The Effects of Edaphic Factors on Riparian Plants in the Middle and Lower Reaches of the Hanjiang River, China
Abstract
:1. Introduction
2. Results
2.1. Community Composition
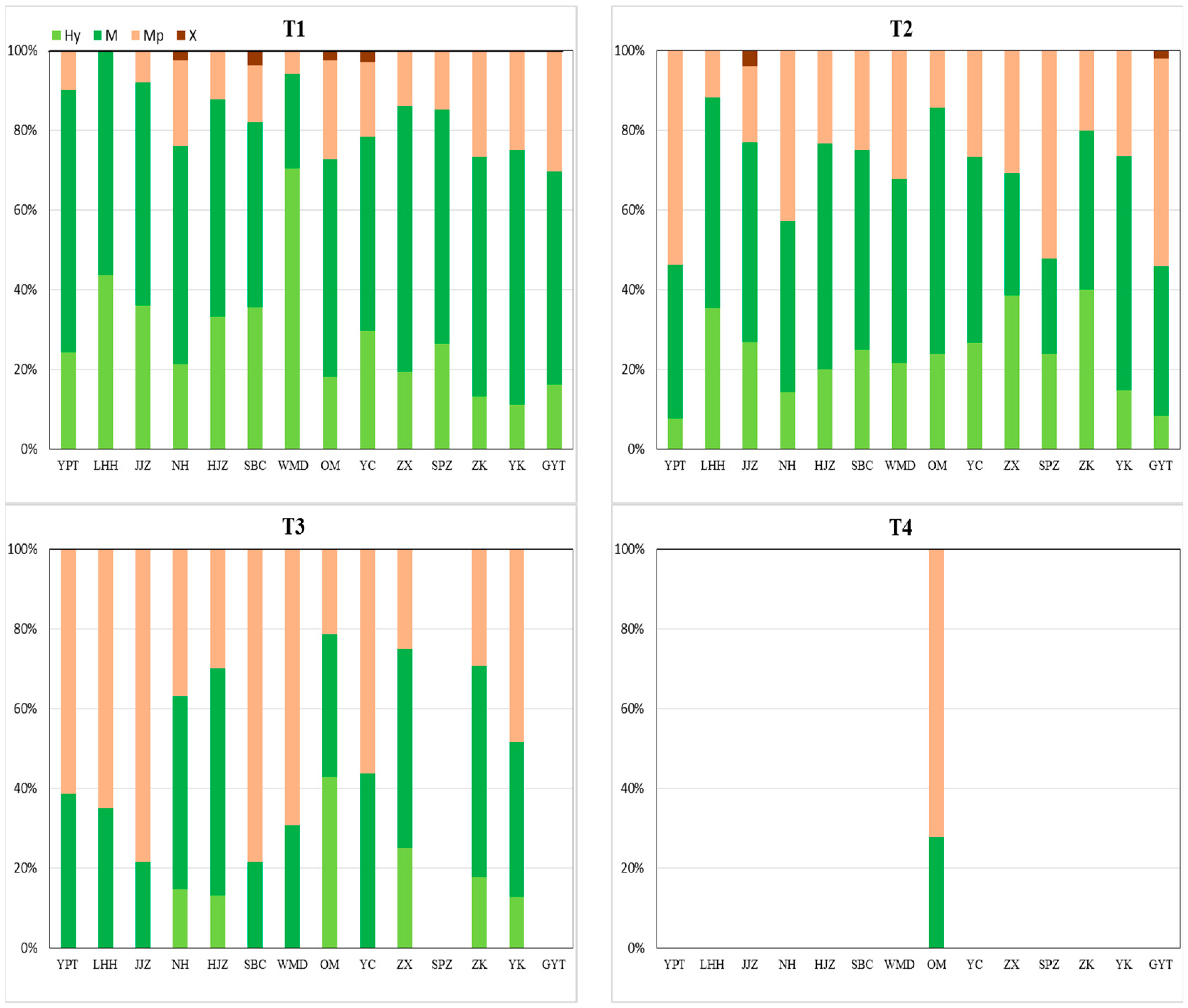
2.2. Community Hydrophilicity and Life History Characteristics
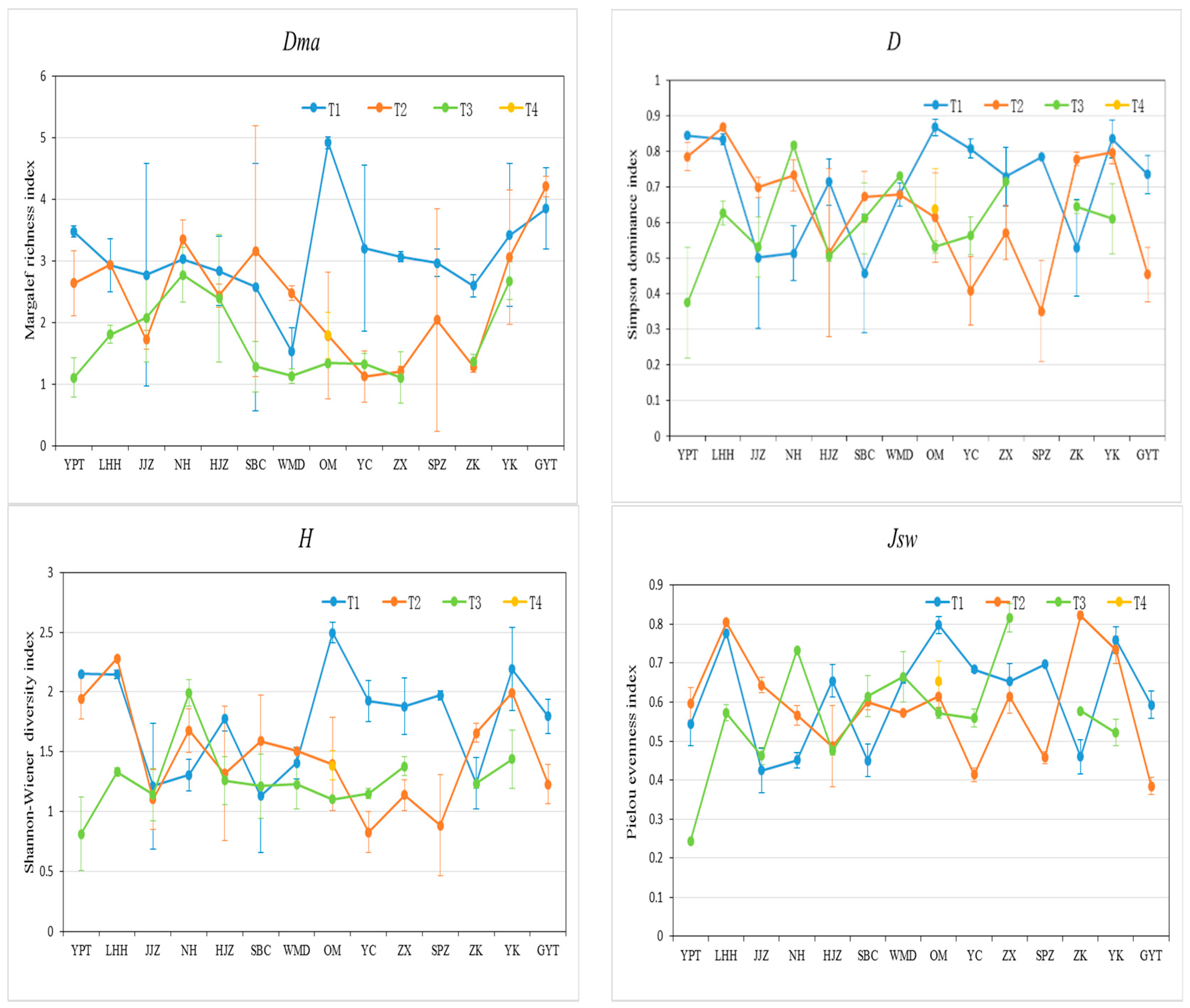
2.3. Species Diversity Characteristics
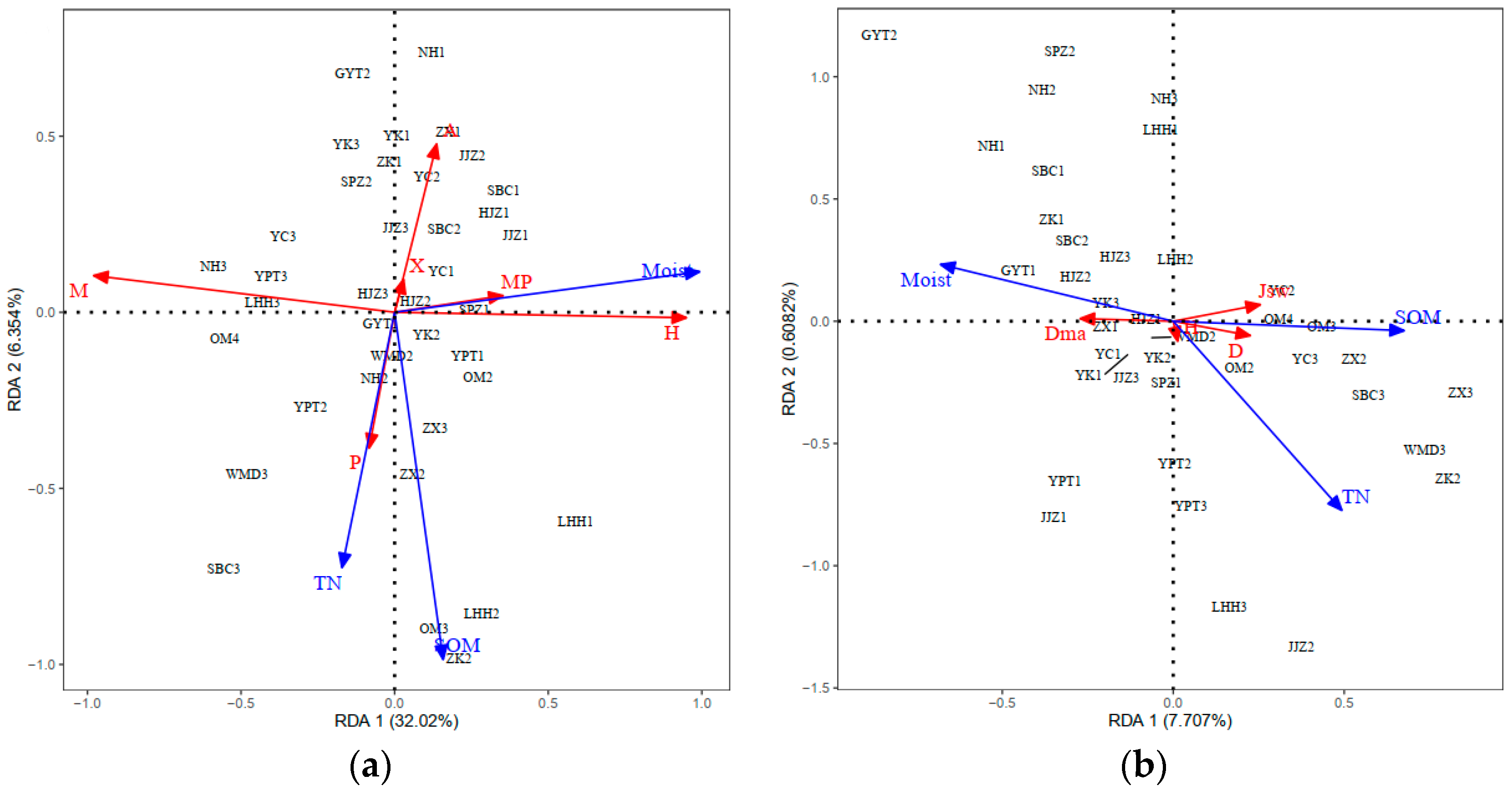
2.4. RDA Ordination and Distribution
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Plant Data Collection
4.3. Edaphic Parameters
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, A.M.; Ayed, F.A.A.; Sheded, M.G.; Alrumman, S.A.; Radwan, T.A.A.; Badry, M.O. Vegetation analysis and environmental relationships of riverain plants in the Aswan Reservoir, Egypt. Plants 2021, 10, 2712. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Svedmark, M. Basic principles and ecological consequences of changing water regimes: Riparian plant communities. Environ. Manag. 2002, 30, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Young-Mathews, A.; Culman, S.W.; Sánchez-Moreno, S.; O’Geen, A.T.; Ferris, H.; Hollander, A.D.; Jackson, L.E. Plant-soil biodiversity relationships and nutrient retention in agricultural riparian zones of the Sacramento valley, California. Agrofor. Syst. 2010, 80, 41–60. [Google Scholar] [CrossRef] [Green Version]
- Mallik, A.U.; Richardson, J.S. Riparian vegetation change in upstream and downstream reaches of three temperate rivers dammed for hydroelectric generation in British Columbia, Canada. Ecol. Eng. 2009, 35, 810–819. [Google Scholar] [CrossRef]
- Stromberg, J.C.; Boudell, J.A. Floods, drought, and seed mass of riparian plant species. J. Arid Environ. 2013, 97, 99–107. [Google Scholar] [CrossRef]
- Li, S.J.; Su, P.X.; Zhang, H.N.; Zhou, Z.J.; Xie, T.T.; Shi, R.; Guo, W. Distribution patterns of desert plant diversity and relationship to soil properties in the Heihe River Basin, China. Ecosphere 2018, 9, e02355. [Google Scholar] [CrossRef]
