Growth Parameters of Various Green Microalgae Species in Effluent from Biogas Reactors: The Importance of Effluent Concentration
Abstract
:1. Introduction
2. Results and Discussion
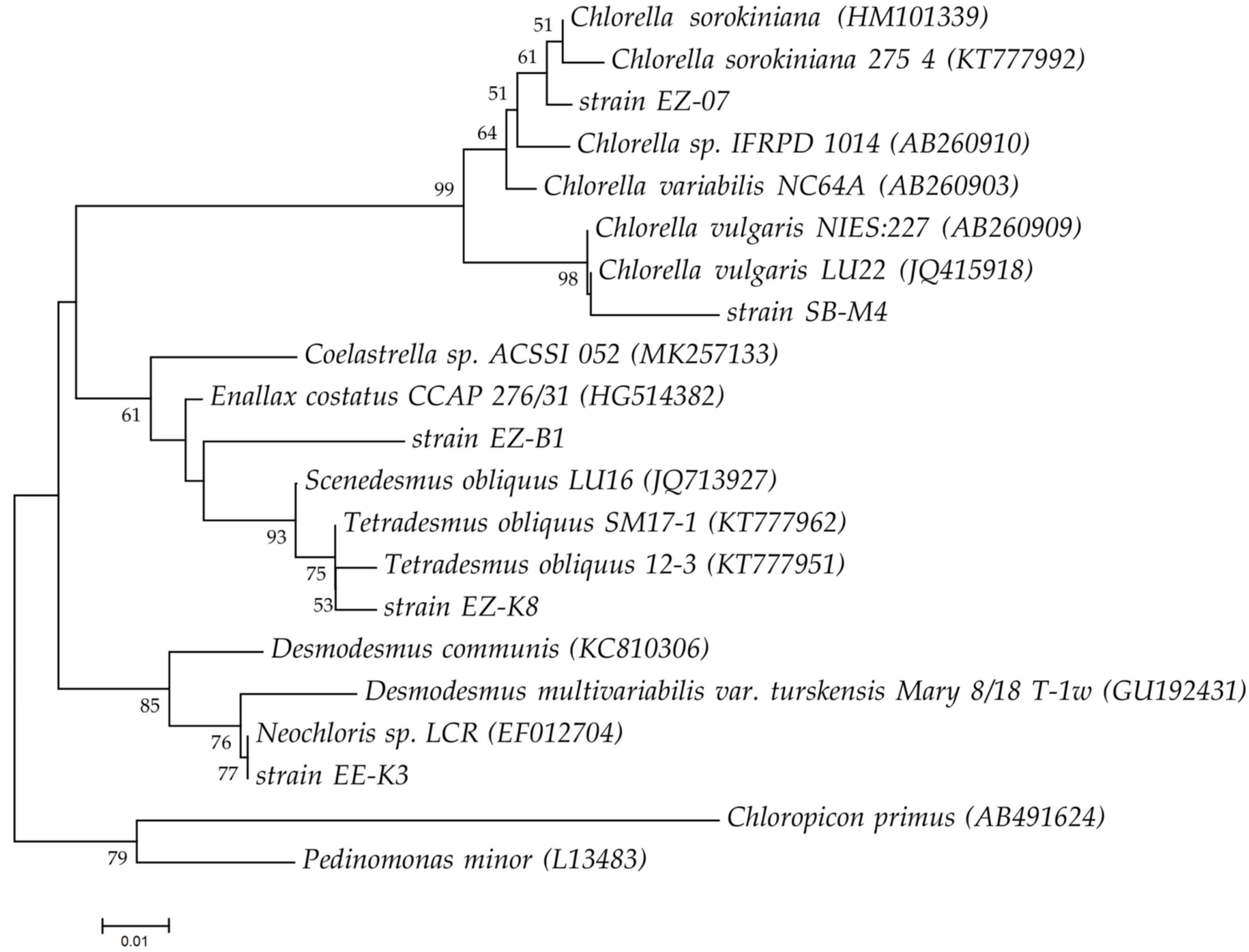
2.1. Tested Microalgal Species
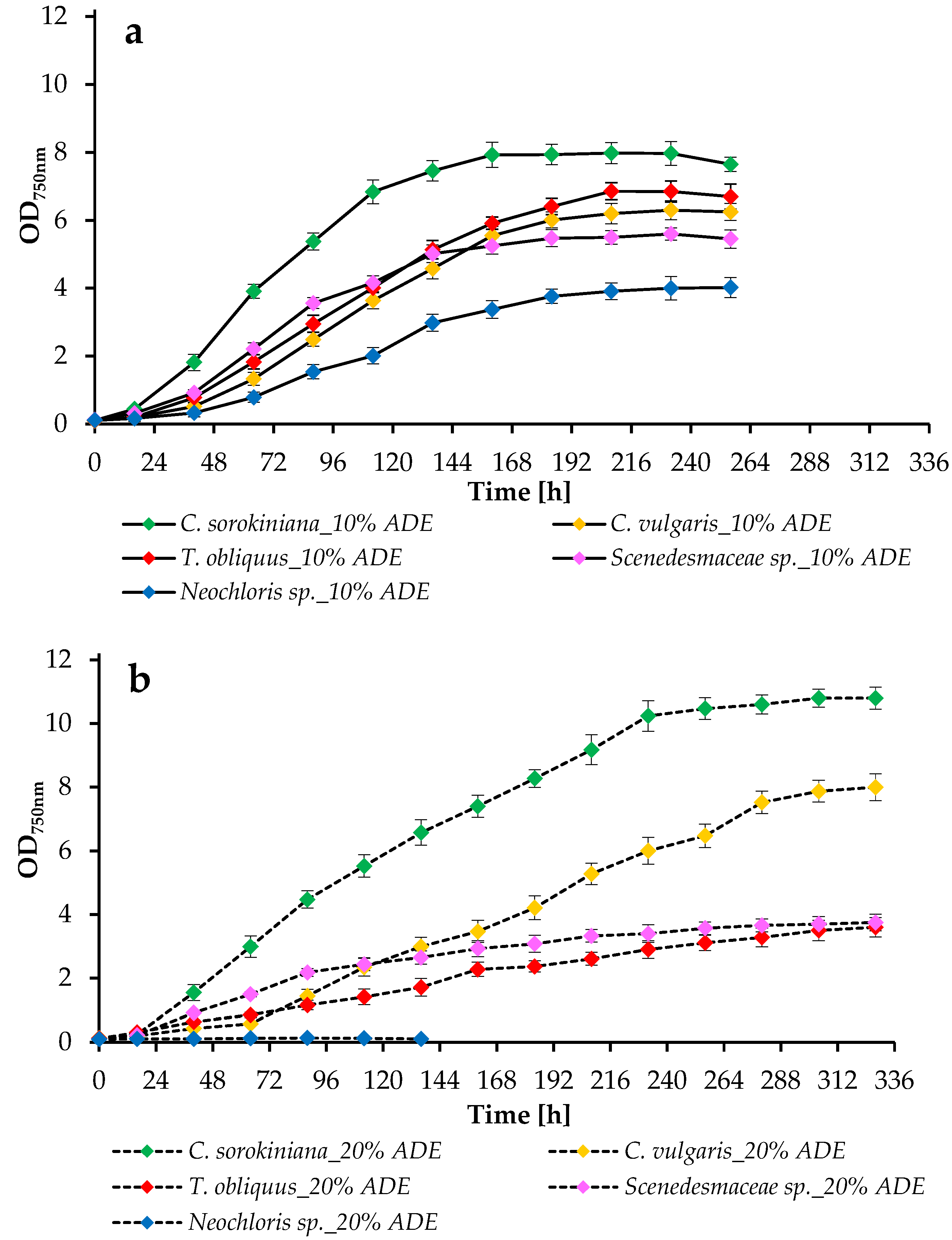
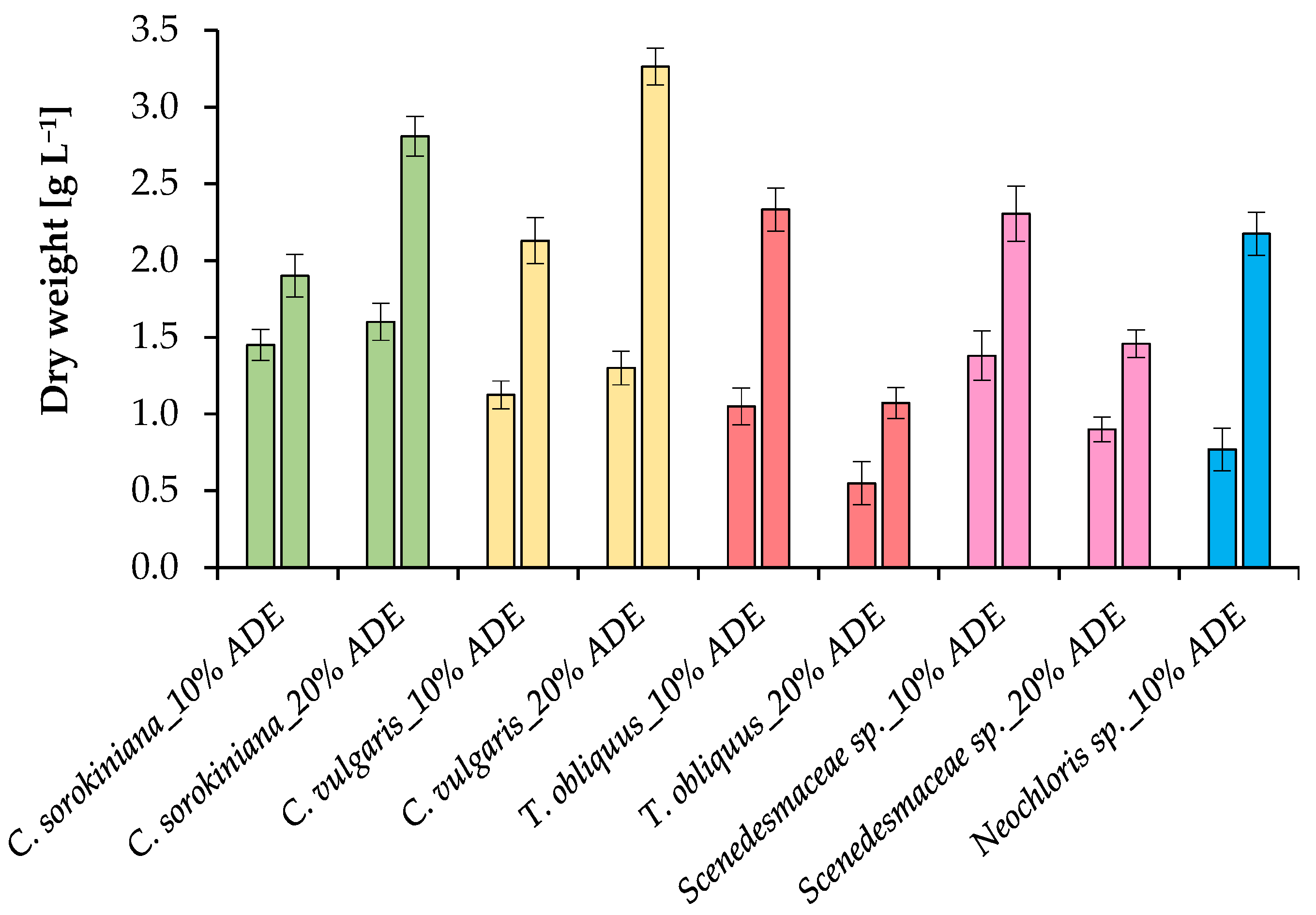
