Abstract
It has been reported that the mitochondrial carrier family proteins of AtMTM1 and AtMTM2 are necessary for manganese superoxide dismutase (MnSOD) activation in Arabidopsis, and are responsive to methyl viologen (MV)-induced oxidative stress. In this study, we showed that MnSOD activity was enhanced specifically by Mn treatments. By using AtMnSOD-overexpressing and AtMnSOD-knockdown mutant plants treated with the widely used oxidative stressors including MV, NaCl, H2O2, and tert-butyl hydroperoxide (t-BH), we revealed that Arabidopsis MnSOD was crucial for root-growth control and superoxide scavenging ability. In addition, it has been reported that E. coli MnSOD activity is inhibited by Fe and that MTM1-mutated yeast cells exhibit elevated Fe content and decreased MnSOD activity, which can be restored by the Fe2+-specific chelator, bathophenanthroline disulfonate (BPS). However, we showed that BPS inhibited MnSOD activity in AtMTM1 and AtMTM2 single- and double-mutant protoplasts, implying that altered Fe homeostasis affected MnSOD activation through AtMTM1 and AtMTM2. Notably, we used inductively coupled plasma-optical emission spectrometry (ICP-OES) analysis to reveal an abnormal Fe/Mn ratio in the roots and shoots of AtMTM1 and AtMTM2 mutants under MV stress, indicating the importance of AtMTM1 in roots and AtMTM2 in shoots for maintaining Fe/Mn balance.
1. Introduction
Superoxide dismutases (SODs) are distributed in the cytoplasm, chloroplasts, and mitochondria of prokaryotic and eukaryotic cells [1,2,3]. They are classified as CuZnSOD, FeSOD, MnSOD, or NiSOD according to the transition metal cofactor ions at the active site [4,5,6]. Cellular superoxide (O2•−) is mainly generated from electron transport chain complexes, and SODs catalyse the dismutation of O2•− to O2 and H2O2. Toxic H2O2 is converted to H2O mainly by catalase, ascorbate peroxidase, and glutathione peroxidase [7]; thus, SODs cooperate with other enzymes to relieve oxidative stress [8,9,10,11]. It has been reported that AtMnSOD-overexpressing plants exhibit increased catalase and peroxidase activities, with decreased malondialdehyde content after NaCl treatment, and maintain a higher germination rate in the presence of oxidative stressors, such as methyl viologen (MV) and H2O2 [12,13]. Plants harboured with antisense AtMnSOD show decreased MnSOD protein levels, with altered tricarboxylic acid cycle enzyme levels and root growth after treatment with NaCl, sorbitol, Fe, and MV [14]. In this study, we established both AtMnSOD-overexpressing (MnSOD-OE) and AtMnSOD-knockdown (msd1) plants to confirm the cofactor specificity and post-translational regulation of MnSOD under conditions of oxidative stress. In addition, antagonism between Fe and Mn has been reported in tomato and rice plants, in which Fe suppresses Mn levels and vice versa [15,16,17]. Therefore, we also investigated the effect of metal ion treatments on MnSOD activity in Arabidopsis.
The MnSOD dimer in E. coli is localised in the cytosol and is regulated by repressors and Fe ion concentrations. Fe suppresses the biosynthesis of MnSOD at the transcriptional and post-translational levels [18,19]. E. coli MnSOD protein and activity levels are inhibited by Fe2+, but not Co2+, Ni2+, or Zn2+, and both protein and activity levels are induced by Mn2+ treatment. It has been suggested that Fe and Mn compete for the metal-binding site of MnSOD, but only Mn can activate the enzyme [19]. X-ray crystallography further shows that MnSOD active sites bind Mn or Fe, but Fe-substituted MnSOD blocks the substrate access channel and inactivates the enzyme [20,21]. Moreover, intracellular O2•− inactivates Fe-S cluster biogenesis enzymes [22], and the interruption in the mitochondrial Fe-S pathway is associated with MnSOD activity in E. coli [23,24].
Yeast Mn transporters of plasma membrane-localized SMF1, intracellular vesicle-localized SMF2, and mitochondrial inner membrane-localized carrier protein MTM1 are involved in mitochondrial MnSOD activation [25,26,27,28]. The yeast MTM1 mutant retains normal MnSOD protein levels after treatment with the metals Mn, Fe, Cu, and Zn, but MnSOD activity is only restored by Mn treatment [25]. Yeast MnSOD acquires its catalytic cofactor, Mn, via MTM1; thus, MnSOD monomers fold into active tetrameric enzymes during post-translational regulation, and unknown factors may also facilitate Mn binding [27]. Intriguingly, yeast MTM1 mutant retains normal mitochondrial Mn levels and exhibits higher mitochondrial Fe content [25]. In addition, MTM1- and SMF2-mutated yeast cells exhibit normal MnSOD protein levels, and the loss of MnSOD activity is fully restored by Mn supplementation [25,27]. Although the yeast SMF2 mutant exhibits decreased Mn content and lower MnSOD activity, mitochondrial Fe levels in the mutant are normal. However, treatment with the Fe2+-specific chelator, bathophenanthroline disulphonate (BPS), decrease mitochondrial Fe levels and increases MnSOD activity [29]. BPS treatment also restores MnSOD activity in the yeast MTM1 mutant [29]. Taken together, these data indicate that the relationship between altered mitochondrial Fe levels and MnSOD activity in yeast is unclear.
Arabidopsis mitochondrial carrier proteins, AtMTM1 and AtMTM2, bind Mn for mitochondrial MnSOD activation. These proteins share a high amino acid sequence homology with yeast MTM1 and they are induced by MV [30,31]. Our previous study showed that AtMTM2 has distinct expression levels from AtMTM1 during development, and that Mn levels are lower in the roots of both mtm1 (miRNA-mediated AtMTM1-knockdown) and mtm2 (T-DNA insertional AtMTM2-knockout) mutants. However, Fe levels are decreased in the roots of the mtm1 mutant, but remain normal in the mtm2 mutant [30]. The role of Mn transporters in plant mitochondria is unclear [32,33], and the effects of Fe metabolism and mitochondrial Fe-S cluster biogenesis proteins on MnSOD activation in Arabidopsis have not been elucidated.
In this study, we observed that MnSOD activity was enhanced significantly with higher Mn concentrations, but not with Fe, Cu, or Zn treatments, implying Mn cofactor specificity for MnSOD activation. Moreover, AtMTM1 and AtMTM2 gene expression levels increased with MnSOD gene expression levels in the presence of the commonly used oxidative stressors including MV, NaCl, H2O2, and tertiary-butyl hydroperoxide (t-BH). We showed MnSOD activity in MnSOD-OE and msd1 mutants corresponded to the representative treatments of MV and NaCl, and revealed MnSOD was crucial for early root growth and plant development. In addition, we treated mtm1, mtm2, and mtm1 mtm2-double mutant with Mn supplementation and the Fe chelator, BPS, and found that Mn and Fe homeostasis affected the primary root length and MnSOD activity via AtMTM1 and AtMTM2. Moreover, Fe/Mn ratio analysis further demonstrated the physiological importance of AtMTM1 in roots and AtMTM2 in shoots for maintaining the Fe/Mn balance under the representative MV treatment.