- Bendix, J.; Hupp, C.R. Hydrological and geomorphological impacts on riparian plant communities. Hydrol. Process. 2000, 14, 2977–2990. [Google Scholar] [CrossRef]
- Mulhouse, J.M.; Burbage, L.E.; Sharitz, R.R. Seed bank-vegetation relationships in herbaceous Carolina bays: Responses to climatic variability. Wetlands 2005, 25, 738–747. [Google Scholar] [CrossRef]
- Stromberg, J.C.; Beauchamp, V.B.; Dixon, M.D.; Lite, S.J.; Paradzick, C. Importance of low-flow and high-flow characteristics to restoration of riparian vegetation along rivers in arid south-western United States. Freshw. Biol. 2007, 52, 651–679. [Google Scholar] [CrossRef]
- Kominoski, J.S.; Shah, J.J.F.; Canhoto, C.; Fischer, D.G.; Giling, D.P.; González, E.; Griffiths, N.A.; Larrañaga, A.; LeRoy, C.; Mineau, M.M.; et al. Forecasting functional implications of global changes in riparian plant communities. Front. Ecol. Environ. 2013, 11, 423–432. [Google Scholar] [CrossRef]
- Wang, M.; Dong, Z.B.; Luo, W.Y.; Lu, J.F.; Li, J.Y. Spatial variability of vegetation characteristics, soil properties and their relationships in and around China’s Badain Jaran Desert. Environ. Earth Sci. 2015, 74, 6847–6858. [Google Scholar] [CrossRef]
- Lite, S.J.; Bagstad, K.J.; Stromberg, J.C. Riparian plant species richness along lateral and longitudinal gradients of water stress and flood disturbance, San Pedro River, Arizona, USA. J. Arid Environ. 2005, 63, 785–813. [Google Scholar] [CrossRef]
- Critchley, C.N.R.; Chambers, B.J.; Fowbert, J.A.; Sanderson, R.A.; Bhogal, A.; Rose, S.C. Association between lowland grassland plant communities and soil properties. Biol. Conserv. 2002, 105, 199–215. [Google Scholar] [CrossRef]
- Taylor, J.P.; Wester, D.B.; Smith, L.M. Soil disturbance, flood management, and riparian woody plant establishment in the Rio Grande floodplain. Wetlands 1999, 19, 372–382. [Google Scholar] [CrossRef]
