Untargeted Phenolic Profiling and Functional Insights of the Aerial Parts and Bulbs of Drimia maritima (L.) Stearn
Abstract
1. Introduction
2. Results
2.1. Untargeted Screening of Polyphenols Content in the Bulb and Aerial Parts of D. maritima
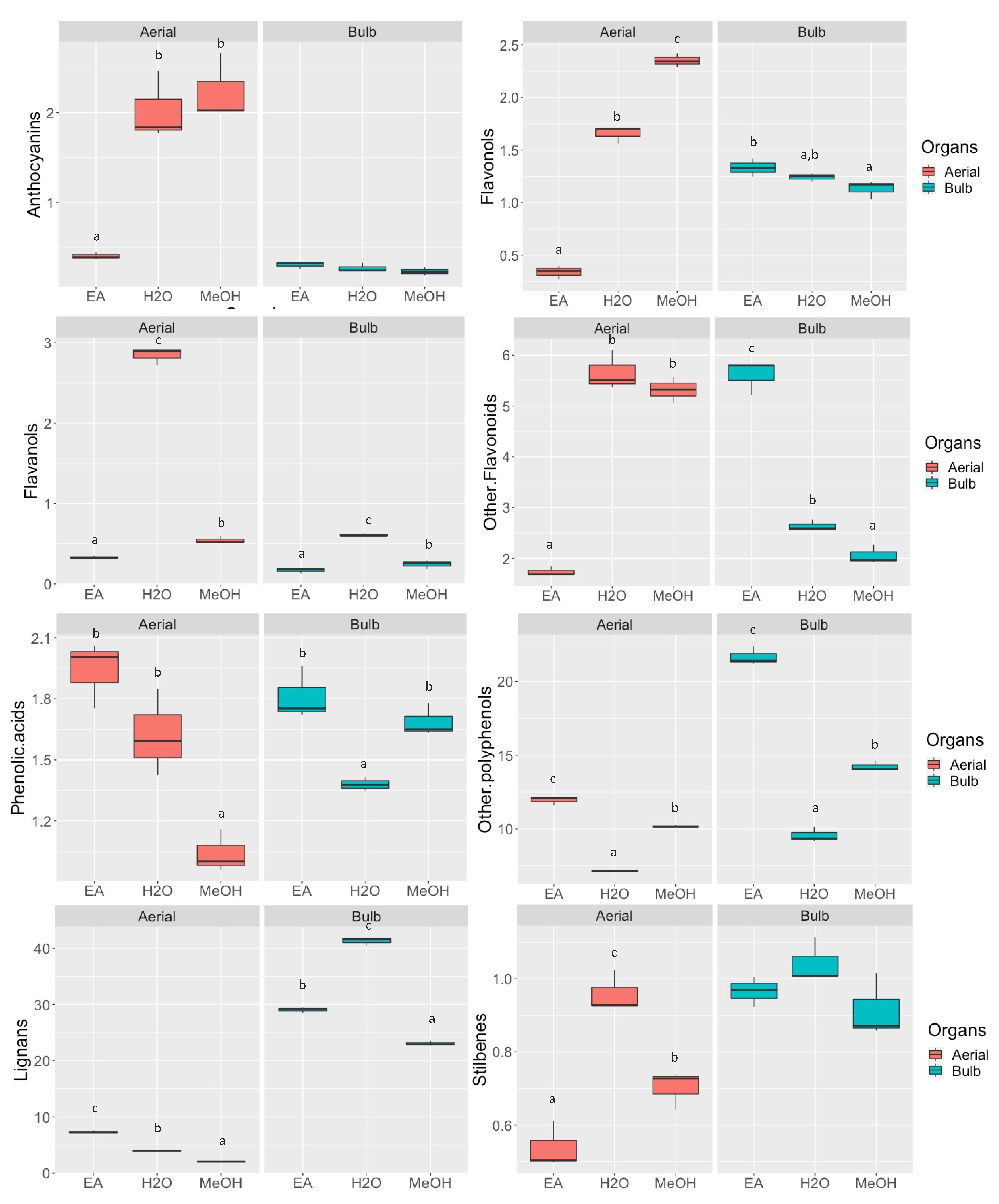
2.2. Effect of the Extraction Solvents on the Phenolic Profile of D. maritima Organ Parts
2.3. In Vitro Antioxidant Activities
2.4. Inhibitory Capacity on Key Enzymes
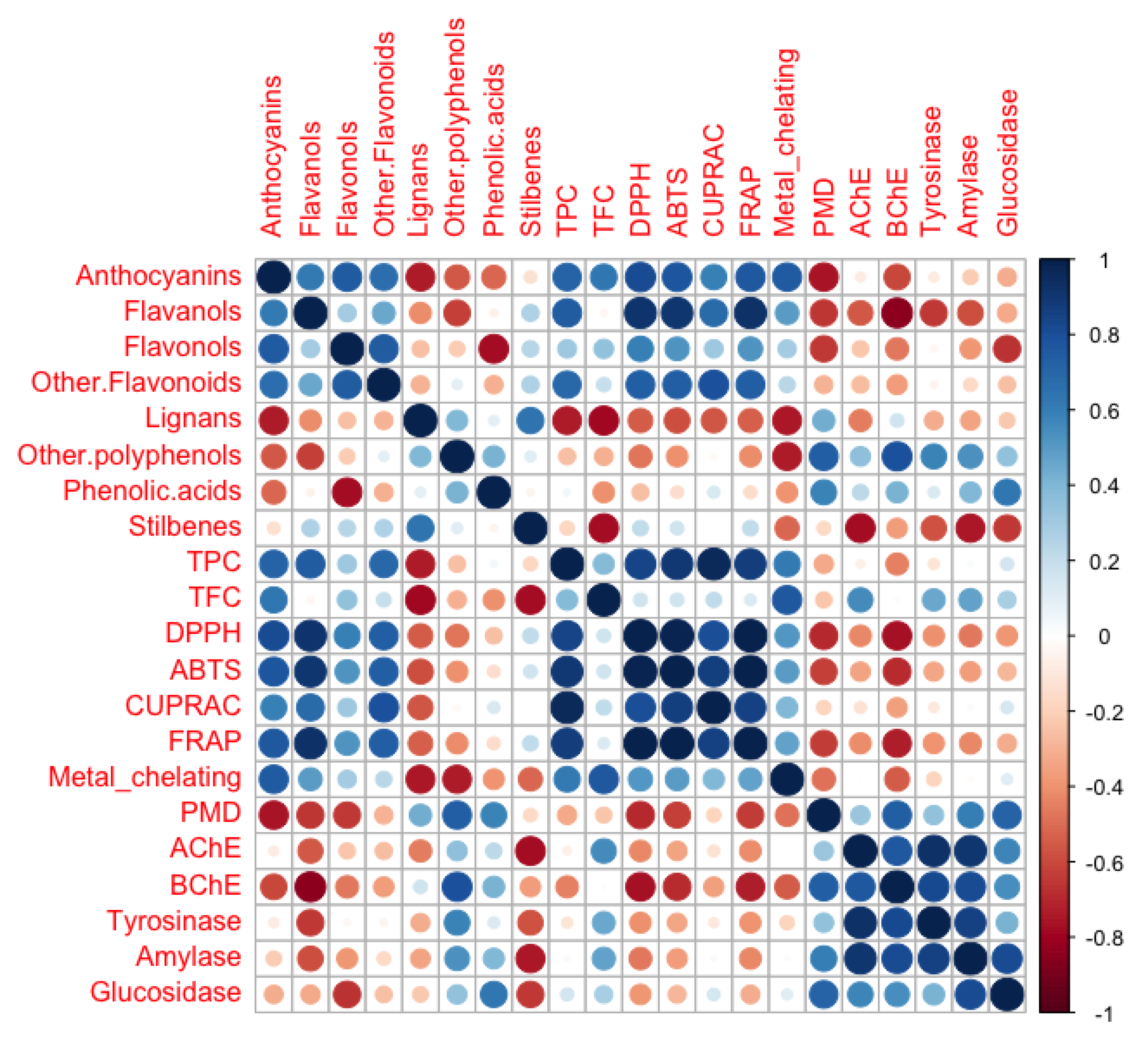
2.5. Pearson’s Correlation between Phenolic Profiles and Biological Activities
3. Discussion
3.1. Untargeted Screening of Polyphenols Content in the D. maritima Extracts
3.2. Antioxidant Activities
3.3. Enzyme Inhibition Activities
3.3.1. Cholinesterase Inhibition Capacity
3.3.2. Tyrosinase Inhibition Capacity
3.3.3. α-Amylase and α-Glucosidase Inhibition Capacity
4. Materials and Methods
4.1. Plant Material
4.2. Extraction Methods
4.3. Determination of Total Bioactive Compounds, In Vitro Antioxidant and Enzyme Inhibitory Activities
4.4. Metabolomics Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Oyedemi, B.O.; Oyedemi, S.O.; Chibuzor, J.V.; Ijeh, I.I.; Coopoosamy, R.M.; Aiyegoro, A.O. Pharmacological evaluation of selected medicinal plants used in the management of oral and skin infections in Ebem-Ohafia District, Abia State, Nigeria. Sci. World J. 2018, 2018, 4757458. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2019, 19, 1199–1209. [Google Scholar] [CrossRef]
- Fedoung, E.F.; Biwole, A.B.; Biyegue, C.F.N.; Tounkam, M.N.; Ntonga, P.A.; Nguiamba, V.P.; Essono, D.M.; Funwi, P.F.; Tonga, C.; Nguenang, G.M. A review of Cameroonian medicinal plants with potentials for the management of the COVID-19 pandemic. Adv. Tradit. Med. 2021, 2021, 1–26. [Google Scholar]
- Singh, S.; Sedha, S. Medicinal plants and their pharmacological aspects. FPI 2018, 1, 156–170. [Google Scholar]
- Aswal, S.; Kumar, A.; Semwal, R.B.; Chauhan, A.; Kumar, A.; Lehmann, J.; Semwal, D.K. Drimia indica: A plant used in traditional medicine and its potential for clinical uses. Medicina 2019, 55, 255. [Google Scholar] [CrossRef] [PubMed]
- Pohl, T.; Koorbanally, C.; Crouch, N.R.; Mulholland, D.A. Bufadienolides from Drimia robusta and Urginea altissima (Hyacinthaceae). Phytochemistry 2001, 58, 557–561. [Google Scholar] [CrossRef]
- Fennell, C.; Light, M.; Sparg, S.; Stafford, G.; Van Staden, J. Assessing African medicinal plants for efficacy and safety: Agricultural and storage practices. J. Ethnopharmacol. 2004, 95, 113–121. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, S.K.; Yadav, A.K. Pharmacological evaluation of antidiabetic activity of Urginea indica in laboratory animals. Int. J. Nutr. Pharmacol. Neurol. Dis. 2015, 5, 63. [Google Scholar]
