Metal Accumulation and Biomass Production in Young Afforestations Established on Soil Contaminated by Heavy Metals
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Biomass and Heavy Metal Stocks in Afforestations
| Topsoil Contamination (Total Extractable, mg kg−1 at Harvest) | Site | Species | Organs | Period (Years) | Extraction (mg m−2 year−1) | Yield (tha−1 year−1) | Reference |
|---|---|---|---|---|---|---|---|
| Zn/Cu/-/Cd = 1158/264/-/2.8 and 650/550/2 | Caslano and Dornach Switzerland | Salix viminalis | LW | 2 and 5 | Zn/-/-/Cd = 330/-/-/0.1 and 155/-/-/0.1 | 5 or 6.6 | [51] |
| Zn/Cu/-/Cd = 650/530/-/2 | Dornach Switzerland | Salix viminalis | LWR | 3 and 1 | Zn/Cu/-/Cd = 128/6.3/-/1.3 | 4.3 and 14 | [57] |
| Zn/Cu/Pb/Cd = 400/180/170/2.5 | Copenhagen recycling center Denmark | Salix viminalis | LW | 1 | Zn/Cu/Pb/Cd = 35/0.8/0.04/10 | 0.9 | [58] |
| Zn/Cu/Pb/Cd = 377/-/-/6.5 | Campine region Belgium | Salix viminalis 8 clones | LW | 4 | Zn/Cu/Pb/Cd = 159/-/-/2 | 3.8 | [49] |
| Zn/Cu/-/Cd = 174/81/-/1.3 | Hradec Kralove Czech Republic | Salix and Populus clones | LWR | 2 | Zn/Cu/-/Cd = 1232/39/-/8.5 | 0.4 | [52] |
| Zn/Cu/Pb/Cd = 295/24/283/2.8 | Litavka River sediments Czech Republic | Salix, Populus | LWR | 3 | Zn/Cu/Pb/Cd = 100/-/2.3/2.7 | 12 | [45] |
| Zn/Cu/Pb/Cd = 1563/112/-/16.7 | Harbour Rotterdam, The Netherlands | Populus ‘Robusta’ | W | 33 | Zn/Cu/Pb/Cd = 15/0.2/-/0.5 | 2 | [56] |