2. Results
2.1. Generation of AtMnSOD-Overexpressing Plants and Characterisation of AtMnSOD T-DNA Insertion Mutants
To increase the effect of Mn, Fe, and oxidative stressors on Arabidopsis AtMnSOD, AtMTM1, and AtMTM2 expression levels, we generated AtMnSOD-overexpressing plants (MnSOD-OE; MnSOD-apoprotein-overexpressing plants) and characterised the AtMnSOD T-DNA insertion (msd1) mutants (Supplementary Figures S1 and S2).
The highest expression line of MnSOD-OE plants with approximately two-fold MnSOD mRNA expression levels was used in the following study; however, MnSOD activity and protein levels in MnSOD-OE plants were not significantly affected. MnSOD-OE plants showed a late-flowering phenotype compared to wild-type (Col) plants (Supplementary Figure S1).
msd1 plants were characterized by genotyping and RT-qPCR. We confirmed that msd1 is a heterologous T-DNA insertional knockdown mutant. The lowest expression line with a 20% reduction in MnSOD transcript level was used in the following study. MnSOD activity and protein levels in the msd1 mutant were lower, while the msd1 mutant showed an early flowering phenotype compared to Col plants (Supplementary Figure S2). Moreover, we are unable to screen the homologous msd1 plants, implying the lethal effect of the null mutant for plant germination.
2.2. Effect of Mn and Fe on Transgenic AtMnSOD-Overexpressing Plants
MnSOD activity in Col and MnSOD-OE plants was analysed. Fourteen-day-old seedlings were treated with 1 mM of the metal ions, Mn, Fe, CuSO4 (Cu), ZnSO4 (Zn), Mn and Fe, Mn and Cu, or Mn and Zn, for 16 h and analyzed by in-gel SOD activity assay (Figure 1). We observed that MnSOD activity was increased after Mn treatment, but was decreased after Fe, Cu, and Zn treatments in Col plants. Treatments of Mn and Fe, Mn and Cu, or Mn and Zn caused intermediate MnSOD activity in Col plants (Figure 1A), indicating that antagonisms between Mn and other metals occurred. These effects were obvious in MnSOD-OE plants (Figure 1B), implying an increase in the activation of MnSOD-apoprotein after Mn treatment in MnSOD-OE plants. Moreover, the protein level in MnSOD-OE and Col plants is similar, implying the saturated level of MnSOD protein inside cells.
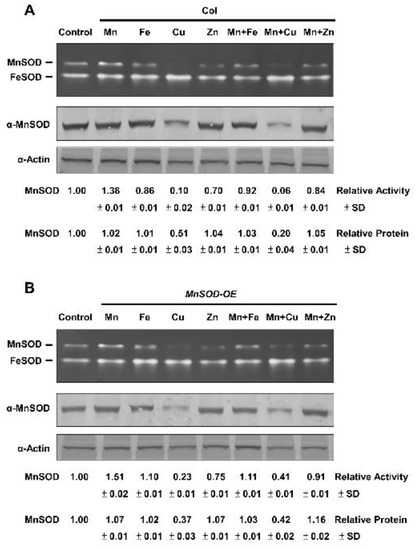
Figure 1.
MnSOD activity and protein levels of Col and MnSOD-OE plants in response to Mn, Fe, and duplex metal ion treatments. (A,B) Fourteen-day-old seedlings were treated with 1 mM of Mn, Fe, Cu, Zn, Mn and Fe, Mn and Cu, or Mn and Zn for 16 h. In-gel SOD activity assay (top) and immunoblotting with α-MnSOD and α-Actin antibodies (bottom) were conducted. Actin was used as a loading control. MnSOD activity and protein levels were measured relative to those in control. Data represent one of three independent repeats.
2.3. Post-Translational Regulation of MnSOD through Oxidative Stressors
AtMTM1 is elevated in response to MV, but not H2O2, when seedlings are grown on plates containing these stressors [31], and we have reported that AtMTM1, AtMTM2, and MnSOD gene expression levels increase after MV treatment [30]. In this study, 14-day-old Col seedlings in 1/2 MS liquid medium were treated with the widely used oxidative stressors including 5 μM MV, 150 mM NaCl, 10 mM H2O2, or 1 mM tert-butyl hydroperoxide (t-BH) with agitation for 2 to 8 h.
RT-qPCR analysis showed that AtMTM1, AtMTM2, and MnSOD gene expression levels were elevated as early as 2 h after all oxidative stress treatments (Figure 2), and we applied MV or NaCl as the representative stressors in the following studies. The mitochondrial oxidation-responsive gene, AOX1A, was used as a reference, as previously reported [30]. AtMTM1 and AtMTM2 expression levels in control without stressors are shown in Supplementary Figure S3. Our results implied that oxidative stressors specifically induced AtMTM1 and AtMTM2.
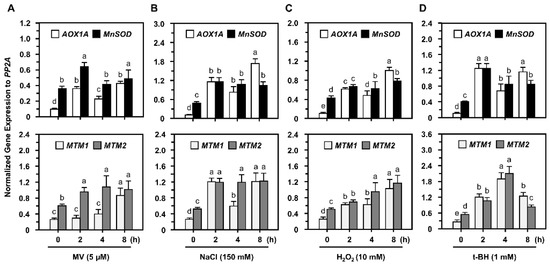
Figure 2.
AtMnSOD, AtMTM1, and AtMTM2 gene expression levels of Col in response to oxidative stressors. (A–D) Fourteen-day-old seedlings were incubated with 5 μM MV, 150 mM NaCl, 10 mM H2O2, or 1 mM t-BH for 2 to 8 h. Gene expressions were normalized to AOX1A which is an oxidation-responsive gene. PP2A was an input control. Data are mean ± SD of three biological replicates. The statistical significances (p < 0.05) are indicated as different letters (Duncan’s multiple range test).
We showed that MnSOD activity was similar between Col and MnSOD-OE plants, and was lower in msd1 plants without oxidative stressors (Supplementary Figures S1 and S2). To clarify the physiological role of MnSOD under oxidative stress, we applied two representative oxidative stressors of MV and NaCl. We incubated 14-day-old seedlings of Col, MnSOD-OE, and msd1 in 1/2 MS liquid medium containing 5 μM MV or 150 mM NaCl with agitation for 24 h, and measured MnSOD activity and protein levels (Figure 3). MnSOD activity was markedly induced in MnSOD-OE and was slightly induced in msd1 plants by MV stressor, and MnSOD activity was still lower in msd1 plants than in Col plants under stress (Figure 3A,B); however, MnSOD protein levels were not significantly different. Similar expression patterns were observed in Col, MnSOD-OE, and msd1 seedlings under NaCl stress (Figure 3C,D). These results indicated the post-translational regulation of MnSOD under oxidative stress in Arabidopsis.
