front. Plant Sci. 2017, 8, 1304. [Google Scholar] [CrossRef] [Green Version]
- Grabov, A.; Ashley, M.; Rigas, S.; Hatzopoulos, P.; Dolan, L.; Vicente-Agullo, F. Morphometric analysis of root shape. New Phytol. 2005, 165, 641–652. [Google Scholar] [CrossRef]
- Pilet, P.E.; Elliott, M.C.; Moloney, M.M. Endogenous and exogenous auxin in the control of root growth. Planta 1979, 146, 405–408. [Google Scholar] [CrossRef]
- Lopez, D.; Tocquard, K.; Venisse, J.-S.; Legué, V.; Roeckel-Drevet, P. Gravity sensing, a largely misunderstood trigger of plant orientated growth. Front. Plant Sci. 2014, 5, 610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutherford, R.; Masson, P.H. Arabidopsis thaliana sku Mutant Seedlings Show Exaggerated Surface-Dependent Alteration in Root Growth Vector. Plant Physiol. 1996, 111, 987–998. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.V.; Holbrook, N.M. Root-Gel Interactions and the Root Waving Behavior of Arabidopsis. Plant Physiol. 2004, 135, 1822–1837. [Google Scholar] [CrossRef] [Green Version]
- Migliaccio, F.; Tassone, P.; Fortunati, A. Circumnutation as an autonomous root movement in plants. Am. J. Bot. 2013, 100, 4–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulia, B.; Fournier, M. The power and control of gravitropic movements in plants: A biomechanical and systems biology view. J. Exp. Bot. 2009, 60, 461–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friml, J. Fourteen Stations of Auxin. Cold Spring Harb. Perspect. Biol. 2021, 14, a039859. [Google Scholar] [CrossRef]
- Konstantinova, N.; Korbei, B.; Luschnig, C. Auxin and Root Gravitropism: Addressing Basic Cellular Processes by Exploiting a Defined Growth Response. Int. J. Mol. Sci. 2021, 22, 2749. [Google Scholar] [CrossRef]
- Band, L.R.; Wells, D.M.; Larrieu, A.; Sun, J.; Middleton, A.M.; French, A.P.; Brunoud, G.; Sato, E.M.; Wilson, M.H.; Péret, B.; et al. Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc. Natl. Acad. Sci. USA 2012, 109, 4668–4673. [Google Scholar] [CrossRef] [Green Version]
- Retzer, K.; Singh, G.; Napier, R.M. It starts with TIRs. Nat. Plants 2018, 4, 410–411. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-González, J.; Lacek, J.; Weckwerth, W.; Retzer, K. Throttling Growth Speed: Evaluation of aux1-7 Root Growth Profile by Combining D-Root system and Root Penetration Assay. Plants 2022, 11, 650. https://doi.org/10.3390/plants11050650
García-González J, Lacek J, Weckwerth W, Retzer K. Throttling Growth Speed: Evaluation of aux1-7 Root Growth Profile by Combining D-Root system and Root Penetration Assay. Plants. 2022; 11(5):650. https://doi.org/10.3390/plants11050650
Chicago/Turabian StyleGarcía-González, Judith, Jozef Lacek, Wolfram Weckwerth, and Katarzyna Retzer. 2022. "Throttling Growth Speed: Evaluation of aux1-7 Root Growth Profile by Combining D-Root system and Root Penetration Assay" Plants 11, no. 5: 650. https://doi.org/10.3390/plants11050650
APA StyleGarcía-González, J., Lacek, J., Weckwerth, W., & Retzer, K. (2022). Throttling Growth Speed: Evaluation of aux1-7 Root Growth Profile by Combining D-Root system and Root Penetration Assay. Plants, 11(5), 650. https://doi.org/10.3390/plants11050650