- Rajput, B.; Golave, A.; Yadav, S.; Jadhav, J.P. Total phenolic concentrations and antioxidant activities in Drimia sp. J. Herbs Spices Med. Plants 2018, 24, 28–36. [Google Scholar] [CrossRef]
- Al Hajj, D.A. Phytochemical Screening of the Rare Species “Urginea Maritima” in Bentael Natural Reserve. Master’s Thesis, Beirut Arab University, Beirut, Lebanon, 2019. [Google Scholar]
- Yadav, P.; Lekhak, U.; Ghane, S.; Lekhak, M. Phytochemicals, antioxidants, estimation of cardiac glycoside (Scillaren A) and detection of major metabolites using LC-MS from Drimia species. S. Afr. J. Bot. 2020, 140, 259–268. [Google Scholar] [CrossRef]
- Langat, L.; Langat, M.K.; Wetschnig, W.; Knirsch, W.; Mulholland, D.A. Antiproliferative Bufadienolides from the Bulbs of Drimia altissima. J. Nat. Prod. 2021, 84, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Bozorgi, M.; Amin, G.; Shekarchi, M.; Rahimi, R. Traditional medical uses of Drimia species in terms of phytochemistry, pharmacology and toxicology. J. Tradit. Chin. Med. 2017, 37, 124–139. [Google Scholar] [CrossRef]
- Foukaridis, G.; Osuch, E.; Mathibe, L.; Tsipa, P. The ethnopharmacology and toxicology of Urginea sanguinea in the Pretoria area. J. Ethnopharmacol. 1995, 49, 77–79. [Google Scholar] [CrossRef]
- Marx, J.; Pretorius, E.; Espag, W.; Bester, M. Urginea sanguinea: Medicinal wonder or death in disguise? Environ. Toxicol. Pharmacol. 2005, 20, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Teshome, M.; Kassa, H.; Charles, K. The toxicity of plant material, drimia altissima (Urginea altissima), against the field rat, Arvicanthis abyssinicus: A potential non-synthetic rodenticide. Ethiop. J. Health Dev. 2010, 24, 175–179. [Google Scholar] [CrossRef][Green Version]
- Nejatbakhsh, F.; Karegar-Borzi, H.; Amin, G.; Eslaminejad, A.; Hosseini, M.; Bozorgi, M.; Gharabaghi, M.A. Squill Oxymel, a traditional formulation from Drimia maritima (L.) Stearn, as an add-on treatment in patients with moderate to severe persistent asthma: A pilot, triple-blind, randomized clinical trial. J. Ethnopharmacol. 2017, 196, 186–192. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kashani, L.M.T.; Mahboubi, M. Squill (Drimia maritima L.) and its novel biological activity. Orient. Pharm. Exp. Med. 2019, 19, 227–234. [Google Scholar] [CrossRef]
- Mammadov, R.; Makasci, A.A.; Uysal, D.D.; Gork, C. Determination of antioxidant activities of different Urginea maritima (L.) Baker plant extracts. Iran. J. Chem. Chem. Eng. 2010, 29, 47–53. [Google Scholar]