| Zn/Cu/Pb/- = 56/17/27/- | Gao country, Sichuan, China | Pinus massoniana | LWR | stand 27 | Zn/Cu/Pb/- = 3095/861/1453/- | [54] | |
| Zn/Cu/Pb/Cd = 243/51/27/- | Erzurum Turkey | Pinus sylvestris | LWR | stand 35 | Zn/Cu/Pb/- = 18′085/8′000/2′886/- | [53] | |
| Zn/Cu/Pb/Cd = 972/173/1687/15 | Chenzhou, China | Populus deltoides | LWR | 5 | Zn/Cu/Pb/Cd = 1410/38/148/61 | 10 | [55] |
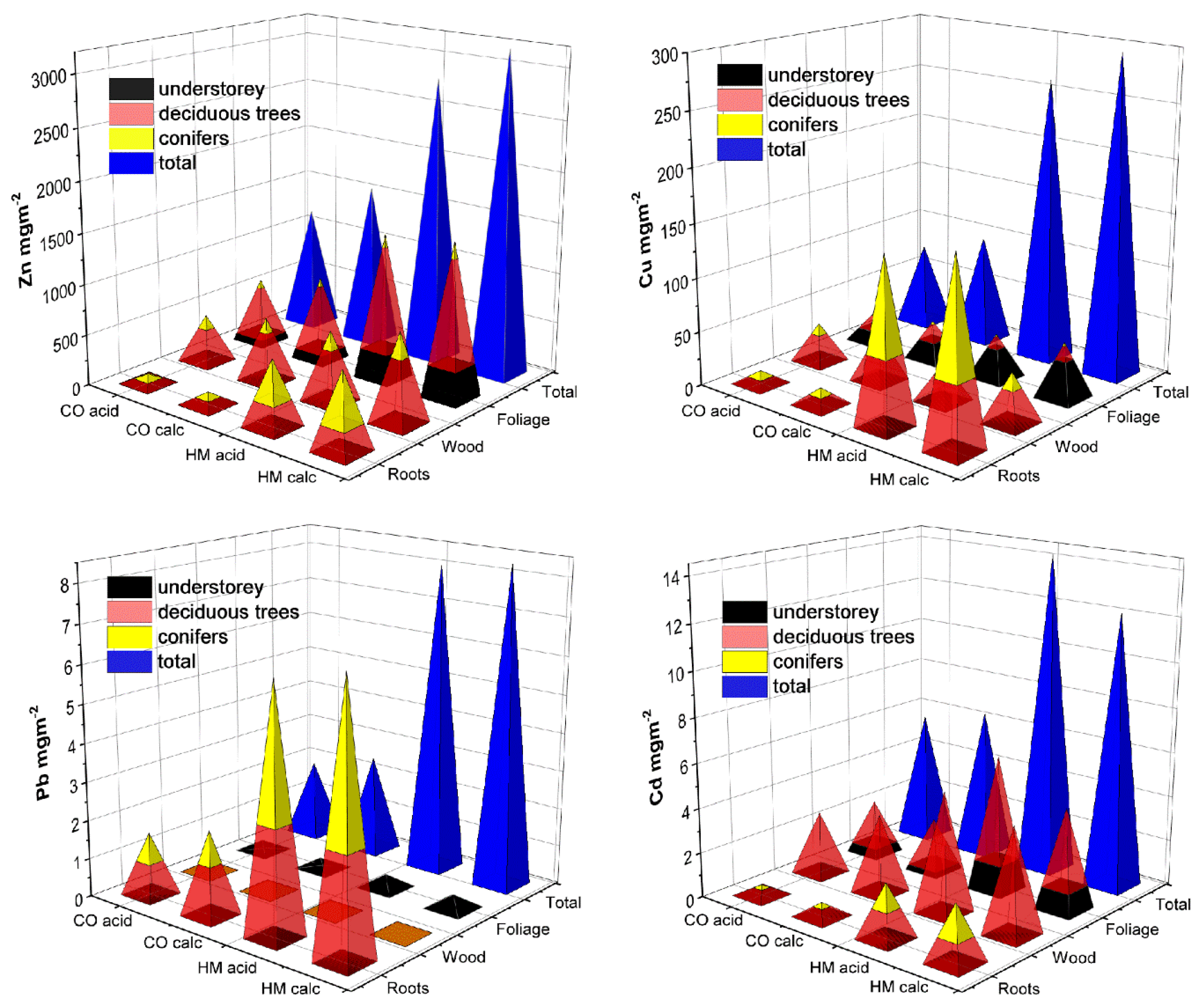
| Zn/Cu/Pb/Cd = 2854/588/103/9.2 | Birmensdorf, Switzerland | Understorey + deciduous + conifer trees | LWR | 4 | Zn/Cu/Pb/Cd = 749/70/2/3 | 14 | Figure 1 and Figure 3 |
3.2. Influence of the Uncontaminated Acidic and Calcareous Subsoil on Metal Uptake
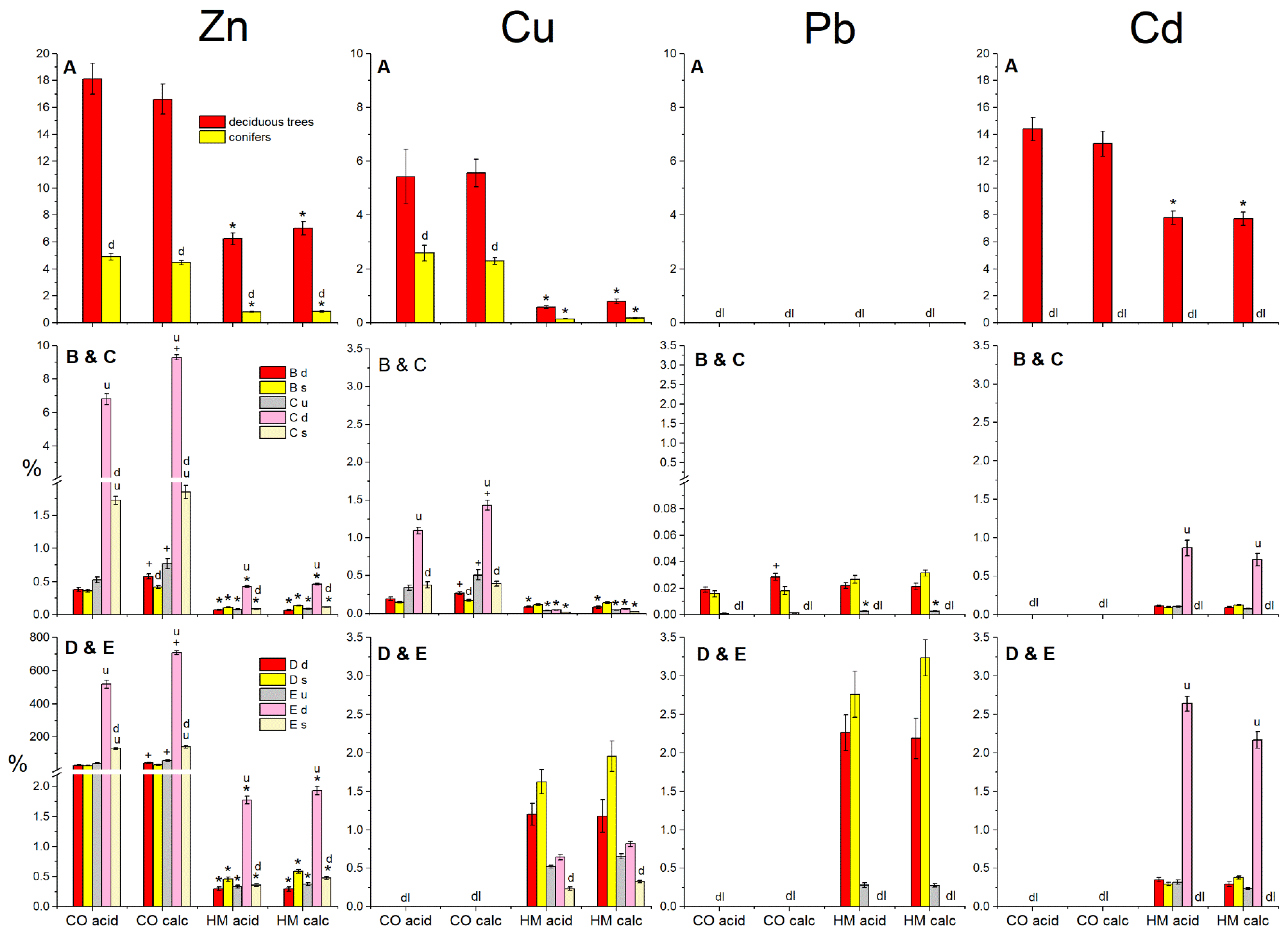
3.3. Metal Allocation Ratios and Soil-to-Plant Metal Transfer
3.4. Soil Metal Solubility in Relation to Plant Uptake and Allocation
3.5. Accumulated Soil Metal Stocks in Foliage and Aboveground Wood
3.6. Phytoremediation Potential of Afforestations for Metal-Contaminated Soils
4. Materials and Methods
- -
- The understorey group (u) consisted of four specimens of tansy (Tanacetum vulgare L.) grown from root cuttings, four small sedge plants of (Carex sylvatica Hudson), one specimen of ransom (Allium ursinum L.) grown from bulb, plus three oak (Quercus pubescens Wild.), three beech (Fagus sylvatica L.) and three spruce seedlings grown from seeds directly sown in the plots.