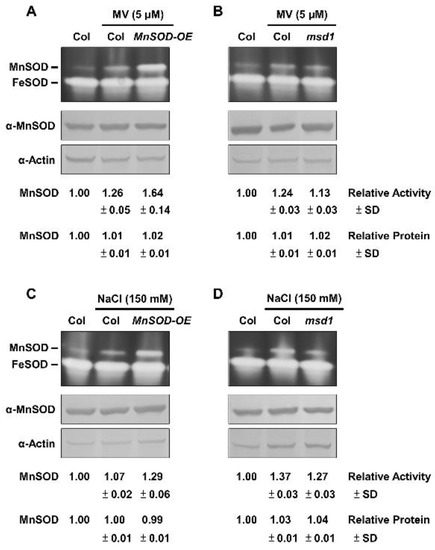
Figure 3.
MnSOD activity and protein levels of MnSOD-OE and msd1 seedlings in response to oxidative stressors. Fourteen-day-old seedlings were incubated with 5 μM MV (A,B) or 150 mM NaCl (C,D) for 24 h, as indicated. In-gel SOD activity assay (top) and immunoblotting with α-MnSOD and α-Actin antibodies (bottom) were conducted. Actin was used as a loading control. MnSOD activity and protein levels were measured relative to those in Col control. Data represent one of three independent repeats.
2.4. Role of MnSOD in the Control of Primary Root Growth during Oxidative Stress
A previous study of the Arabidopsis MnSOD-knockdown mutant oiwa showed that it is a female gametophytic mutant with defective embryo sac development and fertilization, and that the mutation affects reactive oxygen species homeostasis in the mitochondria and cytosol [34,35]. In this study, we focused on the role of MnSOD in early root growth under stress conditions. We applied different stressors in 1/2 MS plates and measured the primary root lengths of Col, MnSOD-OE, and msd1 seedlings (Figure 4).
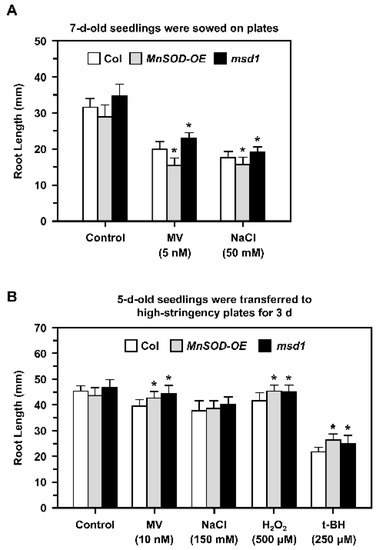
Figure 4.
Root lengths of Col, MnSOD-OE, and msd1 seedlings during oxidative stress. (A) Seedlings were sown on 1/2 MS plates containing 5 nM MV and 50 mM NaCl for 7 days, and the root lengths were measured. (B) Five-day-old seedlings with similar root lengths were transferred from 1/2 MS medium to high-stringency plates containing 10 nM MV, 150 mM NaCl, 500 μM H2O2, or 250 μM t-BH for 3 days, and the root lengths were measured. Data are mean ± SD of three independent repeats. n = 30 seedlings. * Significant at p < 0.05 compared with the Col.
When seedlings were grown on plates containing 5 nM MV or 50 mM NaCl (Figure 4A), root growth was inhibited in all seedlings. In addition, 7-day-old MnSOD-OE seedlings had shorter roots and msd1 had longer roots compared to the roots of Col plants.
Five-day-old seedlings with similar root lengths were then transferred from 1/2 MS plates to high-stringency plates containing 10 nM MV, 150 mM NaCl, 500 μM H2O2, or 250 μM t-BH for 3 days (Figure 4B). Root growth was slightly inhibited by MV, NaCl, and H2O2, and was markedly inhibited by t-BH. In addition, both MnSOD-OE and msd1 seedlings had longer roots in the presence of MV, H2O2, and t-BH, and this may be restricted to the shorter period of treatment. Taken together, we noticed that MnSOD responded to stress conditions during early primary root growth.
2.5. O2•− and H2O2 Accumulation and Distribution in AtMnSOD-Overexpressing Plants under Stress
The O2•− and H2O2 metabolism levels via MnSOD in Arabidopsis are unclear; thus, we used a typical oxidative stressor MV in this study, and treated mature 21-day-old seedlings of Col and MnSOD-OE with 5 μM MV stress for 3 days. The O2•− and H2O2 accumulation was measured by NBT and DAB staining, respectively (Figure 5). Relative O2•− accumulation was increased under MV treatment in Col, but decreased significantly in MnSOD-OE plants (Figure 5A). Relative H2O2 accumulation was increased in both Col and MnSOD-OE seedlings under MV treatment, but MnSOD-OE seedlings exhibited lower H2O2 levels (Figure 5B). These results indicated that mature MnSOD-OE plants retain higher superoxide scavenging ability with decreased O2•− and H2O2 amounts.
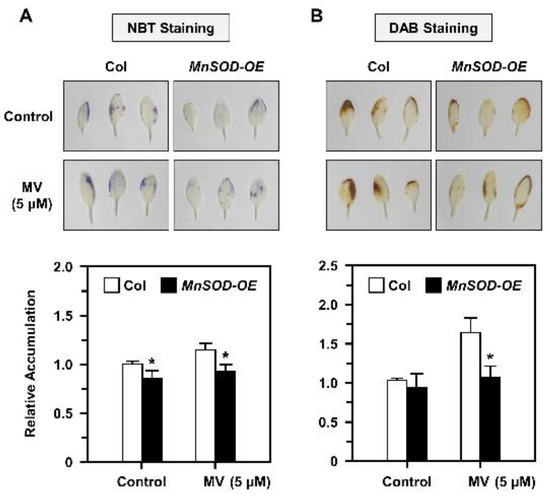
Figure 5.
O2•− and H2O2 accumulation of Col and MnSOD-OE seedlings under MV stress. (A,B) Twenty-one-day-old seedlings were treated with 5 μM MV for 3 days, then O2•− and H2O2 accumulation were analyzed by NBT and DAB staining (top), respectively. Each accumulation in a plant was measured relative to Col (bottom). Data are mean ± SD of three independent repeats. * Significant at p < 0.05 compared with the Col.
2.6. Root-Length Phenotype of AtMTM1- and AtMTM2-Mutated Seedlings Analysed by Extra Mn Supply
The importance of the Mn carrier proteins, AtMTM1 and AtMTM2, for mitochondrial MnSOD activation has been reported using the miRNA-mediated AtMTM1-knockdown mutant (mtm1), the T-DNA insertional AtMTM2-knockout mutant (mtm2), and mtm1 mtm2-double mutants [30]. We have reported that the defective root-length phenotypes of mtm1 and mtm2 single and double mutant seedlings grown in 1/2 MS plates are restored through MnCl2 (Mn) treatment, and AtMTM1 and AtMTM2 are involved in the root-length control with divergent effects [30]. To further examine the effect of defective Mn and increased Mn supply on MnSOD activity in mtm1, mtm2, and mtm1 mtm2-double mutant plants, we monitored the primary root lengths on basal medium plates without Mn (Mn-deficient) or with Mn at 14 μM (normal Mn), 700 μM (50-fold increase), or 1050 μM (75-fold increase) for 6 days (Figure 6).