- Bozcuk, H.; Özdoğan, M.; Aykurt, O.; Topçuoğlu, F.; Öztürk, H.; Ekinci, D.; Karadeniz, A.; Mutlu, A.; Burgucu, D. Urginea maritima (L.) Baker (Liliaceae) extract induces more cytotoxicity than standard chemotherapeutics in the A549 non-small cell lung cancer (NSCLC) cell line. Turk. J. Med. Sci. 2011, 41, 101–108. [Google Scholar]
- Maazoun, A.M.; Hlel, T.B.; Hamdi, S.H.; Belhadj, F.; Jemâa, J.M.B.; Marzouki, M.N. Screening for insecticidal potential and acetylcholinesterase activity inhibition of Urginea maritima bulbs extract for the control of Sitophilus oryzae (L.). J. Asia-Pac. Entomol. 2017, 20, 752–760. [Google Scholar] [CrossRef]
- Hamzeloo-Moghadam, M.; Aghaei, M.; Abdolmohammadi, M.H.; Khalaj, A.; Fallahian, F. Cytotoxic effect of Drimia maritima bulb extract and induction of mitochondrial apoptotic signaling in human breast cancer cells, MCF-7 and MDA-MB-468. OncoTargets Ther. 2018, 11, 7669. [Google Scholar] [CrossRef] [PubMed]
- Belhaddad, O.E.; Charef, N.; Amamra, S.; Zerargui, F.; Baghiani, A.; Khennouf, S.; Arrar, L. Chromatographic fractionation, antioxidant and antibacterial activities of Urginea maritima methanolic extract. Pak. J. Pharm. Sci. 2017, 30, 127–134. [Google Scholar] [PubMed]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- Lankatillake, C.; Huynh, T.; Dias, D.A. Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods 2019, 15, 1–35. [Google Scholar] [CrossRef]
- Phuyal, N.; Jha, P.K.; Raturi, P.P.; Rajbhandary, S. Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. Sci. World J. 2020, 2020, 8780704. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Truong, D.-H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Vega, F.A.; Martin, C. Anthocyanins of the Squill. Nature 1963, 197, 382–383. [Google Scholar] [CrossRef]
- Kakouri, E.; Kanakis, C.; Trigas, P.; Tarantilis, P.A. Characterization of the chemical composition of Drimia numidica plant parts using high-resolution mass spectrometry: Study of their total phenolic content and antioxidant activity. Anal. Bioanal. Chem. 2019, 411, 3135–3150. [Google Scholar] [CrossRef]
- Rhimi, W.; Ben Salem, I.; Camarda, A.; Saidi, M.; Boulila, A.; Otranto, D.; Cafarchia, C. Chemical characterization and acaricidal activity of Drimia maritima (L.) bulbs and Dittrichia viscosa leaves against Dermanyssus gallinae. Vet. Parasitol. 2019, 268, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; Elsohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid. Based Complement Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef] [PubMed]