- -
- The deciduous tree group (d) consisted of two birch (Betula pendula Roth), two willow (Salix viminalis L.) and four poplar (Populus tremula L.) trees grown from cuttings.
- -
- The conifer group (s) consisted of six spruce trees (P. abies (L.) Karst., six provenances from 500 to 1800 m a.s.l.) grown from three-year-old nursery seedlings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kastrati, G.; Paçarizi, M.; Sopaj, F.; Tašev, K.; Stafilov, T.; Mustafa, M. Investigation of Concentration and Distribution of Elements in Three Environmental Compartments in the Region of Mitrovica, Kosovo: Soil, Honey and Bee Pollen. Int. J. Environ. Res. Public Health 2021, 18, 2269. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tu, J.; Liu, H.; Hua, M.; Liao, Q.; Feng, J.; Weng, Z.; Huang, G. Multivariate analysis of trace element concentrations in atmospheric deposition in the Yangtze River Delta, East China. Atmos. Environ. 2009, 43, 5781–5790. [Google Scholar] [CrossRef]
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated Sites in Europe: Review of the Current Situation Based on Data Collected through a European Network. J. Environ. Public Health 2013, 2013, 158764. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Lu, X.; Qiannan, D.; Lei, H.; Bi, J. A review of soil heavy metal pollutionfrom industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Shammi, S.A.; Salam, A.; Khan, M.A.H. Assessment of heavy metal pollution in the agricultural soils, plants, and in the atmospheric particulate matter of a suburban industrial region in Dhaka, Bangladesh. Environ. Monit. Assess. 2021, 193, 104:1–104:12. [Google Scholar] [CrossRef]
- Hadjipanagiotou, C.; Christou, A.; Zissimos, A.M.; Chatzitheodoridis, E.; Varnavas, S.P. Contamination of stream waters, sediments, and agricultural soil in the surroundings of an abandoned copper mine by potentially toxic elements and associated environmental and potential human health-derived risks: A case study from Agrokipia, Cyprus. Environ. Sci. Pollut. Res. 2020, 27, 41279–41298. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Ippolito, J.A.; Xing, W.; Qiu, K.; Yang, H. Lead smelting effects heavy metal concentrations in soils, wheat, and potentially humans. Environ. Pollut. 2020, 257, 113641. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.; Qureshi, S.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Sterckeman, T.; Thomine, S. Mechanisms of cadmium accumulation in plants. Crit. Rev. Plant Sci. 2020, 39, 322–359. [Google Scholar] [CrossRef]
- Hanczaruk, R.; Kompała-Bąba, A. Changes in the vascular flora of a postflotation zinc-lead ore spoil heap of the “Orzeł Biały” mining and smelting works in Bytom (Silesian Upland) after 15 years. Acta Agrobot. 2019, 72, 1762. [Google Scholar] [CrossRef]
- Christou, A.; Theologides, C.P.; Costa, C.; Kalavrouziotis, I.K.; Varnavas, S.P. Assessment of toxic heavy metals concentrations in soils and wild and cultivated plant species in Limni abandoned copper mining site, Cyprus. J. Geochem. Explor. 2017, 178, 16–22. [Google Scholar] [CrossRef]
- Xing, W.; Liu, H.; Banet, T.; Wang, H.; Ippolito, J.A.; Li, L. Cadmium, copper, lead and zinc accumulation in wild plant species near a lead smelter. Ecotoxicol. Environ. Saf. 2020, 198, 110683. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Donaldson, E.; Kaste, J.; Friedland, A. Forest floor lead, copper and zinc concentrations across the northeastern United States: Synthesizing spatial and temporal responses. Sci. Total Environ. 2014, 505, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Wang, J.; Sun, L.; Xu, Z.; Tang, J.; Yan, J.; Zeng, X. Profiles and potential health risks of heavy metals in soil and crops from the watershed of Xi River in Northeast China. Ecotoxicol. Environ. Saf. 2018, 169, 442–448. [Google Scholar] [CrossRef]
- Nowack, B.; Rais, D.; Frey, B.; Menon, M.; Schulin, R.; Günthardt-Goerg, M.S.; Luster, J. Influence of metal contamination on soil parameters in a lysimeter experiment designed to evaluate phytostabilization by afforestation. For. Snow Landsc. Res. 2006, 80, 201–211. [Google Scholar]
- Shaheen, A.; Javed, I. Spatial distribution and mobility assessment of carcinogenic heavy metals in soil profiles using geostatistics and random forest, Boruta Algorithm. Sustainability 2018, 10, 799. [Google Scholar] [CrossRef]
- Zhao, R.; Li, J.; Ma, Y.; Lv, Y. A field study of vertical mobility and relative bioavailability of Cu and Ni in calcareous soil. Environ. Pollut. Bioavailab. 2020, 32, 121–130. [Google Scholar] [CrossRef]
