Boron, Manganese, and Zinc Sorption and Leaf Uptake on Citrus Cultivated on a Sandy Soil
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Description of the Study Site
2.2. Experimental Design and Treatment Application
2.3. Adsorption Study
2.4. Soil and Leaf Sampling and Analysis
2.5. Statistical Analysis
3. Result and Discussion
3.1. Adsorption Study in a Sandy Soil
3.2. Soil Micronutrients Distribution in a Sandy Soil
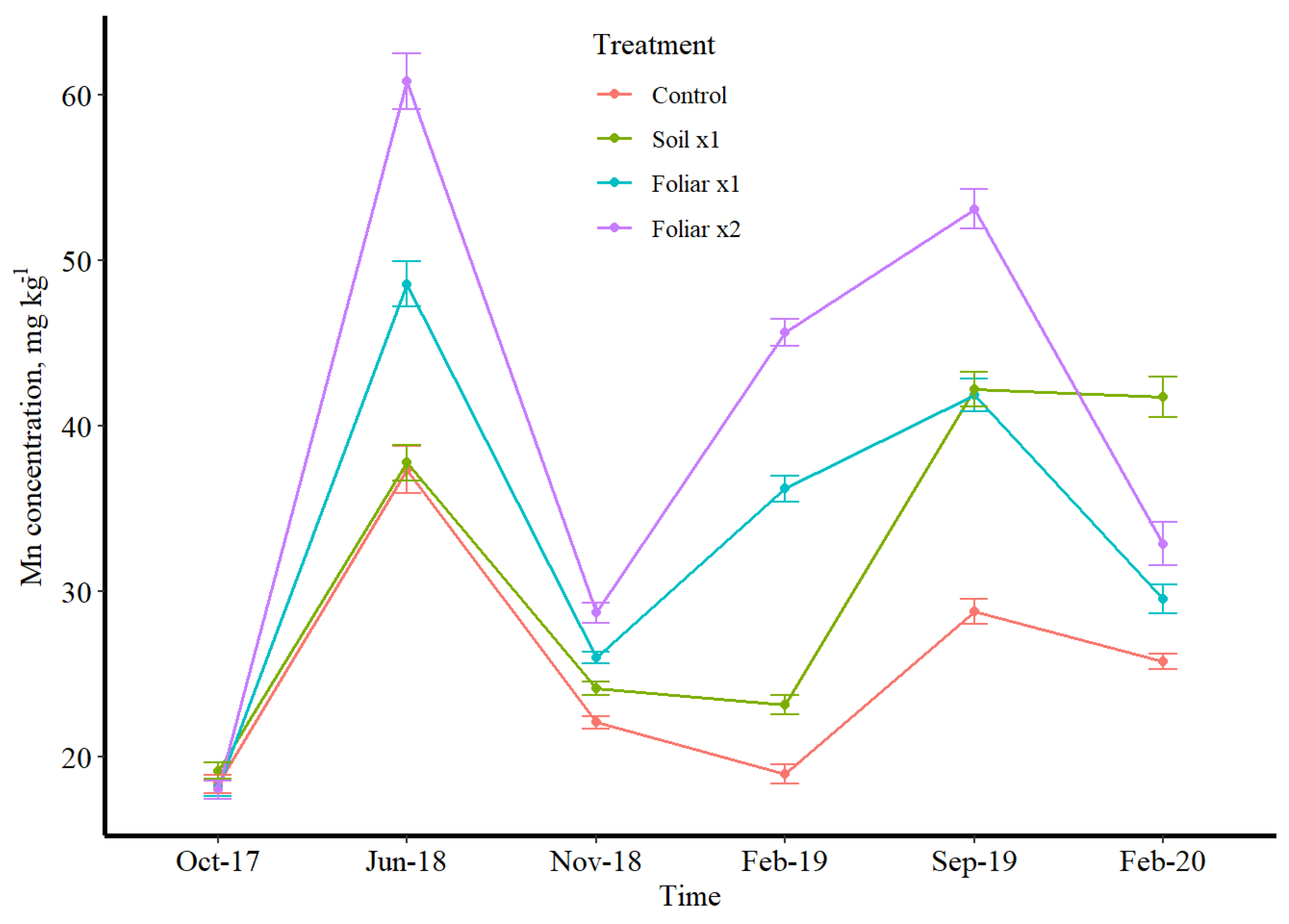
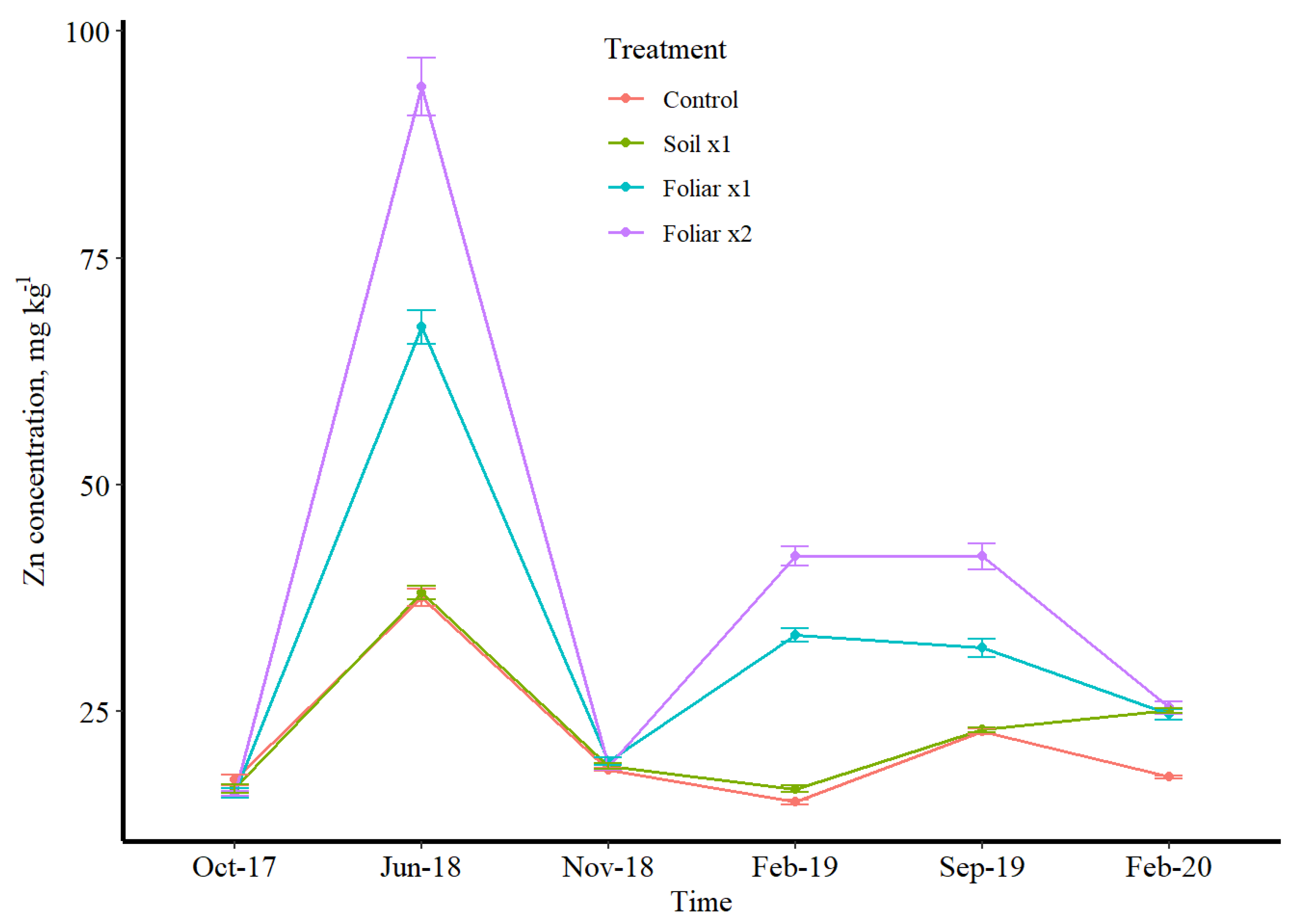
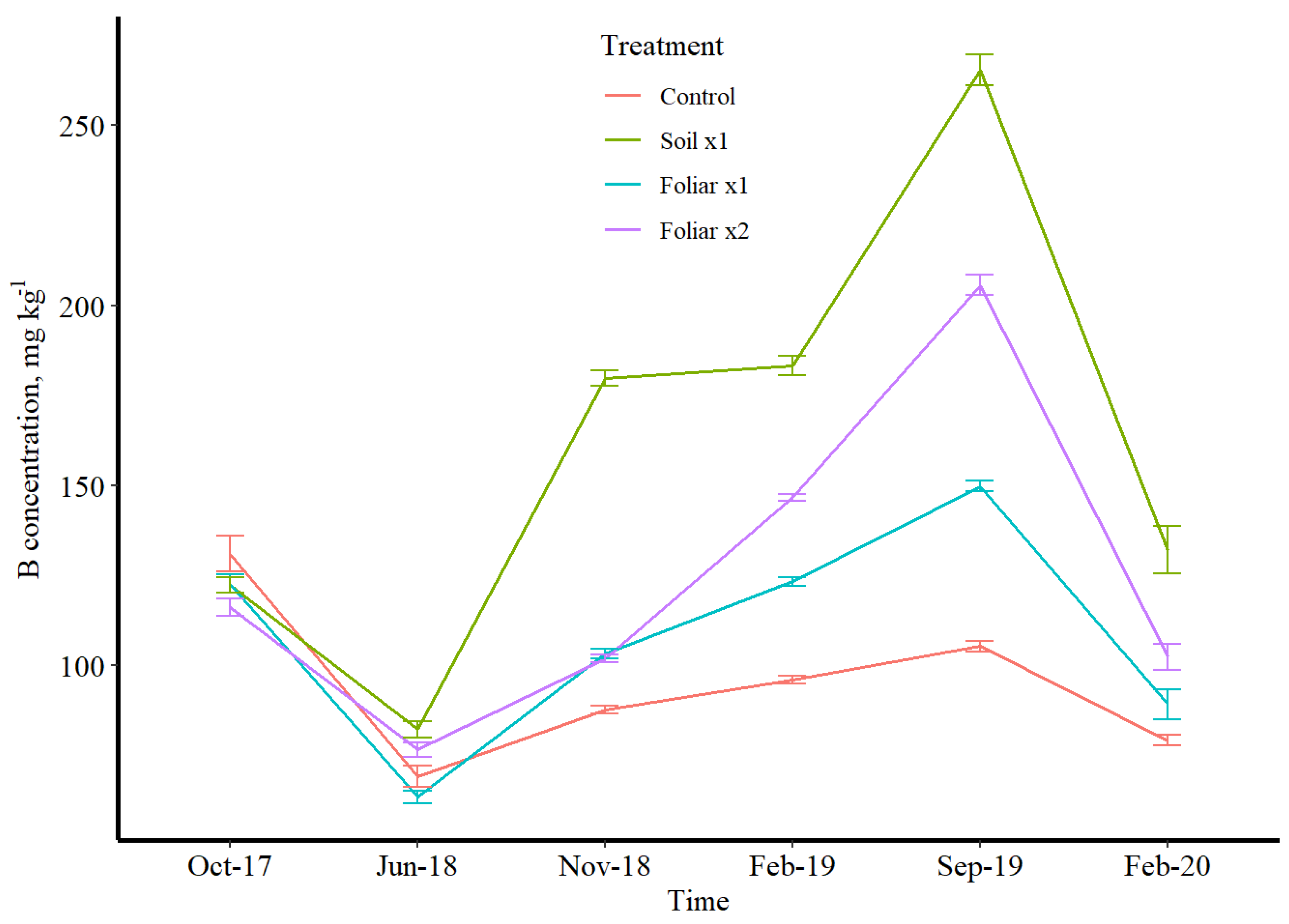
3.3. Seasonal Leaf Response to Micronutrients
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooda, P.S. Assessing Bioavailability of Soil Trace Elements; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 227–265. [Google Scholar]
- Shaheen, S.M.; Tsadilas, C.D.; Rinklebe, J. A review of the distribution coefficients of trace elements in soils: Influence of sorption system, element characteristics, and soil colloidal properties. Adv. Colloid Interface Sci. 2013, 201–202, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L. Environmental Soil Chemistry: An Overview; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1–42. [Google Scholar]
- Gupta, U.C.; Wu, K.; Liang, S. Micronutrients in soils, crops, and livestock. Earth Sci. Front. 2008, 15, 110–125. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Wutscher, H.K.; Smith, P.F. Citrus. In Nutrient Deficiencies and Toxicities in Crop Plants; Bennett, W.F., Paul, M.N., Eds.; APS Press: Eagan, MN, USA, 1993; pp. 165–170. [Google Scholar]