- Maazoun, A.; Belhadj, F.; Jemâa, J.B.; Marzouki, M. Assessment of antioxidant potential and α-amylase and acetylcholinesterase inhibitory activities of Urginea maritima (L.) Baker bulbs. J. Mater. Environ. Sci. 2018, 9, 3197–3205. [Google Scholar]
- Tahri, Y.; Koubaa, I.; Frikha, D.; Maalej, S.; Allouche, N. Chemical Investigation and Biological Valorization of Two Essential Oils Newly Extracted from Different Parts of Drimia maritima. J. Essent. Oil Bear. Plants 2020, 23, 1022–1034. [Google Scholar] [CrossRef]
- Rezzagui, A.; Senator, A.; Benbrinis, S.; Bouriche, H. Free Radical Scavenging Activity, Reducing Power and Anti-Hemolytic Capacity of Algerian Drimia maritima Baker Flower Extracts. J. Drug Deliv. Ther. 2020, 10, 70–78. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- González-Paramás, A.M.; Esteban-Ruano, S.; Santos-Buelga, C.; De Pascual-Teresa, S.; Rivas-Gonzalo, J.C. Flavanol Content and Antioxidant Activity in Winery Byproducts. J. Agric. Food Chem. 2004, 52, 234–238. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kähkönen, M.P.; Törrönen, A.R.; Heinonen, I.M. Catechins and Procyanidins in Berries of Vaccinium Species and Their Antioxidant Activity. J. Agric. Food Chem. 2005, 53, 8485–8491. [Google Scholar] [CrossRef]
- Deka, P.; Kumar, A.; Nayak, B.; Eloziia, N. Some plants as a source of acetyl cholinesterase inhibitors: A review. Int. Res. J. Pharm. 2017, 8, 5–13. [Google Scholar] [CrossRef]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.M. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 121. [Google Scholar] [CrossRef]
- Tundis, R.; Bonesi, M.; Menichini, F.; Loizzo, R.M. Recent knowledge on medicinal plants as source of cholinesterase inhibitors for the treatment of dementia. Mini Rev. Med. Chem. 2016, 16, 605–618. [Google Scholar] [CrossRef]
- Islam, M.T.; Da Silva, C.B.; De Alencar, M.V.O.B.; Paz, M.F.C.J.; Almeida, F.R.d.C.; Melo-Cavalcante, A.A.d.C. Diterpenes: Advances in Neurobiological Drug Research. Phytother. Res. 2016, 30, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Suarez, A.I.; Bec, N.; Armijos, C.; Gilardoni, G.; Larroque, C.; Vidari, G. Carnosol from Lepechinia mutica and tiliroside from Vallea stipularis: Two promising inhibitors of BuChE. Rev. Bras. Farmacogn. 2018, 28, 559–563. [Google Scholar] [CrossRef]
- Topcu, G.; Öztürk, M.; Kuşman, T.; Demırkoz, A.A.B.; Kolak, U.; Ulubelen, A. Terpenoids, essential oil composition, fatty acid profile, and biological activities of Anatolian Salvia fruticosa Mill. Turk. J. Chem. 2013, 37, 619–632. [Google Scholar]