- Pesonen, J.; Kuokkanen, T.; Rautio, P.; Lassi, U. Bioavailability of nutrients and harmful elements in ash fertilizers: Effect of granulation. Biomass Bioenergy 2017, 100, 92–97. [Google Scholar] [CrossRef]
- Romero-Baena, A.J.; Barba-Brioso, C.; Ross, A.; González, I.; Aparicio, P. Mobility of potentially toxic elements in family garden soils of the Riotinto mining area. Appl. Clay Sci. 2021, 203, 105999. [Google Scholar] [CrossRef]
- Misra, V.; Tiwari, A.; Shukla, B.; Seth, C.S. Effects of soil amendments on the bioavailability of heavy metals from zinc mine tailings. Environ. Monit. Assess. 2008, 155, 467–475. [Google Scholar] [CrossRef]
- Fifi, U.; Winiarski, T.; Emmanuel, E. Assessing the Mobility of Lead, Copper and Cadmium in a Calcareous Soil of Port-au-Prince, Haiti. Int. J. Environ. Res. Public Health 2013, 10, 5830–5843. [Google Scholar] [CrossRef] [PubMed]
- André, O.; Vollenweider, P.; Günthardt-Goerg, M.S. Foliage response to heavy metal contamination in Sycamore Maple (Acer pseudoplatanus L.). For. Snow Landsc. Res. 2006, 80, 275–288. [Google Scholar]
- Hermle, S.; Günthardt-Goerg, M.S.; Schulin, R. Effects of metal-contaminated soil on the performance of young trees growing in model ecosystems under field conditions. Environ. Pollut. 2006, 144, 703–714. [Google Scholar] [CrossRef]
- Martin, D.; Vollenweider, P.; Buttler, A.; Günthardt-Goerg, M.S. Bioindication of heavy metal contamination in vegetable gardens. For. Snow Landsc. Res. 2006, 80, 169–180. [Google Scholar]
- Cosio, C.; Vollenweider, P.; Keller, C. Localization and effects of cadmium in leaves of a cadmium-tolerant willow (Salix viminalis L.): I. Macrolocalization and phytotoxic effects of cadmium. Environ. Exp. Bot. 2006, 58, 64–74. [Google Scholar] [CrossRef][Green Version]
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef]
- Fahr, M.; Laplaze, L.; Bendaou, N.; Hocher, V.; El Mzibri, M.; Bogusz, D.; Smouni, A. Effect of lead on root growth. Front. Plant Sci. 2013, 4, 175:1–175:7. [Google Scholar] [CrossRef]
- Hasnaoui, S.E.L.; Fahr, M.; Keller, C.; Levard, C.; Angeletti, B.; Chaurand, P.; Triqui, Z.E.A.; Guedira, A.; Rhazi, L.; Colin, F.; et al. Screening of Native Plants Growing on a Pb/Zn Mining Area in Eastern Morocco: Perspectives for Phytoremediation. Plants 2020, 9, 1458. [Google Scholar] [CrossRef]
- Holeksa, J.; Błońska, A.; Kompała-Bąba, A.; Woźniak, G.; Kurek, P.; Szarek-Łukaszewska, G.; Grodzińska, K.; Żywiec, M. The vegetation of the Olkusz ore-bearing region. Nat. Hist. Values Olkusz Ore-Bear. Reg. 2015, 105–128. [Google Scholar]
- French, C.J.; Dickinson, N.M.; Putwain, P.D. Woody biomass phytoremediation of contaminated brownfield land. Environ. Pollut. 2006, 141, 387–395. [Google Scholar] [CrossRef]
- Robinson, B.H.; Bañuelos, G.; Conesa, H.M.; Evangelou, M.W.H.; Schulin, R. The Phytomanagement of Trace Elements in Soil. Crit. Rev. Plant Sci. 2009, 28, 240–266. [Google Scholar] [CrossRef]
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilization: A green approach to contaminant containment. Adv. Agron. 2011, 112, 145–204. [Google Scholar]
- Conesa, H.M.; Evangelou, M.W.H.; Robinson, B.; Schulin, R. A critical view of current state of phytotechnologies to remediarte soils: Still a promising tool? Sci. World J. 2012, 2012, 173829:1–17382:10. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, M.W.; Conesa, H.M.; Robinson, B.; Schulin, R. Biomass Production on Trace Element–Contaminated Land: A Review. Environ. Eng. Sci. 2012, 29, 823–839. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Conesa, H.M.; Robinson, B.H.; Schulin, R. Phytomanagement: Phytoremediation and the production of biomass for economic revenue on contaminated land. In Phytoremediation: Management of Environmental Contaminats; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 115–132. [Google Scholar]
- Cundy, A.; Bardos, R.; Puschenreiter, M.; Mench, M.; Bert, V.; Friesl-Hanl, W.; Müller, I.; Li, X.; Weyens, N.; Witters, N.; et al. Brownfields to green fields: Realising wider benefits from practical contaminant phytomanagement strategies. J. Environ. Manag. 2016, 184, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, R.; Pirzad, A. Hyperaccumulatio of Potential Toxic Micronutrients by Plants. In Plant Micronutrients; Aftab, T., Hakeen, K.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 345–365. [Google Scholar]
- Dal Corso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy Metal Pollutions: State of the Art and Innovation in Phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef]
- Mleczek, M.; Rutkowski, P.; Rissmann, I.; Kaczmark, Z.; Goliński, P.; Szetner, K.; Strażyńska, K.; Stachowiak, A. Biomass productivity and phytoremediation potential of Salix alba and Salix viminalis. Biomass Bioenergy 2010, 34, 1410–1418. [Google Scholar] [CrossRef]