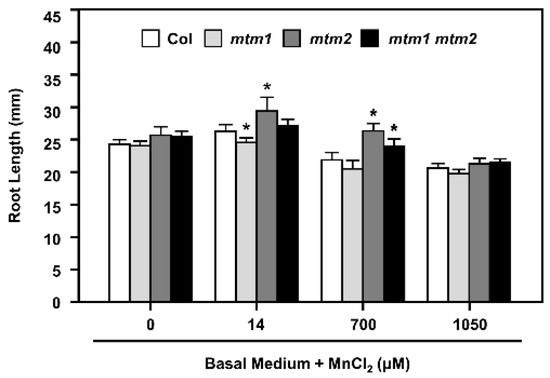
Figure 6.
Root lengths of Col, mtm1, mtm2, and mtm1 mtm2-double mutants with Mn deficiency or by extra Mn supply. Seedlings were grown on the basal medium plates without MnCl2 supply or with extra MnCl2 of 14 μM (normal Mn), 700 μM (50-fold increase), or 1050 μM (75-fold increase) for 6 days, then the root lengths were measured. Data are mean ± SD of three independent repeats. n = 30 seedlings. * Significant at p < 0.05 compared with the Col.
Col plants and all mutant lines showed similar root lengths under Mn-deficient conditions. A normal Mn supply resulted in abnormal root length in the single mutants; mtm1 and mtm2 plants showed shorter and longer root-length phenotypes, respectively. A 50-fold increase in Mn supply inhibited root growth, but mtm2 and mtm1 mtm2-double mutant plants retained longer root lengths compared to Col plants. A 75-fold increase in Mn supply inhibited root growth in all seedlings. Based on the toxicity of increased Mn concentration and its inhibition of root growth in Col seedlings, the altered root lengths of mutant plants indicated that AtMTM1 participates more in the control of Mn homeostasis than AtMTM2. Overall, these results confirmed that AtMTM1 and AtMTM2 coordinate Mn homeostasis with divergence.
2.7. MnSOD Activity in AtMTM1- and AtMTM2-Mutated Protoplasts after Fe Chelation
Our previous study showed that AtMTM1 and AtMTM2 are necessary for AtMnSOD activation; AtMTM1-mutant plants have lower Fe levels in the roots, but AtMTM2-mutant plants have similar Fe levels in the shoots compared to wild-type plants [30]. To delineate the role of Fe in MnSOD activation in mtm1, mtm2, and mtm1 mtm2-double mutants, we applied the Fe2+-specific chelator, BPS, to mesophyll protoplasts at 100 to 1000 μM for 16 h (Figure 7).
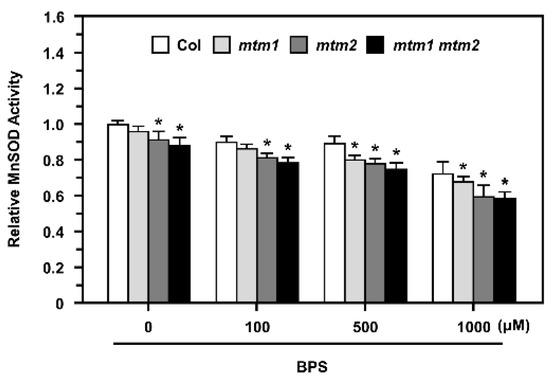
Figure 7.
MnSOD activity of Col, mtm1, mtm2, and mtm1 mtm2-double mutant protoplasts after Fe2+-specific chelator (BPS) treatment. Protoplasts were treated without or with 100, 500, or 1000 μM BPS for 16 h. MnSOD activity in protoplasts was measured relative to Col without BPS treatment. Data are mean ± SD of three independent repeats. * Significant at p < 0.05 compared with the Col.
The mtm1 mtm2-double mutant protoplasts showed lower MnSOD activity than Col protoplasts without treatment, which is consistent with the results of a previous study [30]. In this study, we revealed that mtm1 and mtm2 protoplasts exhibited slightly decreased MnSOD activity compared to Col protoplasts. The 100 and 500 μM BPS treatments slightly inhibited MnSOD activity in Col protoplasts, and the 1000 μM BPS treatment markedly decreased MnSOD activity. By comparing relative MnSOD activity within each treatment, mtm1 mutant protoplasts exhibited decreased MnSOD activity after 500 μM BPS treatment, and mtm2 and mtm1 mtm2-double mutant protoplasts showed significantly decreased MnSOD activity from 100 μM BPS, implying that AtMTM2 was more sensitive to BPS treatment than AtMTM1. Overall, these results indicated that the altered Fe homeostasis is involved in AtMTM1 and AtMTM2-mediated MnSOD activity.
2.8. Fe/Mn Ratio in AtMTM1- and AtMTM2-Mutant Seedlings Treated with Methyl Viologen (MV)
We further elucidated the balance between Mn and Fe in mtm1, mtm2, and mtm1 mtm2-double mutants using inductively coupled plasma-optical emission spectrometry (ICP-OES). To reveal the Fe/Mn ratio in root and shoot tissues, we used MV as a representative stressor, and applied higher MV dosage with long-term treatment in this study, since AtMTM1 and AtMTM2 was altered slightly in short-term MV treatment as shown in Figure 2. Fourteen-day-old seedlings were incubated in 1/2 MS liquid medium containing 10 μM MV with agitation for 3 days (Figure 8). Before treatment, the Fe/Mn ratio was lower in the roots of mtm1, but higher in the roots of mtm2 and mtm1 mtm2-double mutants compared to the ratio in Col seedlings. In addition, the Fe/Mn ratios were similar in the shoots of all seedlings. However, MV-induced stress caused a decrease in the Fe/Mn ratio in the roots of mtm1 seedlings (Figure 8A) and an increase in the Fe/Mn ratio in the shoots of mtm2 seedlings compared to the ratios in Col seedlings (Figure 8B). Taken together, these findings indicated that AtMTM1 and AtMTM2 are involved in Fe/Mn balance in the roots and shoots, respectively.
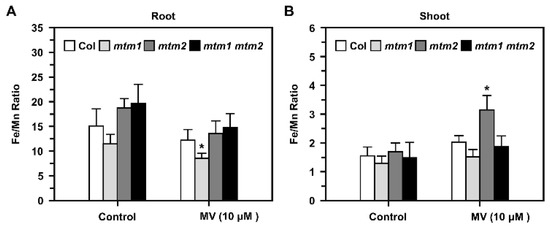
Figure 8.
Fe/Mn ratios in roots and shoots of Col, mtm1, mtm2, and mtm1 mtm2-double mutants in response to MV. (A,B) Fourteen-day-old seedlings were incubated with 10 μM MV for 3 days. Fe and Mn contents in roots and shoots were measured by ICP-OES, and converted to Fe/Mn ratio. Data are mean ± SD of three independent repeats. * Significant at p < 0.05 compared with the Col.