- Wu, L.; Chen, C.; Cheng, C.; Dai, H.; Ai, Y.; Lin, C.; Chung, Y. Evaluation of tyrosinase inhibitory, antioxidant, antimicrobial, and antiaging activities of Magnolia officinalis extracts after Aspergillus niger fermentation. BioMed Res. Int. 2018, 2018, 201786. [Google Scholar] [CrossRef]
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef]
- Opperman, L.; De Kock, M.; Klaasen, J.; Rahiman, F. Tyrosinase and Melanogenesis Inhibition by Indigenous African Plants: A Review. Cosmetics 2020, 7, 60. [Google Scholar] [CrossRef]
- Namjoyan, F.; Jahangiri, A.; Azemi, M.E.; Mousavi, H. Inhibitory Effects of Urginea maritima (L.) Baker, Zhumeria majdae Rech. F. and Wendelbo and Physalis divaricata D. Don Ethanolic Extracts on Mushroom Tyrosinase. Pharm. Sci. 2016, 22, 81–86. [Google Scholar] [CrossRef]
- Di Petrillo, A.; González-Paramás, A.M.; Era, B.; Medda, R.; Pintus, F.; Santos-Buelga, C.; Fais, A. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complement. Altern. Med. 2016, 16, 453. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Prakash, O. Enzymes inhibition and antidiabetic effect of isolated constituents from Dillenia indica. BioMed Res. Int. 2013, 2013, 382063. [Google Scholar] [CrossRef]
- Arumugam, G.; Manjula, P.; Paari, N. A review: Anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013, 2, 196–200. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Valere Tsouh Fokou, P.; Kobarfard, F.; Amiruddin Zakaria, Z. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Koorbanally, N.A.; Islam, M.S. Anti-oxidative activity and inhibition of key enzymes linked to type 2 diabetes (α-glucosidase and α-amylase) by Khaya senegalensis. Acta Pharm. 2014, 64, 311–324. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maiti, K.; Mukherjee, K.; Houghton, P.J. Leads from Indian medicinal plants with hypoglycemic potentials. J. Ethnopharmacol. 2006, 106, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Galicia, E.; Aguilar-Contreras, A.; Aguilar-Santamaria, L.; Roman-Ramos, R.; Chavez-Miranda, A.; Garcia-Vega, L.; Flores-Saenz, J.; Alarcon-Aguilar, F. Studies on Hypoglycemic Activity of Mexican Medicinal Plants. Proc. West. Pharmacol. Soc. 2002, 45, 118–124. [Google Scholar] [PubMed]
- Barbosa-Filho, J.M.; Vasconcelos, T.H.; Alencar, A.A.; Batista, L.M.; Oliveira, R.A.; Guedes, D.N.; Falcão, H.d.S.; Moura, M.D.; Diniz, M.F.; Modesto-Filho, J. Plants and their active constituents from South, Central, and North America with hypoglycemic activity. Rev. Bras. Farmacogn. 2005, 15, 392–413. [Google Scholar] [CrossRef]
- Aslan, M.; Orhan, N.; Orhan, D.D.; Ergun, F. Hypoglycemic activity and antioxidant potential of some medicinal plants traditionally used in Turkey for diabetes. J. Ethnopharmacol. 2010, 128, 384–389. [Google Scholar] [CrossRef]