- Baum, S.; Bolte, A.; Weih, M. High value of short rotation coppice plantations for phytodiversity in rural landscapes. GCB Bioenergy 2012, 4, 728–738. [Google Scholar] [CrossRef]
- Mleczek, M.; Rutkowski, P.; Rissmann, I.; Kaczmarek, Z.; Goliński, P.; Szentner, K.; Strażyńska, K.; Stachowiak, A. Phytoextraction of potentially toxic elements by six tree species growing on hazardous mining sludge. Environ. Sci. Pollut. Res. 2017, 24, 22183–22195. [Google Scholar] [CrossRef]
- Madejón, P.; Dominguez, M.T.; Madejón, E.; Cabrera, F.; Marañón, T.; Murillo, J.M. Soil-plant relationships and contamination by trace elements: A review of twenty years of experimentation and monitoring after the Aznalcóllar (SW Spain) mine accident. Sci. Total Environ. 2018, 625, 50–63. [Google Scholar] [CrossRef]
- Preston, M.D.; Brummell, M.E.; Smenderovac, E.; Rantala-Sykes, B.; Rumney, R.H.; Sherman, G.; Basiliko, N.; Beckett, P.; Hebert, M. Tree restoration and ecosystem carbon storage in an acid and metal impacted landscape: Chronosequence and resampling approaches. For. Ecol. Manag. 2020, 463, 118012. [Google Scholar] [CrossRef]
- Al-Lami, M.K.; Nguyen, D.; Oustriere, N.; Burken, J.G. High throughput screening of native species for tailings eco-restoration using novel computer visualization for plant phenotyping. Sci. Total Environ. 2021, 780, 146490. [Google Scholar] [CrossRef] [PubMed]
- Mayerová, M.; Petrová, Š; Madaras, M.; Lipavský, J.; Šimon, T.; Vaněk, T. Non-enhanced phytoextraction of cadmium, zinc, and lead by high-yielding crops. Environ. Sci. Pollut. Res. 2017, 24, 14706–14716. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Ni, J.C.; Zhou, Q.X. Uptake of Heavy Metals by Trees: Prospects for Phytoremediation. Mater. Sci. Forum 2013, 743–744, 768–781. [Google Scholar] [CrossRef]
- Günthardt-Goerg, M.S.; Vollenweider, P.; Hermle, S.; Schulin, R. Growth and metal accumulation of young forest trees and understorey plants on contaminated topsoil: Influence of subsoil and time. Plant Soil 2019, 437, 375–395. [Google Scholar] [CrossRef]
- Li, B.; Chen, D.; Yang, Y.; Li, X. Effects of soil properties on accumulation characteristics of copper, manganese, zinc, and cadmium in Chinese turnip. Plant Divers. 2019, 41, 340–346. [Google Scholar] [CrossRef]
- Van Slycken, S.; Witters, N.; Meiresonne, L.; Meers, E.; Ruttens, A.; Van Peteghem, P.; Weyens, N.; Tack, F.M.G.; Vangronsveld, J. Field Evaluation of Willow Under Short Rotation Coppice for Phytomanagement of Metal-Polluted Agricultural Soils. Int. J. Phytoremediation 2013, 15, 677–689. [Google Scholar] [CrossRef]
- Perlein, A.; Bert, V.; Desannaux, O.; de Souza, M.F.; Papin, A.; Gaucher, R.; Zdanevitch, I.; Meers, E. The Use of Sorghum in a Phytoattenuation Strategy: A Field Experiment on a TE-Contaminated Site. Appl. Sci. 2021, 11, 3471. [Google Scholar] [CrossRef]
- Hammer, D.; Kayser, A.; Keller, C. Phytoextraction of Cd and Zn with Salix viminalis in field trials. Soil Use Manag. 2003, 19, 187–192. [Google Scholar] [CrossRef]
- Kacálková, L.; Tlustoš, P.; Száková, J. Phytoextraction of risk ements by willow and poplar trees. Int. J. Phytoremediat. 2015, 17, 414–421. [Google Scholar] [CrossRef]
- Çomaklı, E.; Bingöl, M.S. Heavy metal accumulation of urban Scots pine (Pinus sylvestris L.) plantation. Environ. Monit. Assess. 2021, 193, 192. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, L.; You, C.; Tan, B.; Yang, W. Dynamics of heavy metal uptake and soil heavy metal stocks across a series of Masson pine plantations. J. Clean. Prod. 2020, 269, 122395. [Google Scholar] [CrossRef]
- Suo, Y.; Tang, N.; Li, H.; Corti, G.; Jiang, L.; Huang, Z.; Zhang, Z.; Huang, J.; Wu, Z.; Feng, C.; et al. Long-term effects of phytoextraction by a poplar clone on the concentration, fractionation, and transportation of heavy metals in mine tailings. Environ. Sci. Pollut. Res. 2021, 28, 47528–47539. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.; Van Nevel, L.; De Schrijver, A.; Piesschaert, F.; Oosterbaan, A.; Tack, F.M.; Verheyen, K. Tree species effect on the redistribution of soil metals. Environ. Pollut. 2007, 149, 173–181. [Google Scholar] [CrossRef]
- Keller, C.; Hammer, D.; Kayser, A.; Richner, W.; Brodbeck, M.; Sennhauser, M. Root development and heavy metal phytoextraction efficiency: Comparison of different plant species in the field. Plant Soil 2003, 249, 67–81. [Google Scholar] [CrossRef]
- Jensen, J.K.; Holm, P.; Nejrup, J.; Larsen, M.B.; Borggaard, O.K. The potential of willow for remediation of heavy metal polluted calcareous urban soils. Environ. Pollut. 2009, 157, 931–937. [Google Scholar] [CrossRef]