3. Discussion
The plant mitochondrial carrier family (MCF) contains approximately 60 proteins that coordinate metabolic and ionic homeostasis between the cytosol and mitochondria [36,37,38,39]. The mitochondrial MCF proteins, AtMTM1 and AtMTM2, are Mn-specific carrier proteins involved in MnSOD activation in Arabidopsis [30,31]. In a previous study, yeast cytosol-localised MnSOD was inactive, but its activity could be restored by treatment with a high concentration of Mn [27], and mitochondrial MnSOD was markedly enhanced by Mn treatment. Moreover, wild-type yeast cells treated with Fe show increased mitochondrial Fe levels and MnSOD protein levels, but retain normal MnSOD activity [29]. In this study, MnSOD activity was largely increased under higher concentrations of Mn treatment (Figure 1). This result indicated the Mn cofactor specificity of Arabidopsis MnSOD activation and the increased apoprotein levels in MnSOD-OE plants occurred through Mn treatment. It is worthy to adjust metal concentration based on the normal range in 1/2 MS media. In addition, we observed antagonisms between Mn and other metals, including Fe, Cu, and Zn, for MnSOD activity in Arabidopsis, which agrees with the results of an earlier study on the effect of Mn and Fe on MnSOD activity in E. coli [19].
It has been reported that AtMTM1 is an oxidation-responsive gene [31,40]. In this study, we showed that both AtMTM1 and AtMTM2 gene expression levels respond to MV, NaCl, H2O2, and t-BH-induced oxidative stress in Col plants (Figure 2), and that elevated AtMTM2 gene expression levels can be detected earlier than elevated AtMTM1 levels. In addition, MnSOD-OE plants exhibited significantly enhanced MnSOD activity, but msd1 plants showed slightly decreased MnSOD activity compared to MnSOD activity in Col plants under MV and NaCl treatments (Figure 3), indicating the post-translational regulation of MnSOD. It is worthy to investigate the post-translation regulation such as phosphorylation or ubiquitination, in order to elucidate the mechanism of MnSOD activation. In a previous study, AtMnSOD antisense plants displayed shorter root lengths under the high-stringency oxidative stress conditions of 0.5 μM MV [14]. The AtMnSOD mutant, oiwa-1, shows defective embryo sac formation, but the auxin gradient is not altered in this mutant [34,35]. Plants with AtMnSOD overexpression driven by the seed-specific promoter, At2S3, have higher germination rates after 10 μM MV and 10 mM H2O2 treatment [13]. In this study, we examined the root growth phenotype of 7-day-old MnSOD-OE and msd1 seedlings on plates containing 5 nM MV or 50 mM NaCl (Figure 4). The different root lengths reflected the participation of MnSOD in early root growth. However, after transferring 5-day-old seedlings from 1/2 MS to higher-stringency conditions for 3 days, both MnSOD-OE and msd1 seedlings showed longer roots. Thus, it is worth monitoring the root length for a longer period. Since MnSOD is the only reported enzyme that has the ability to catalyse superoxide (O2•−) in mitochondria, we quantified O2•− and H2O2 levels in mature (21-day-old) MnSOD-OE seedlings, and demonstrated that the overexpression of MnSOD enhanced the superoxide scavenging ability and maintained the cellular levels of H2O2 (Figure 5). Overall, we demonstrated that MnSOD is crucial for the control of early root growth under stress conditions and that it scavenges superoxide radicals during plant development. Moreover, it is worthy to elucidate MnSOD-mediated oxidative stress and the accompanied shorter primary root length, as well as the phenotypes of branch roots and root hairs.
In this study, we used basal medium, with and without Mn treatment, and monitored the root length of AtMTM1 and AtMTM2 single and double mutants (Figure 6). The results reflected the different sensitivities of AtMTM1 and AtMTM2 to Mn, as previously described [30]. These experiments revealed that mtm1 had a greater ability than mtm2 to restore root length in the presence of Mn, indicating the importance of AtMTM1 in root growth. It is worthy to investigate the relationship of the primary root phenotype between msd1 seedlings and mtm1 mtm2-double mutant, since both plants shared the similar longer primary root length. In a previous study, we used mtm1 mtm2-double mutant protoplasts to show that the mitochondrial carrier proteins, AtMTM1 and AtMTM2, are crucial for MnSOD activation [30]. In this study, we found that AtMTM1 and AtMTM2-single mutant protoplasts exhibited slightly lower MnSOD activity than control protoplasts without treatment. These findings increased the evidence for the physiological roles of these carrier proteins, and indicated that AtMTM1 and AtMTM2 have different sensitivities for MnSOD activation. Moreover, we applied an Fe chelator and found that MnSOD activity was lower in mtm2 than in mtm1 plants, and it clearly decreased in mtm1 mtm2-double mutant protoplasts (Figure 7). These results indicated that AtMTM1 and AtMTM2-mediated MnSOD activation is affected by Fe homeostasis, and we suggested that the disrupted systems by Fe homeostasis include MnSOD apoprotein synthesis in the cytosol, Mn binding to MnSOD via mitochondrial AtMTM1 and AtMTM2, or tetrameric MnSOD activation in the mitochondrial matrix.
It has been reported that the yeast MnSOD promoter region contains heme and stress-related regulatory sites, and that MnSOD transcription is regulated by heme [41]. The associations between mitochondrial MnSOD levels; Fe levels; the expression levels of the Fe/S cluster biogenesis genes, MRS3, MRS4, SSQ1, GRX5, and YFH1, have been investigated [29,42], and elevated mitochondrial Fe levels do not correlate well with MnSOD activity in yeast. Since mitochondrial heme synthesis and Fe-S cluster biogenesis involve Fe utilisation and Fe homeostasis [33,43], we suggest that Arabidopsis MnSOD transportation or activation via AtMTM1 and AtMTM2 are affected by unknown Fe-S proteins. Moreover, a previous study of the Fe/Mn ratio in plants revealed an antagonistic relationship between Mn and Fe levels in root absorption and translocation from roots to shoots [16,44]. In this study, we observed that the Fe/Mn ratio was altered in the roots of mtm1 plants and the shoots of mtm2 plants under MV stress (Figure 8), implying physiological roles of AtMTM1 and AtMTM2 in Fe/Mn balance. It is possible that AtMTM1 or AtMTM2 are also involved in Fe regulation, and it is worth monitoring the root length of mtm1 and mtm2 plants grown on the basal medium with Fe added.
4. Materials and Methods
4.1. Plants and Growth Conditions
Arabidopsis thaliana accession Columbia-0 (Col) was the wild-type plant. AtMnSOD heterozygote T-DNA-inserted knockdown mutant (msd1; SALK 122275) was requested from Arabidopsis Biological Resource Center (ABRC). mtm1 (miRNA-mediated AtMTM1-knockdown), mtm2 (T-DNA insertional AtMTM2-knockout), and mtm1 mtm2-double mutants were established in our previous study [30]. Plants were grown in soil or on 1/2 MS (Sigma-Aldrich, St. Louis, MO, USA) plates supplied with 1% sucrose and 0.8% Phytagel (Sigma-Aldrich) with illumination at 80–100 μmol m−2 s−1 under standard long day condition (16 h light/8 h dark) at 22–24 °C.