- Sutton-Grier, A.E.; Wright, J.P.; Richardson, C.J. Different plant traits affect two pathways of riparian nitrogen removal in a restored freshwater wetland. Plant Soil 2013, 365, 41–57. [Google Scholar] [CrossRef]
- Yu, M.F.; Tao, Y.X.; Liu, W.Z.; Xing, W.; Liu, G.H.; Wang, L.; Ma, L. C, N, and P stoichiometry and their interaction with different plant communities and soils in subtropical riparian wetlands. Environ. Sci. Pollut. R. 1998, 27, 1024–1034. [Google Scholar] [CrossRef]
- Schwoertzig, E.; Ertlen, D.; Trémolières, M. Are plant communities mainly determined by anthropogenic land cover along urban riparian corridors? Urban Ecosyst. 2016, 19, 1767–1786. [Google Scholar] [CrossRef]
- Guo, W.; Gong, X.S.; Deng, X.W.; Wang, Z.X.; Li, Z.Q. Community succession of macrophytes in the middle and lower reaches of the Hanjiang River. Chin. Bull. Bot. 2016, 51, 782–789. [Google Scholar] [CrossRef]
- Li, L.C.; Zhang, L.P.; Xia, J.; Gippel, C.J.; Wang, R.C.; Zeng, S.D. Implications of modelled climate and land cover changes on runoff in the Middle Route of the South to North Water Transfer Project in China. Water Resour. Manag. 2015, 29, 2563–2579. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Z.H.; Yu, D.; Liu, C.H.; Li, Z.Q.; Xiao, K.Y. Diversity of aquatic plants in Hanjiang River basin and possible effects of the engineering of transferring water from south China to north China on it. Acta Ecol. Sin. 2002, 22, 1933–1938. [Google Scholar] [CrossRef]
- Xu, J.C.; Du, H.C.; Wang, X.N.; Qin, J.H.; Xia, C.X.; Hou, J.; Fan, Z.Y.; Wu, H.; Wang, J.; He, X.G. Current situation and changing trend of aquatic organisms resources in main stream of Hanjiang River from 2017 to 2020 (in Chinese). J. Huazhong Agric. Univ. 2021, 40, 126–137. [Google Scholar] [CrossRef]
- Hu, Y.X.; Huang, J.L.; Du, Y.; Han, P.P.; Wang, J.L.; Huang, W. Monitoring wetland vegetation pattern response to water-level change resulting from the three gorges project in the two largest freshwater lakes of China. Ecol. Eng. 2015, 74, 274–285. [Google Scholar] [CrossRef]