- Menon, M.; Hermle, S.; Abbaspour, K.C.; Günthardt-Goerg, M.S.; Oswald, S.E.; Schulin, R. Water regime of metal-contaminated soil under juvenile forest vegetation. Plant Soil 2005, 271, 227–241. [Google Scholar] [CrossRef][Green Version]
- Pehoiu, G.; Murarescu, O.; Radulescu, C.; Dulama, I.D.; Teodorescu, S.; Stirbescu, R.M.; Bucurica, I.A.; Stanescu, S.G. Heavy metals accumulation and translocation in native plants grown on tailing dumps and human health risk. Plant Soil 2020, 456, 405–424. [Google Scholar] [CrossRef]
- Brunetti, G.; Soler-Rovira, P.; Farrag, K.; Senesi, N. Tolerance and accumulation of heavy metals by wild plant species grown in contaminated soils in Apulia region, Southern Italy. Plant Soil 2008, 318, 285–298. [Google Scholar] [CrossRef]
- De la Fuente, C.; Pardo, T.; Alburquerque, J.; Martínez-Alcalá, I.; Bernal, M.P.; Clemente, R. Assessment of native shrubs for stabilisation of a trace elements-polluted soil as the final phase of a restoration process. Agric. Ecosyst. Environ. 2014, 196, 103–111. [Google Scholar] [CrossRef]
- Bidar, G.; Pelfrêne, A.; Schwartz, C.; Waterlot, C.; Sahmer, K.; Marot, F.; Douay, F. Urban kitchen gardens: Effect of the soil contamination and parameters on the trace element accumulation in vegetables—A Review. Sci. Total Environ. 2020, 738, 139569. [Google Scholar] [CrossRef]
- Čudić, V.; Stojiljković, D.; Jovović, A. Phytoremediation potential of wild plants growing on soil contaminated with heavy metals. Arch. Ind. Hyg. Toxicol. 2016, 67, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Bidar, G.; Pruvot, C.; Garçon, G.; Verdin, A.; Shirali, P.; Douay, F. Seasonal and annual variations of metal uptake, bioaccumulation, and toxicity in Trifolium repens and Lolium perenne growing in a heavy metal-contaminated field. Environ. Sci. Pollut. Res. 2008, 16, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Bech, J.; Duran, P.; Roca, N.; Poma, W.; Sánchez, I.; Barceló, J.; Boluda, R.; Roca-Pérez, L.; Poschenrieder, C. Shoot accumulation of several trace elements in native plant species from contaminated soils in the Peruvian Andes. J. Geochem. Explor. 2012, 113, 106–111. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Migeon, A.; Richaud, P.; Guinet, F.; Chalot, M.; Blaudez, D. Metal Accumulation by Woody Species on Contaminated Sites in the North of France. Water Air Soil Pollut. 2009, 204, 89–101. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation of Metal-Contaminated Soil in Temperate Humid Regions of British Columbia, Canada. Int. J. Phytoremediation 2009, 11, 575–590. [Google Scholar] [CrossRef]
- Lorenc-Plucińska, G.; Walentynowicz, M.; Niewiadomska, A. Capabilities of alders (Alnus incana and A. glutinosa) to grow in metal-contaminated soil. Ecol. Eng. 2013, 58, 214–227. [Google Scholar] [CrossRef]
- Ciadamidaro, L.; Madejón, P.; Puschenreiter, M. Growth of Populus alba and its influence on soil trace element availability. Sci. Total Environ. 2013, 454–455, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-K.; Liu, Z.-Y.; Wang, H. Use of Single Extraction Methods to Predict Bioavailability of Heavy Metals in Polluted Soils to Rice. Commun. Soil Sci. Plant Anal. 2010, 41, 820–831. [Google Scholar] [CrossRef]
- Anjos, C.; Magalhães, C.; Abreu, M.M. Metal (Al, Mn, Pb and Zn) soils extractable reagents for available fraction assessment: Comparison using plants, and dry and moist soils from the Braçal abandoned lead mine area, Portugal. J. Geochem. Explor. 2012, 113, 45–55. [Google Scholar] [CrossRef]
- Galhardi, J.A.; Leles, B.P.; de Mello, J.W.; Wilkinson, K. Bioavailability of trace metals and rare earth elements (REE) from the tropical soils of a coal mining area. Sci. Total Environ. 2019, 717, 134484. [Google Scholar] [CrossRef] [PubMed]
- Ciadamidaro, L.; Madejón, E.; Robinson, B.; Madejón, P. Soil plant interactions of Populus alba in contrasting environments. J. Environ. Manag. 2014, 132, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Unterbrunner, R.; Puschenreiter, M.; Sommer, P.; Wieshammer, G.; Tlustoš, P.; Zupan, M.; Wenzel, W. Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environ. Pollut. 2007, 148, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, M.W.; Deram, A.; Gogos, A.; Studer, B.; Schulin, R. Assessment of suitability of tree species for the production of biomass on trace element contaminated soils. J. Hazard. Mater. 2012, 209–210, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Swiss Federal Council. Ordinance on Pollution of the Soil. Available online: https://www.fedlex.admin.ch/eli/cc/1998/2261_2261_2261/en (accessed on 22 December 2021).