4.2. Generation of AtMnSOD-Overexpressing Plants
The coding region of Arabidopsis AtMnSOD was amplified by RT-PCR and ligated into the yT&A vector (Yeastern Biotech, Taipei, Taiwan) for sequencing, then subcloned into the SacI and XbaI sites of the destination vector pPZP200GB with CaMV 35S promoter [45]. AtMnSOD-overexpressing (MnSOD-OE) plants were generated by Agrobacterium tumefaciens GV3101-mediated transformation and the floral dip method [46], then selected by Basta.
4.3. Genotyping, RT-qPCR, In-Gel SOD Activity, and Immunoblotting Assay
Plant genomic DNA was extracted for genotyping by PCR, as previously described [47]. Total RNA was prepared with TRIZOL reagent (Invitrogen) and TURBO DNA-free Kit (Applied Biosystems, Foster City, CA, USA). cDNA synthesis was performed by High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems). Transcription levels were monitored by RT-qPCR with KAPA SYBR FAST q-PCR Kit (KAPA Biosystems, Wilmington, MA, USA). Protein Phosphatase 2A subunit A3 (PP2A) was an internal control [48,49] for RT-qPCR.
Fourteen-day-old seedlings were incubated in 1/2 MS liquid medium containing MnCl2 (Mn), Fe citrate (Fe), Mn plus Fe, or oxidative stressors of methyl viologen (MV), NaCl, H2O2, or tert-butyl hydroperoxide (t-BH) with agitation. The gene expression levels were analyzed by q-PCR. AOX1A, a mitochondrial stress gene, was a reference [30].
Protein was extracted by ice-cold griding buffer (150 mM Tris-HCl, pH 7.2), and supernatant was purified twice by centrifugation for 10 min at 16,000× g at 4 °C [30]. Protein concentration was determined by Bio-Rad protein assay reagent (Bio-Rad, Techview, Singapore). In-gel SOD activity assay was performed as previously described [50]. MnSOD protein level was examined by immunoblotting with α-MnSOD (Agrisera, Västerbäck, Vännäs, Sweden) antibody. Actin protein was used as an internal control and analyzed with α-Actin (Agrisera) antibody.
4.4. Analysis of O2•− and H2O2 Accumulations by Nitrobule Tetrazolium (NBT) and Diaminobenzidine (DAB) Staining
Twenty-one-day-old seedlings were incubated in 1/2 MS liquid medium containing 5 μM MV with gentle shaking for 3 days, then stained with 1 mg/mL NBT or 1 mg/mL DAB in dark overnight. Leaves were fixed with decolorizing solution containing ethanol, lactic acid, and glycerol (3:1:1) for 1 h, then washed with 70% ethanol for 1 h to remove chlorophyll completely. O2•− and H2O2 accumulation were detected by NBT and DAB staining, respectively [51,52,53]. The relative accumulation was quantified by image J system (https://imagej.nih.gov/ij/download.html accessed on 24 February 2022).
4.5. Root Length Assay in Response to Oxidative Stressors and Mn Treatment
The root lengths of 7-day-old seedlings on 1/2 MS plates containing mild-stringency stressors of 5 nM MV or 50 mM NaCl were measured. In addition, 5-day-old seedlings were transferred from 1/2 MS medium to plates containing higher-stringency stressors of 10 nM MV or 150 mM NaCl for 3 days were conducted.
Moreover, the root lengths of seedlings in the Mn deficiency condition were analyzed by using the basal medium containing 5 mM KNO3, 2 mM MgSO4, 2 mM Ca (NO3)2, 2.5 mM KH2PO4, 70 μM H3BO3, 40 μM Fe EDTA, 1 μM ZnSO4, 0.5 μM CuSO4, 0.2 μM Na2MoO4, 4.7 mM MES (pH 5.5) with 43 mM sucrose, then solidified with 0.8% Phytagel [54]. Seedlings were grown on plates supplied without MnCl2 (Mn deficient) or with MnCl2 at 14 μM (normal Mn), 700 μM (50-fold increase), or 1050 μM (75-fold increase) for 6 days.
4.6. Fe chelation in Arabidopsis Protoplasts
The Fe2+-specific chelator bathophenanthroline disulfonate, BPS (Sigma-Aldrich), can decrease Fe contents in yeast cytosol and mitochondria, and the effect of BPS on MnSOD activity was analyzed [29,42,55]. Arabidopsis mesophyll protoplasts were isolated from four-week-old plants [56]. An amount of 3 × 105 protoplasts were treated with BPS at 100 μM, 500 μM, or 1000 μM for 16 h [55,57,58], and were examined for its effect on MnSOD activity.
4.7. Fe/Mn Ratios in Roots and Shoots in Response to MV
The inductively coupled plasma-optical emission spectrometry (ICP-OES) (PerkinElmer OPTIMA 5300) analysis was applied to measure Mn and Fe metal contents in plants [59,60]. Fourteen-day-old seedlings were incubated in 1/2 MS liquid medium containing 10 μM MV with agitation for 3 days, then shoots and roots were separated. An amount of 0.1 g dried samples were used for ICP-OES analysis [30]. Spinach and tomato leaves (NIST SRM-1570a and NIST SRM-1573a) were the references. The output Mn and Fe contents by ICP-OES were converted to Fe/Mn ratio.
4.8. Statistical Analysis
All experiments were repeated independently at least three times. Statistical analysis involved Student’s t-test and Duncan’s multiple range test. p value < 0.05 was considered statistically significant.
4.9. PCR Primers and GenBank Accession Numbers
Primers and gene accession numbers are listed in Supplementary Table S1.
5. Conclusions
This study strengthened the importance of MnSOD activation through its carrier proteins AtMTM1 and AtMYM2 by metal ion treatments and oxidative stressors. We showed that MnSOD activity was specifically enhanced by Mn treatment, and antagonism occurred between Mn and other metals for MnSOD activation. We clarified the post-translational regulation of MnSOD during oxidative stress and demonstrated that MnSOD participates in the control of early root growth and enhances superoxide scavenging efficiency in mature seedlings. It is worthy to connect the altered root-length phenotype and elevated MnSOD enzyme activity. We also revealed that altered Fe homeostasis inhibited MnSOD activity through the carrier proteins AtMTM1 and AtMTM2. Especially, AtMTM1 and AtMTM2 participate in Fe/Mn regulation with tissue specificity. It would be interesting to investigate the mechanism of MnSOD post-translational regulation through phosphorylation site mutation, and to elucidate the substrate affinity of AtMTM1 and AtMTM2 through in vitro studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11050619/s1. Figure S1: Characterizations of AtMnSOD-overexpressing (MnSOD-OE) plant; Figure S2: Characterizations of AtMnSOD T-DNA knockdown (msd1) mutant; Figure S3: Control experiment of AtMTM1 and AtMTM2 gene expression levels in Col plants; Table S1: Primers for cloning, genotyping, RT-qPCR, and accession numbers of genes.