- Alluri, N.; Majumdar, M. Evaluation of α-glucosidase inhibition of Drimia nagarjunae, a medicinal plant from South India. Bangladesh J. Pharmacol. 2015, 10, 635–636. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef]
- Kalita, D.; Holm, D.G.; LaBarbera, D.V.; Petrash, J.M.; Jayanty, S.S. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLoS ONE 2018, 13, e0191025. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.-Y.; Cho, K.-S.; Lee, H.-S. α-amylase and α-glucosidase inhibitors isolated from Triticum aestivum L. sprouts. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 47–51. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Martinelli, E.; Senizza, B.; Miras-Moreno, B.; Yildiztugay, E.; Arikan, B.; Elbasan, F.; Ak, G.; Balci, M.; Zengin, G.; et al. The Combination of Mild Salinity Conditions and Exogenously Applied Phenolics Modulates Functional Traits in Lettuce. Plants 2021, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Miras-Moreno, B.; Yildiztugay, E.; Ozfidan-Konakci, C.; Arikan, B.; Elbasan, F.; Ak, G.; Rouphael, Y.; Zengin, G.; Lucini, L. Metabolomics and Physiological Insights into the Ability of Exogenously Applied Chlorogenic Acid and Hesperidin to Modulate Salt Stress in Lettuce Distinctively. Molecules 2021, 26, 6291. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.; Pérez-Jiménez, J.; Neveu, V.; Medina-Ramon, A.; M’Hiri, N.; Garcia Lobato, P.; Manach, C.; Knox, K.; Eisner, R.; Wishart, D. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordoñez, M.; Llorach, R.; Farran-Codina, A.; Barupal, D.K.; Neveu, V.; Manach, C.; Andres-Lacueva, C.; Scalbert, A. Systematic analysis of the polyphenol metabolome using the Phenol-Explorer database. Mol. Nutr. Food Res. 2016, 60, 203–211. [Google Scholar] [CrossRef]
- Salek, R.M.; Neumann, S.; Schober, D.; Hummel, J.; Billiau, K.; Kopka, J.; Correa, E.; Reijmers, T.; Rosato, A.; Tenori, L.; et al. COordination of Standards in MetabOlomicS (COSMOS): Facilitating integrated metabolomics data access. Metabolomics 2015, 11, 1587–1597. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pagnossa, J.P.; Blasi, F.; Cossignani, L.; Hilsdorf Piccoli, R.; Zengin, G.; Montesano, D.; Cocconcelli, P.S.; Lucini, L. Phenolic profiling and in vitro bioactivity of Moringa oleifera leaves as affected by different extraction solvents. Food Res. Int. 2020, 127, 108712. [Google Scholar] [CrossRef]

| Parts | Solvents | DPPH | ABTS | CUPRAC | FRAP | MCA | PBD |
|---|---|---|---|---|---|---|---|
| (mg TE/g) | (mg EDTAE/g) | (mmol TE/g) | |||||
| Aerial parts | EA | 4.75 ± 0.40 a | 26.52 ± 0.77 a | 47.44 ± 0.72 a | 15.26 ± 0.75 a | 21.18 ± 1.76 a | 1.35 ± 0.02 c |