- Obreza, T.A.; Morgan, K.T. Nutrition of Florida Citrus Trees, 2nd ed.; EDIS: Washington, DC, USA, 2008; pp. 1–100. [Google Scholar]
- McLaren, R.G.; Swift, R.S.; Quin, B.F. EDTA-extractable copper, zinc, and manganese in soils of the canterbury plains. N. Z. J. Agric. Res. 1984, 27, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Zekri, M. Micronutrient Deficiencies in Citrus: Iron, Zinc, and Manganese; This Document is sl 204, A Fact Sheet; Soil and Water Science Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2003. [Google Scholar]
- Morgan, K.T.; Rouse, R.E.; Ebel, R.C. Foliar applications of essential nutrients on growth and yield of ‘Valencia’sweet orange infected with huanglongbing. HortScience 2016, 51, 1482–1493. [Google Scholar] [CrossRef]
- Survey, W.S. Soil Survey Staff, Natural Resources Conservation Service; United States Department of Agriculture: Washington, DC, USA, 2011. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk Density; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 2018; pp. 363–375. [Google Scholar]
- Uthman, Q.O.; Kadyampakeni, D.M.; Nkedi-Kizza, P. Boron availability and uptake in huanglongbing-affected citrus trees on a florida entisol. J. Plant Nutr. 2020, 43, 1248–1258. [Google Scholar] [CrossRef]