- Ruttens, A.; Boulet, J.; Weyens, N.; Smeets, K.; Adriaensen, K.; Meers, E.; Van Slycken, S.; Tack, F.M.G.; Meiresonne, L.; Thewys, T.; et al. Short Rotation Coppice Culture of Willows and Poplars as Energy Crops on Metal Contaminated Agricultural Soils. Int. J. Phytoremediat. 2011, 13, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Witters, N.; Mendelsohn, R.; Van Slycken, S.; Weyens, N.; Schreurs, E.; Meers, E.; Tack, F.M.G.; Carleer, R.; Vangronsveld, J. Phytoremediation, a sustainable remediation technology? Conclusions from a case study. I: Energy production and carbon dioxide abatement. Biomass Bioenergy 2012, 39, 454–469. [Google Scholar] [CrossRef]
- Desai, M.; Haigh, M.; Walkington, H. Phytzoremediation: Metal decontamination of soils after the sequential forestation of former opencast coal land. Sci. Total Environ. 2019, 656, 670–680. [Google Scholar] [CrossRef]
- Kouhi, S.M.M.; Moudi, M. Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted saline-sodic soil. Environ. Sci. Pollut. Res. 2020, 27, 10027–10038. [Google Scholar] [CrossRef]
- Nirola, R.; Megharaj, M.; Beecham, S.; Aryal, R.; Thavamani, P.; Vankateswarlu, K.; Saint, C. Remediation of metalliferous mines, revegetation challenges and emerging prospects in semi-arid and arid conditions. Environ. Sci. Pollut. Res. 2016, 23, 20131–20150. [Google Scholar] [CrossRef]
- Grodzińska, K.; Szarek-Lukaszewska, G. Heavy metal vegetation in the Olkusz region (southern Poland)—Preliminary Studies. Pol. Bot. J. 2009, 1, 105–112. [Google Scholar]
- Blennow, K.; Persson, E.; Lindner, M.; Faias, S.P.; Hanewinkel, M. Forest owner motivations and attitudes towards supplying biomass for energy in Europe. Biomass Bioenergy 2014, 67, 223–230. [Google Scholar] [CrossRef]
- De Besi, M.; McCormick, K. Towards a Bioeconomy in Europe: National, Regional and Industrial Strategies. Sustainability 2015, 7, 10461–10478. [Google Scholar] [CrossRef]

| Zn | Cu | Pb | Cd | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HM | CO | HM | CO | HM | CO | HM | CO | |||||||||
| Acid | Calc | Acid | Calc | Acid | Calc | Acid | Calc | Acid | Calc | Acid | Calc | Acid | Calc | Acid | Calc | |
| u R | 1544 ± 61.2 ds | 1432 ± 53.5 ds | 109.7 ± 14.4 *ds | 103.0 ± 13.9 *ds | 579.4 ± 56.5 ds | 505.3 ± 23.8 ds | 31.9 ± 10.5 * | 30.7 ± 7.7 * | 10.9 ± 0.7 ds | 10.4 ± 0.7 d | 2.0 ± 0.09 *s | 2.1 ± 0.16 * | 4.8 ± 0.19 d | 4.6 ± 0.26 d | 0.4 ± 0.03 *d | 0.4 ± 0.03 *d |
| u W | 147.2 ± 9.5 ds | 132.5 ± 13.2 ds | 65.6 ± 6.6 *ds | 76.5 ± 12.8 *s | 16.8 ± 0.6 ds | 22.6 ± 3.2 +ds | 14.6 ± 0.4 *ds | 13.4 ± 0.6 *ds | nd | nd | nd | nd | nd | nd | nd | nd |
| u L | 253.2 ± 13.5 Wds | 187.1 ± 9.4 +Wd | 76.2 ± 3.2 *d | 74.3 ± 1.9 *ds | 16.5 ± 1.0 ds | 17.5 ± 1.4 ds | 14.5 ± 1.8 s | 12.8 ± 0.7 *ds | 3.7 ± 0.7 | 3.0 ± 0.6 | nd | nd | 1.2 ± 0.06 d | 0.7 ± 0.06 +d | 0.5 ± 0.03 *d | 0.3 ± 0.02 *+d |
| d R | 876.2 ± 59.4 us | 598.4 ± 40.2 +us | 158.8 ± 5.0 *u | 141.6 ± 3.6 *+u | 181.9 ± 11.9 us | 131.7 ± 11.9 +us | 16.7 ± 0.4 | 16.5 ± 0.7 * | 7.7 ± 0.4 us | 6.0 ± 0.3 +us | 2.5 ± 0.15 * | 2.3 ± 0.16 * | 3.3 ± 0.16 u | 1.9 ± 0.01 +us | 1.0 ± 0.04 *us | 0.7 ± 0.02 *+us |