| MeOH | 19.44 ± 0.31 b | 48.34 ± 0.51 b | 53.18 ± 0.65 b | 29.93 ± 0.46 b | 25.28 ± 0.33 b | 0.82 ± 0.06 b | |
| Water | 36.99 ± 0.38 c | 85.96 ± 1.13 c | 87.37 ± 0.16 c | 55.43 ± 0.39 c | 22.91 ± 0.30 a | 0.72 ± 0.04 a | |
| Bulbs | EA | 11.44 ± 0.35 C | 38.62 ± 0.25 C | 66.92 ± 1.69 B | 24.49 ± 0.39 B | 4.68 ± 0.26 B | 1.45 ± 0.09 B |
| MeOH | 6.81 ± 0.28 A | 27.67 ± 0.20 B | 25.60 ± 0.27 A | 16.52 ± 0.24 A | 3.78 ± 0.23 A | 0.99 ± 0.05 A | |
| Water | 7.67 ± 0.23 B | 23.51 ± 0.62 A | 24.31 ± 0.50 A | 16.38 ± 0.29 A | 13.21 ± 0.40 C | 1.09 ± 0.10 A | |
| Parts | Solvents | AChE | BChE | Tyrosinase | Amylase | Glucosidase |
|---|---|---|---|---|---|---|
| (mg GALAE/g) | (mg KAE/g) | (mmol ACAE/g) | ||||
| Aerial parts | EA | 2.36 ± 0.09 c | 4.77 ± 0.40 b | 48.59 ± 0.65 b | 1.00 ± 0.01 c | 0.66 ± 0.08 b |
| MeOH | 1.89 ± 0.01 b | 2.80 ± 0.24 a | 54.64 ± 0.44 c | 0.63 ± 0.01 b | 0.06 ± 0.02 a | |
| Water | 0.36 ± 0.04 a | na | 6.44 ± 0.93 a | 0.17 ± 0.01 a | 0.04 ± 0.01 a | |
| Bulbs | EA | 1.39 ± 0.19 A | 5.10 ± 0.27 B | 50.18 ± 0.51 A | 0.76 ± 0.01 C | 0.33 ± 0.05 A |
| MeOH | 1.86 ± 0.09 B | 4.72 ± 0.23 B | 51.38 ± 0.57 B | 0.53 ± 0.02 B | na | |
| Water | na | 1.65 ± 0.13 A | na | 0.09 ± 0.01 A | na | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zengin, G.; Mahomoodally, M.F.; Yıldıztugay, E.; Jugreet, S.; Simal-Gandara, J.; Rouphael, Y.; Pannico, A.; Lucini, L. Untargeted Phenolic Profiling and Functional Insights of the Aerial Parts and Bulbs of Drimia maritima (L.) Stearn. Plants 2022, 11, 600. https://doi.org/10.3390/plants11050600
Zhang L, Zengin G, Mahomoodally MF, Yıldıztugay E, Jugreet S, Simal-Gandara J, Rouphael Y, Pannico A, Lucini L. Untargeted Phenolic Profiling and Functional Insights of the Aerial Parts and Bulbs of Drimia maritima (L.) Stearn. Plants. 2022; 11(5):600. https://doi.org/10.3390/plants11050600
Chicago/Turabian StyleZhang, Leilei, Gokhan Zengin, Mohamad Fawzi Mahomoodally, Evren Yıldıztugay, Sharmeen Jugreet, Jesus Simal-Gandara, Youssef Rouphael, Antonio Pannico, and Luigi Lucini. 2022. "Untargeted Phenolic Profiling and Functional Insights of the Aerial Parts and Bulbs of Drimia maritima (L.) Stearn" Plants 11, no. 5: 600. https://doi.org/10.3390/plants11050600
APA StyleZhang, L., Zengin, G., Mahomoodally, M. F., Yıldıztugay, E., Jugreet, S., Simal-Gandara, J., Rouphael, Y., Pannico, A., & Lucini, L. (2022). Untargeted Phenolic Profiling and Functional Insights of the Aerial Parts and Bulbs of Drimia maritima (L.) Stearn. Plants, 11(5), 600. https://doi.org/10.3390/plants11050600