- Liu, W.Z.; Liu, G.H.; Zhang, Q.F. Shoreline vegetation in the Danjiangkou reservoir: Characteristics, related factors, and differences with adjacent riverine wetlands. Clean 2014, 42, 1014–1021. [Google Scholar] [CrossRef]
- Bornman, T.G.; Adams, J.B.; Bate, G.C. Environmental factors controlling the vegetation zonation patterns and distribution of vegetation types in the Olifants Estuary, South Africa. S. Afr. J. Bot. 2008, 74, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, N.E.; Richardson, C.; Ho, M.C. Connecting differential responses of native and invasive riparian plants to climate change and environmental alteration. Ecol. Appl. 2015, 25, 753–767. [Google Scholar] [CrossRef] [Green Version]
- Xiu, C.; Gerisch, M.; Ilg, C.; Henle, K.; Ouyang, Z.Y. Effects of hydrologic modifications to riparian plant communities in a large river system in northern China. Ecol. Res. 2015, 30, 461–469. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Colmer, T.D.; Pierik, R.; Millenaar, F.F.; Peeters, A.J.M. How plants cope with complete submergence. New Phytol. 2006, 170, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; Kroon, H. Plasticity in morphology and biomass allocation in Cynodon dactylon, a grass species forming stolons and rhizomes. Oikos 1994, 70, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Hughes, F.M.R. Floodplain biogeomorphology. Prog. Phys. Geog. 1997, 21, 501–529. [Google Scholar] [CrossRef]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef]
- Osawa, T.; Mitsuhashi, H.; Ushimaru, A. River confluences enhance riparian plant species diversity. Plant Ecol. 2010, 209, 95–108. [Google Scholar] [CrossRef]
- Greet, J.; Cousens, R.D.; Webb, J.A. Seasonal timing of inundation affects riparian plant growth and flowering: Implications for riparian vegetation composition. Plant Ecol. 2013, 214, 87–101. [Google Scholar] [CrossRef]