Author Contributions
Formal analysis, writing, and visualization—S.-H.H. and T.-L.J.; investigation—S.-H.H.; validation, data curation, supervision, project administration, and funding acquisition—T.-L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science and Technology, Taiwan, grant number 107-2923-B-002-003-MY3 and 109-2311-B-002-022-MY3 and National Taiwan University, grant number NTU-CC-110L893602.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article and supplementary materials.
Acknowledgments
We appreciate Wolfgang Schmidt for sharing root length assay protocol, Kuo-Chen Yeh for helping with ICP-OES analysis, and NTU Technology Commons for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fridovich, I. Superoxide dismutases. Adv. Enzymol. Relat. Areas Mol. Biol. 1986, 58, 61–97. [Google Scholar] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Pauling, L. The discovery of the superoxide radical. Trends Biochem. Sci. 1979, 4, N270–N271. [Google Scholar] [CrossRef]
- Bowler, C.; Camp, W.V.; Montagu, M.V.; Inzé, D. Superoxide dismutase in plants. Crit. Rev. Plant Sci. 1994, 13, 199–218. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Monde, R.A.; Last, R.L. Superoxide dismutase in Arabidopsis: An eclectic enzyme family with disparate regulation and protein localization. Plant Physiol. 1998, 118, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.F. Superoxide dismutases: Ancient enzymes and new insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinalducci, S.; Murgiano, L.; Zolla, L. Redox proteomics: Basic principles and future perspectives for the detection of protein oxidation in plants. J. Exp. Bot. 2008, 59, 3781–3801. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Bowler, C.; Montagu, M.; Inzé, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Møller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. Rep. 2001, 52, 561–591. [Google Scholar] [CrossRef] [Green Version]
- Taylor, N.L.; Tan, Y.F.; Jacoby, R.P.; Millar, A.H. Abiotic environmental stress induced changes in the Arabidopsis thaliana chloroplast, mitochondria and peroxisome proteomes. J. Proteom. 2009, 72, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ying, Y.; Chen, J.; Wang, X. Transgenic Arabidopsis overexpressing Mn-SOD enhanced salt-tolerance. Plant Sci. 2004, 167, 671–677. [Google Scholar] [CrossRef]
- Xi, D.M.; Liu, W.S.; Yang, G.D.; Wu, C.A.; Zheng, C.C. Seed-specific overexpression of antioxidant genes in Arabidopsis enhances oxidative stress tolerance during germination and early seedling growth. Plant Biotechnol. J. 2010, 8, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Lehmann, M.; Schwarzländer, M.; Baxter, C.J.; Sienkiewicz-Porzucek, A.; Williams, T.C.R.; Schauer, N.; Fernie, A.R.; Fricker, M.D.; Ratcliffe, R.G.; et al. Decreased in manganese superoxide dismutase leads to reduced root growth and affects tricarboxylic acid cycle flux and mitochondrial redox homeostasis. Plant Physiol. 2008, 147, 101–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Tinaut, M.C.; Leal, A.; Recalde Martínez, L. Iron-manganese interaction and its relation to boron levels in tomato plants. Plant Soil 1980, 55, 377–388. [Google Scholar] [CrossRef]
- Tanaka, A.; Navasero, S.A. Interaction between iron and manganese in the rice plant. Soil Sci. Plant Nutr. 1966, 12, 29–33. [Google Scholar] [CrossRef]
- Twyman, E.S. The iron and manganese requirements of plants. New Phytol. 1951, 50, 210–226. [Google Scholar] [CrossRef]
- Hassan, H.M.; Sun, H.C. Regulatory roles of Fnr, Fur, and Arc in expression of manganese-containing superoxide dismutase in Escherichia coli. Proc. Natl. Acad. Sci. USA 1992, 89, 3217–3221. [Google Scholar] [CrossRef] [Green Version]
- Privalle, C.; Fridovich, I. Transcriptional and maturational effects of manganese dismutase 2 is Saccharomyces serevisiae requires MTM1, a member of the mitochondrial carrier family. Proc. Natl. Acad. Sci. USA 1992, 100, 10353–10357. [Google Scholar]
- Whittaker, J.W. The irony of manganese superoxide dismutase. Biochem. Soc. Trans. 2003, 31, 1318–1321. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, J.W. Metal uptake by manganese superoxide dismutase. Biochem. Biophys. Acta 2010, 1804, 298–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, D.H.; Tuminello, J.F.; Emptage, M.H. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J. Biol. Chem. 1993, 268, 22369–22376. [Google Scholar] [CrossRef]
- Aguirre, J.D.; Culotta, V.C. Battles with iron: Manganese in oxidative stress protection. J. Biol. Chem. 2012, 287, 13541–13548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maringanti, S.; Imlay, J.A. An intracellular iron chelator pleiotropically suppresses enzymatic and growth defects of superoxide dismutase-deficient Escherichia coli. J. Bacteriol. 1999, 181, 3792–3802. [Google Scholar] [CrossRef] [Green Version]
- Luk, E.; Carroll, M.; Baker, M.; Culotta, V.C. Manganese activation of superoxide dismutase 2 in Saccharomyces cerevisiae requires MTM1, a membrane of the mitochondrial carrier family. Proc. Natl. Acad. Sci. USA 2003, 100, 10353–10357. [Google Scholar] [CrossRef] [Green Version]
- Luk, E.; Culotta, V.C. Manganese superoxide dismutase in Saccharomyces cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transporter, Smf2p. J. Biol. Chem. 2001, 276, 47556–47562. [Google Scholar] [CrossRef] [Green Version]
- Luk, E.; Yang, M.; Jensen, L.T.; Bourbonnais, Y.; Culotta, V.C. Manganese activation of superoxide dismutase 2 in the mitochondria of Saccharomyces cerevisiae. J. Biol. Chem. 2005, 280, 22715–22720. [Google Scholar] [CrossRef] [Green Version]
- Portnoy, M.E.; Liu, X.F.; Culotta, V.C. Saccharomyces cerevisiae expresses three functionally distinct homologues of the Nramp family of metal transporters. Mol. Cell. Biol. 2000, 20, 7893–7902. [Google Scholar] [CrossRef]
- Yang, M.; Cobine, P.A.; Molik, S.; Naranuntarat, A.; Lill, R.; Winge, D.R.; Culotta, V.C. The effects of mitochondrial iron homeostasis on cofactor specificity of superoxide dismutase 2. EMBO J. 2006, 25, 1775–1783. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.H.; Lin, S.F.; Huang, Y.C.; Huang, C.H.; Kuo, W.Y.; Jinn, T.L. Significance of AtMTM1 and AtMTM2 for mitochondrial MnSOD activation in Arabidopsis. Front. Plant Sci. 2021, 12, 690064. [Google Scholar] [CrossRef]