- Uthman, Q.O.; Kadyampakeni, D.M.; Nkedi-Kizza, P. Manganese adsorption, availability, and uptake in citrus under microsprinkler irrigation. Agrosyst. Geosci. Environ. 2020, 3, e20061. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. Lmertest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Scott, H.D.; Beasley, S.D.; Thompson, L.F. Effect of lime on boron transport to and uptake by cotton. Soil Sci. Soc. Am. J. 1975, 39, 1116–1121. [Google Scholar] [CrossRef]
- Mualem, Y. A new model for predicting the hydraulic conductivity of unsaturated porous media. Water Resour. Res. 1976, 12, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Hippler, F.W.R.; Boaretto, R.; Quaggio, J.; Azevedo, R.A.; Mattos, D. Towards soil management with Zn and Mn: Estimates of fertilisation efficacy of citrus trees. Ann. Appl. Biol. 2015, 166, 484–495. [Google Scholar] [CrossRef]
- Marschner, H. Zinc Uptake from Soils; Springer: Heidelberg, The Netherlands, 1993; pp. 59–77. [Google Scholar]
- Harris, W.G.; Carlisle, V.W.; Chesser, S.L. Clay mineralogy as related to morphology of Florida soils with sandy epipedons. Soil Sci. Soc. Am. J. 1987, 51, 1673–1677. [Google Scholar] [CrossRef]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Kadyampakeni, D.M.; Morgan, K.T. Nutrition of Florida Citrus Trees, 3rd ed.; EDIS: Washington, DC, USA, 2020. [Google Scholar]
- Bukovac, M.J.; Wittwer, S.H. Absorption and mobility of foliar applied nutrients. Plant Physiol. 1957, 32, 428–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittwer, S.; Teubner, F. Foliar absorption of mineral nutrients. Annu. Rev. Plant Physiol. 1959, 10, 13–30. [Google Scholar] [CrossRef]