- Stromberg, J.C. Restoration of riparian vegetation in the south-western United States: Importance of flow regimes and fluvial dynamism. J. Arid Environ. 2001, 49, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Pettit, N.E.; Froend, R.H.; Davies, P.M. Identifying the natural flow regime and the relationship with riparian vegetation for two contrasting western Australian rivers. Regul. River. 2001, 17, 201–215. [Google Scholar] [CrossRef]
- McIntyre, S.; Lavorel, S.; Tremont, R.M. Plant life-history attributes: Their relationship to disturbance response in herbaceous vegetation. J. Ecol. 1995, 83, 31–44. [Google Scholar] [CrossRef]
- Wang, S.Y.; Pi, Y.X.; Jiang, Y.Y.; Pan, H.W.; Wang, X.X.; Wang, X.M.; Zhou, J.M.; Zhu, G.B. Nitrate reduction in the reed rhizosphere of a riparian zone: From functional genes to activity and contribution. Environ. Res. 2020, 180, 108867. [Google Scholar] [CrossRef]
- Pang, Q.Z.; Guo, J.C.; Wei, C.; Zhao, P.; Wang, H.W.; Han, J.X.; Liu, H.S. Migration regularity and Removal efficiency of nitrogen and phosphorus by four different wetland plants. J. Northw. For. Uni. 2019, 34, 68–73. [Google Scholar] [CrossRef]
- Wang, Y.D.; Yang, J.; Liang, J.P.; Qiang, Y.F.; Fang, S.Q.; Gao, M.X.; Fan, X.Y.; Yang, G.H.; Zhang, B.W.; Feng, Y.Z. Analysis of the environmental behavior of farmers for non-point source pollution control and management in a water source protection area in China. Sci. Total Environ. 2018, 633, 1126–1135. [Google Scholar] [CrossRef]
- Li, J.; Loneragan, W.A.; Duggin, J.A.; Grant, C.D. Issues affecting the measurement of disturbance response patterns in herbaceous vegetation: A test of the intermediate disturbance hypothesis. Plant Ecol. 2004, 172, 11–26. [Google Scholar] [CrossRef]
- Castelli, R.M.; Chambers, J.C.; Tausch, R.J. Soil-plant relations along a soil-water gradient in great basin riparian meadows. Wetlands 2000, 20, 251–266. [Google Scholar] [CrossRef]
- Yang, H.J.; Li, Y.; Wu, M.Y.; Zhang, Z.; Li, L.H.; Wan, S.Q. Plant community responses to nitrogen addition and increased precipitation: The importance of water availability and species traits. Glob. Chang. Biol. 2011, 17, 2936–2944. [Google Scholar] [CrossRef]