- Su, Z.; Chai, M.F.; Lu, P.L.; An, R.; Chen, J.; Wang, X.C. AtMTM1, a novel mitochondrial protein, may be involved in activation of the manganese-containing superoxide dismutase in Arabidopsis. Planta 2007, 226, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 26, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Connolly, E.L. Mitochondrial iron transport and homeostasis in plants. Front. Plant Sci. 2013, 4, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.V.; Fiol, D.F.; Sundaresan, V.; Zabaleta, E.J.; Pagnussat, G.C. oiwa, a female gametophytic mutant impaired in a mitochondrial manganese-superoxide dismutase, reveals crucial roles for reactive oxygen species during embryo sac development and fertilization in Arabidopsis. Plant Cell 2013, 25, 1573–1591. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.V.; Distéfano, A.M.; Zabaleta, E.J.; Pagnussat, G.C. New insights into the functional roles of reactive oxygen species during embryo sac development and fertilization in Arabidopsis thaliana. Plant Signal Behav. 2013, 8, e25714. [Google Scholar] [CrossRef] [Green Version]
- Millar, A.H.; Heazlewood, J.L. Genomic and proteomic analysis of mitochondrial carrier proteins in Arabidopsis. Plant Physiol. 2003, 131, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Møller, I.M.; Rasmusson, A.G.; Van Aken, O. Plant mitochondria—Past, present and future. Plant J. 2021, 108, 912–959. [Google Scholar] [CrossRef]
- Palmieri, F.; Pierri, C.L.; Grassi, A.D.; Nunes-Nesi, A.; Fernie, A.R. Evolution, structure and function of mitochondrial carriers: A review with new sights. Plant J. 2011, 66, 161–181. [Google Scholar] [CrossRef]
- Picault, N.; Hodges, M.; Palmieri, L.; Palmieri, F. The growing family of mitochondrial carriers in Arabidopsis. Trends Plant Sci. 2004, 9, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Van Aken, O.; Zhang, B.; Carrie, C.; Uggalla, V.; Paynter, E.; Giraud, E.; Whelan, J. Defining the mitochondrial stress response in Arabidopsis thaliana. Mol. Plant 2009, 2, 1310–1324. [Google Scholar] [CrossRef]
- Pinkham, J.L.; Wang, Z.; Alsina, J. Heme regulates SOD2 transcription by activation and repression in Saccharomyces cerevisiae. Curr. Genet. 1997, 1997, 281–291. [Google Scholar] [CrossRef]
- Naranuntarat, A.; Jensen, L.T.; Panicni, S.; Penner-Hahn, J.E.; Culotta, V.C. The interaction of mitochondrial iron with manganese superoxide dismutase. J. Biol. Chem. 2009, 284, 22633–22640. [Google Scholar] [CrossRef] [Green Version]
- Busi, M.V.; Maliandi, M.V.; Valdez, H.; Clemente, M.; Zabaleta, E.J.; Araya, A.; Gomez-Casati, D.F. Deficiency of Arabidopsis thaliana frataxin alters activity of mitochondrial Fe-S proteins and induces oxidative stress. Plant J. 2006, 48, 873–882. [Google Scholar] [CrossRef]
- Shive, J.W. Significant roles of trace elements in the nutrition of plants. Plant Physiol. 1941, 16, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.C.; Lee, W.C.; Guo, W.Y.; Pan, S.M.; Chen, L.J.; Li, H.M.; Jinn, T.L. A copper chaperone for superoxide dismutase that confers three types of copper/zinc superoxide dismutase activity in Arabidopsis. Plant. Physiol. 2005, 139, 425–436. [Google Scholar] [CrossRef] [Green Version]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Edwards, K.; Johnstone, C.; Thompson, C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acid. Res. 1991, 19, 1349. [Google Scholar] [CrossRef]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.K.; Scheible, W.R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Remans, T.; Smeets, K.; Opdenakker, K.; Mathijsen, D.; Vangronsveld, J.; Cuypers, A. Normalisation of real-time RT-PCR gene expression measurements in Arabidopsis thaliana exposed to increased metal concentrations. Planta 2008, 227, 1343–1349. [Google Scholar] [CrossRef] [Green Version]
- Kuo, W.Y.; Huang, C.H.; Shih, C.; Jinn, T.L. Cellular extract preparation for superoxide dismutase (SOD) activity assay. Bio Protoc. 2013, 3, e811. [Google Scholar] [CrossRef]
- Rodríguez-Celma, J.; Pan, I.C.; Li, W.; Lan, P.; Buckhout, T.J.; Schmidt, W. The transcriptional response of Arabidopsis leaves to Fe deficiency. Front. Plant Sci. 2013, 4, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Li, C.; Chang, P.P.; Ghebremariam, K.M.; Qin, L.; Liang, Y. Overexpression of tomato SpMPK3 gene in Arabidopsis rnhances the osmotic tolerance. Biochem. Biophys. Res. Commun. 2014, 443, 357–362. [Google Scholar] [CrossRef]
- Song, X.; Fang, J.; Han, X.; He, X.; Liu, M.; Hu, J.; Zhau, R. Overexpression of quinine reductase from Salix matsudana Koidz enhances salt tolerance in transgenic Arabidopsis thaliana. Gene 2016, 576, 520–527. [Google Scholar] [CrossRef]
- Zhai, Z.; Gayomba, S.R.; Jung, H.I.; Vimalakumari, N.K.; Piñeros, M.; Craft, E.; Rutzke, M.A.; Danku, J.; Lahner, B.; Punshon, T.; et al. OPT3 is a phloem-specific iron transporter that is essential for systemic iron signaling and redistribution of iron and cadmium in Arabidopsis. Plant Cell 2014, 26, 2249–2264. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [Green Version]
- Farid, M.; Ali, S.; Shakoor, M.B.; Bharwana, S.A.; Rizvi, H.; Ehsan, S.; Tauqeer, H.M.; Iftikhar, U.; Hannan, F. EDTA assisted phytoremediation of Cadmium, Lead and Zinc. Intl. J. Agron. Plant. Prod. 2013, 4, 2833–2846. [Google Scholar]
- Kociałkowski, W.Z.; Diatta, J.B.; Grzebisz, W. Evaluation of chelating agents as heavy metals extractants in agricultural soils under threat of contamination. Pol. J. Environ. Stud. 1999, 8, 149–154. [Google Scholar]
- Lin, Y.F.; Liang, H.M.; Yang, S.Y.; Boch, A.; Clemens, S.; Chen, C.C.; Wu, J.F.; Huang, J.L.; Yeh, K.C. Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytol. 2009, 182, 392–404. [Google Scholar] [CrossRef]
- Shanmugam, V.; Lo, J.C.; Wu, C.L.; Wang, S.L.; Lai, C.C.; Connolly, E.L.; Huang, J.L.; Yeh, K.C. Differential expression and regulation of iron-regulated metal transporters in Arabidopsis halleri and Arabidopsis thaliana-the role in zinc tolerance. New Phytol. 2011, 190, 125–137. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).