- Orgell, W.H. The isolation of plant cuticle with pectic enzymes. Plant Physiol. 1955, 30, 78–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey-Wyssling, A. Submicroscopic Morphology of Protoplasm and Its Derivatives; Elsevier: Amsterdam, The Netherlands, 1948. [Google Scholar]
- Swanson, C.A.; Whitney, J.B. Studies on the translocation of foliar-applied p32 and other radioisotopes in bean plants. Am. J. Bot. 1953, 40, 816–823. [Google Scholar] [CrossRef]
- Wallihan, E.F.; Heymann-Herschberg, L. Some factors affecting absorption and translocation of zinc in citrus plants. Plant Physiol. 1956, 31, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, M.H.F.; Machado, B.A.; Rodrigues, E.S.; Montanha, G.S.; Rossi, M.L.; Otto, R.; Linhares, F.S.; Carvalho, H.W.P. In vivo evaluation of zn foliar uptake and transport in soybean using X-ray absorption and fluorescence spectroscopy. J. Agric. Food Chem. 2019, 67, 12172–12181. [Google Scholar] [CrossRef] [PubMed]

| Soil Depth | ρs | ρb | θs | θFC |
|---|---|---|---|---|
| g cm−3 | g cm−3 | cm3 cm−3 | cm3 cm−3 | |
| 0–15 | 2.65 | 1.56 | 0.41 | 0.07 |
| 15–30 | 2.65 | 1.67 | 0.37 | 0.10 |
| 30–45 | 2.65 | 1.61 | 0.39 | 0.10 |
| 45–60 | 2.65 | 1.63 | 0.38 | 0.09 |
| Soil Depth | KD, cm3 g−1 | R (θs) | R (θFC) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B | Mn | Zn | B | Mn | Zn | B | Mn | Zn | |
| 0–15 | 0.10 | 2.20 | 6.47 | 1.38 | 9.34 | 25.54 | 3.23 | 50.03 | 145.19 |