- Hocking, M.D.; Reynolds, J.D. Impacts of salmon on riparian plant diversity. Science 2011, 331, 1609–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, M.; Thoms, M.; Reid, M. Determining the ecohydrological character of aquatic refugia in a dryland river system: The importance of temporal scale. Ecohydrol. Hydrobiol. 2012, 12, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Azami, K.; Takemoto, M.; Otsuka, Y.; Yamagishi, S.; Nakazawa, S. Meteorology and species composition of plant communities, birds and fishes before and after initial impoundment of Miharu Dam Reservoir, Japan. Landsc. Ecol. Eng. 2012, 8, 81–105. [Google Scholar] [CrossRef]
- Colonnello, G.; Medina, E. Vegetation changes induced by dam construction in a tropical estuary: The case of the Mánamo River, Orinoco Delta (Venezuela). Plant Ecol. 1998, 139, 145–154. [Google Scholar] [CrossRef]
- Yu, M.X.; Wood, P.; Giesen, N.V.; Liu, X.L.; Li, Q.F.; Wang, G.Q.; Zhang, J.Y. Enhanced potential ecological risk induced by a large scale water diversion project. Stoch. Environ. Res. Risk A. 2020, 34, 2125–2138. [Google Scholar] [CrossRef]
- Yang, Q.; Xie, P.; Shen, H.; Xu, J.; Wang, P.L.; Zhang, B. A novel flushing strategy for diatom bloom prevention in the lower-middle Hanjiang River. Water Res. 2012, 46, 2525–2534. [Google Scholar] [CrossRef]
- Xu, X.Z.; Song, G.D.; Dang, T.M.; Liu, J.W.; Zhang, H.W.; Gao, H.; Liu, Y.K. Environment and sustainability of the Middle Route, South-to-North Water Transfer Project in China: A close look. Environ. Dev. Sustain. 2018, 20, 2415–2426. [Google Scholar] [CrossRef]
- Qian, J.L.; Zhang, L.D.; Le, M.L. Total nitrogen and phosphorus in soil were determined by persulfate digestion. Soil 1990, 22, 258–262. [Google Scholar] [CrossRef]
- Curtis, J.T.; Mcintosh, R.P. An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]