| d W | 257.4 ± 5.0 us | 171.6 ± 5.9 +us | 127.5 ± 5.1 *u | 103.4 ± 4.1 *+ | 7.1 ± 0.2 us | 6.1 ± 0.1 +us | 6.4 ± 0.2 *us | 6.0 ± 0.2 us | nd | nd | nd | nd | 1.5 ± 0.04 | 0.8 ± 0.06 + | 0.8 ± 0.06 * | 0.5 ± 0.02 *+ |
| d L | 1270 ± 44.8 Wus | 905.7 ± 29.5 +Wus | 560 ± 13.6 *Wus | 424.5 ± 11.1 *+Wus | 11.2 ± 02 Wus | 10.6 ± 0.4 Wus | 10.8 ± 0.2 Ws | 10.2 ± 0.2 Wus | nd | nd | nd | nd | 3.8 ± 0.17 Wu | 2.0 ± 0.10 Wu | 2.1 ± 0.10 Wu | 1.2 ± 0.07 Wu |
| s R | 1731 ± 88.4 du | 1874 ± 96.7 du | 164.1 ± 4.3 *u | 157.7 ± 5.7 *u | 365.5 ± 24.0 du | 322.7 ± 32.4 du | 19.7 ± 0.7 * | 18.4 ± 0.8 * | 14.6 ± 1.0 du | 12.4 ± 1.0 d | 2.9 ± 0.33 *u | 2.3 ± 0.26 * | 5.0 ± 0.25 d | 4.6 ± 0.27 d | 0.5 ± 0.03 *d | 0.5 ± 0.06 *d |
| s W | 178.5 ± 9.2 du | 180.7 ± 9.5 du | 127.7 ± 4.7 *u | 122.3 ± 10.4 *u | 9.6 ± 0.5 du | 11.1 ± 0.8 du | 9.0 ± 0.4 du | 9.3 ± 0.5 du | nd | nd | nd | nd | nd | nd | nd | nd |
| s L | 185.8 ± 8.3 du | 191.3 ± 5.9 d | 101.6 ± 3.9 *Wd | 108.9 ± 2.9 *du | 3.60 ± 0.3 Wdu | 4.5 ± 0.2 Wdu | 3.5 ± 0.3 Wdu | 4.3 ± 0.1 Wdu | nd | nd | nd | nd | nd | nd | nd | nd |
| Zn | Cu | Pb | Cd | Biomass | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group/ Organ | HM | Subsoil | HM | Subsoil | HM | Subsoil | HM | Subsoil | HM | Subsoil |
| u | <0.0001 | 0.0083 | <0.0001 | 0.0011 | <0.0001 | ns | <0.0001 | 0.0002 | ns | <0.0001 |
| d R | <0.0001 | 0.0399 HM: soil 0.0189 | <0.0001 | ns | <0.0001 | ns | <0.0001 | HM: soil 0.0309 | 0.0011 | <0.0001 |
| d W | <0.0001 | <0.0001 | ns | <0.0001 | nd | <0.0001 | HM: soil 0.0024 | <0.0001 | <0.0001 | |
| d L | <0.0001 | 0.0030 HM: soil 0.0068 | 0.0046 | 0.0003 | nd | <0.0001 | 0.0001 | <0.0001 | ||
| s R | <0.0001 | 0.0042 | <0.0001 | ns | <0.0001 | ns | <0.0001 | 0.002 | ns | <0.0001 HM: soil 0.0289 |
| s W | <0.0001 | 0.0077 | ns | 0.0088 | nd | nd | ns | <0.0001 HM: soil 0.0053 | ||
| s L | <0.0001 | 0.0035 | ns | <0.0001 HM: soil 0.0357 | nd | nd | 0.0072 | <0.0001 HM: soil 0.0004 | ||
| total | <0.0001 | <0.0001 HM: soil 0.028 | <0.0001 | 0.0018 | <0.0001 | ns | <0.0001 | 0.0022 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günthardt-Goerg, M.S.; Vollenweider, P.; Schulin, R. Metal Accumulation and Biomass Production in Young Afforestations Established on Soil Contaminated by Heavy Metals. Plants 2022, 11, 523. https://doi.org/10.3390/plants11040523
Günthardt-Goerg MS, Vollenweider P, Schulin R. Metal Accumulation and Biomass Production in Young Afforestations Established on Soil Contaminated by Heavy Metals. Plants. 2022; 11(4):523. https://doi.org/10.3390/plants11040523
Chicago/Turabian StyleGünthardt-Goerg, Madeleine Silvia, Pierre Vollenweider, and Rainer Schulin. 2022. "Metal Accumulation and Biomass Production in Young Afforestations Established on Soil Contaminated by Heavy Metals" Plants 11, no. 4: 523. https://doi.org/10.3390/plants11040523
APA StyleGünthardt-Goerg, M. S., Vollenweider, P., & Schulin, R. (2022). Metal Accumulation and Biomass Production in Young Afforestations Established on Soil Contaminated by Heavy Metals. Plants, 11(4), 523. https://doi.org/10.3390/plants11040523