| 15–30 | 0.03 | 0.13 | 2.68 | 1.14 | 1.59 | 13.12 | 1.50 | 3.17 | 45.81 |
| 30–45 | 0.04 | 0.10 | 0.05 | 1.16 | 1.41 | 1.21 | 1.64 | 2.61 | 1.81 |
| 45–60 | 0.03 | 0.09 | 0.05 | 1.13 | 1.38 | 1.21 | 1.54 | 2.63 | 1.91 |
| Source of Variation | Effect | B | Mn | Zn |
|---|---|---|---|---|
| Treatment | FE | ** | *** | *** |
| Time | FE | *** | *** | *** |
| Treatment × Time | FE | *** | *** | *** |
| Depth | RE | * | *** | *** |
| Rep × Depth | RE | ns | *** | *** |
| B | Mn | Zn | ||||
|---|---|---|---|---|---|---|
| Soil Depth, cm | Control | Soil 1 | Control | Soil | Control | Soil |
June 2018 | __________________________________mg kg−1______________________________________ | |||||
| 0–15 | 0.4 ± 0.004 | 0.5 ± 0.005 | 49.9 ± 0.7 | 72.6 ± 1.2 | 24.7 ± 0.5 | 35.7 ± 0.7 |
| 15–30 | 0.3 ± 0.002 | 0.4 ± 0.002 | 15.2 ± 0.4 | 19.4 ± 0.4 | 10.5 ± 0.6 | 8.7 ± 0.3 |
| 30–45 | 0.3 ± 0.002 | 0.4 ± 0.002 | 8.5 ± 0.2 | 11.5 ± 0.3 | 5.1 ± 0.2 | 4.7 ± 0.1 |
| 45–60 | 0.3 ± 0.005 | 0.4 ± 0.002 | 8.2 ± 0.2 | 10.1 ± 0.2 | 5.6 ± 0.2 | 4.8 ± 0.1 |
| February 2019 | ||||||
| 0–15 | 0.3 ± 0.002 | 0.4 ± 0.004 | 45.4 ± 0.6 | 55.3 ± 0.8 | 28.8 ± 0.5 | 31.6 ± 0.6 |
| 15–30 | 0.3 ± 0.003 | 0.3 ± 0.003 | 15.4 ± 0.3 | 17.7 ± 0.4 | 13.9 ± 0.5 | 15.2 ± 0.6 |
| 30–45 | 0.2 ± 0.002 | 0.3 ± 0.004 | 8.7 ± 0.2 | 8.7 ± 0.2 | 8.9 ± 0.4 | 6.9 ± 0.3 |
| 45–60 | 0.2 ± 0.001 | 0.2 ± 0.003 | 7.1 ± 0.1 | 6.4 ± 0.1 | 6.4 ± 0.4 | 4.1 ± 0.1 |
| September 2019 | ||||||