| Index | Transect | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Hydrophilicity group | ||||
| Hydric species | 19 | 12 | 8 | 2 |
| Mesic species | 64 | 58 | 37 | 8 |
| Mesophytes | 27 | 36 | 40 | 14 |
| Xerophytes | 1 | 1 | 1 | 0 |
| Life-history | ||||
| Perennial species | 49 | 46 | 36 | 12 |
| Annual species | 62 | 61 | 50 | 12 |
| Axis 1 | Axis 2 | Axis 3 | Axis 4 | Random Permutation Test | ||
|---|---|---|---|---|---|---|
| R2 | p | |||||
| Altitude | 0.011 | 0.108 | −0.068 | −0.122 | 0.084 | 0.215 |
| Soil moisture | 0.560 | −0.060 | −0.199 | −0.082 | 0.539 | 0.001 *** |
| TN | −0.001 | −0.049 | −0.570 | −0.107 | 0.1673 | 0.027 * |
| TP | −0.347 | 1.207 | 0.381 | −1.514 | 0.0071 | 0.888 |
| SOM | 0.012 | 0.525 | 0.467 | 0.366 | 0.445 | 0.001 *** |
| Eigenvalues | 0.018 | 0.006 | 0.001 | 0.0001 | ||
| Species-environment correlations | 0.738 | 0.581 | 0.431 | 0.314 | ||
| Significance test for all axes | F = 4.6202 p = 0.001 *** | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Li, E.; Zhou, R.; Xia, Y.; Yang, C.; Zhang, Y. The Effects of Edaphic Factors on Riparian Plants in the Middle and Lower Reaches of the Hanjiang River, China. Plants 2022, 11, 531. https://doi.org/10.3390/plants11040531
Yang J, Li E, Zhou R, Xia Y, Yang C, Zhang Y. The Effects of Edaphic Factors on Riparian Plants in the Middle and Lower Reaches of the Hanjiang River, China. Plants. 2022; 11(4):531. https://doi.org/10.3390/plants11040531
Chicago/Turabian StyleYang, Jiao, Enhua Li, Rui Zhou, Ying Xia, Chao Yang, and Yingying Zhang. 2022. "The Effects of Edaphic Factors on Riparian Plants in the Middle and Lower Reaches of the Hanjiang River, China" Plants 11, no. 4: 531. https://doi.org/10.3390/plants11040531
APA StyleYang, J., Li, E., Zhou, R., Xia, Y., Yang, C., & Zhang, Y. (2022). The Effects of Edaphic Factors on Riparian Plants in the Middle and Lower Reaches of the Hanjiang River, China. Plants, 11(4), 531. https://doi.org/10.3390/plants11040531