| 0–15 | 0.3 ± 0.002 | 0.3 ± 0.006 | 45.8 ± 0.7 | 109.2 ± 2.5 | 23.0 ± 0.6 | 74.6 ± 2.6 |
| 15–30 | 0.3 ± 0.001 | 0.3 ± 0.006 | 19.9 ± 0.6 | 44.2 ± 0.9 | 17.5 ± 0.7 | 34.7 ± 1.1 |
| 30–45 | 0.3 ± 0.002 | 0.3 ± 0.009 | 9.6 ± 0.2 | 34.3 ± 1.0 | 11.2 ± 0.6 | 29.5 ± 0.8 |
| 45–60 | 0.3 ± 0.002 | 0.3 ± 0.012 | 5.9 ± 0.1 | 18.5 ± 0.7 | 8.6 ± 0.5 | 15.7 ± 0.6 |
| Element | r | p-Value |
|---|---|---|
| Boron | −0.76 | 0.0045 |
| Zinc | −0.69 | 0.0127 |
| Iron | 0.49 | 0.1041 |
| Copper | 0.65 | 0.0215 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uthman, Q.O.; Kadyampakeni, D.M.; Nkedi-Kizza, P.; Kwakye, S.; Barlas, N.T. Boron, Manganese, and Zinc Sorption and Leaf Uptake on Citrus Cultivated on a Sandy Soil. Plants 2022, 11, 638. https://doi.org/10.3390/plants11050638
Uthman QO, Kadyampakeni DM, Nkedi-Kizza P, Kwakye S, Barlas NT. Boron, Manganese, and Zinc Sorption and Leaf Uptake on Citrus Cultivated on a Sandy Soil. Plants. 2022; 11(5):638. https://doi.org/10.3390/plants11050638
Chicago/Turabian StyleUthman, Qudus O., Davie M. Kadyampakeni, Peter Nkedi-Kizza, Samuel Kwakye, and Neriman Tuba Barlas. 2022. "Boron, Manganese, and Zinc Sorption and Leaf Uptake on Citrus Cultivated on a Sandy Soil" Plants 11, no. 5: 638. https://doi.org/10.3390/plants11050638
APA StyleUthman, Q. O., Kadyampakeni, D. M., Nkedi-Kizza, P., Kwakye, S., & Barlas, N. T. (2022). Boron, Manganese, and Zinc Sorption and Leaf Uptake on Citrus Cultivated on a Sandy Soil. Plants, 11(5), 638. https://doi.org/10.3390/plants11050638