2.2. Growth Parameters of Microalgae in ADE
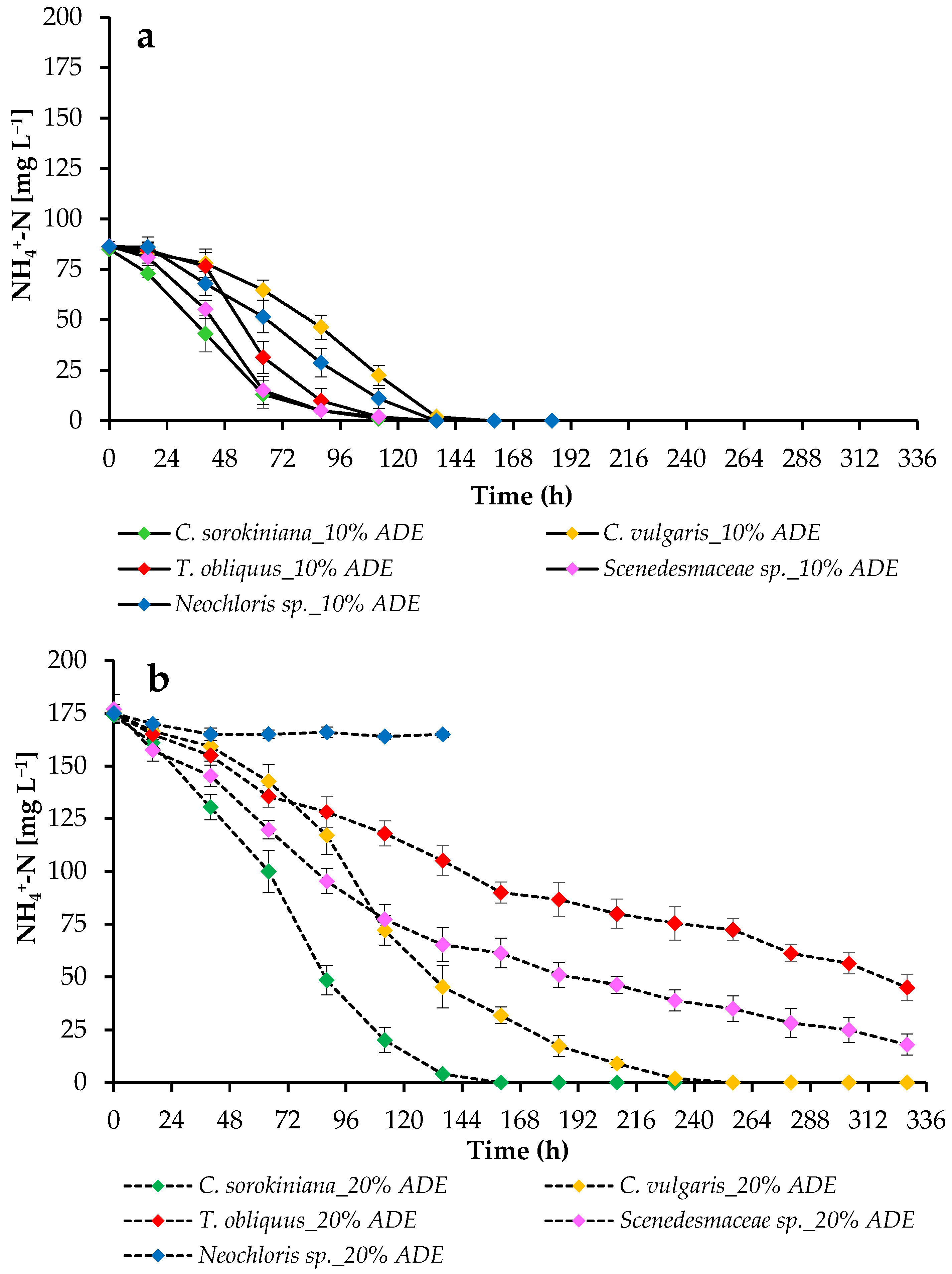
2.3. Ammonium Nitrogen Uptake by Strains and pH Change during Cultivation at Different Effluent Loads
2.4. Comparison of Protein Contents in Algal Cells
3. Materials and Methods
3.1. Microalgal Strains: Isolation and Identification
3.2. Pre-Culture Conditions
3.3. Experimental Organization: Effluent-Based Media Preparation
3.4. Cultivation in the Photobioreactor
3.5. Growth Measurements and Analytical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Tolboom, S.N.; Carrillo-Nieves, D.; de Jesús Rostro-Alanis, M.; de la Cruz Quiroz, R.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldivar, R. Algal-based removal strategies for hazardous contaminants from the environment—A review. Sci. Total Environ. 2019, 665, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Chen, P.; Addy, M.; Zhang, R.; Deng, X.; Ma, Y.; Cheng, Y.; Hussain, F.; Chen, C.; Liu, Y.; et al. Carbon-dependent alleviation of ammonia toxicity for algae cultivation and associated mechanisms exploration. Bioresour. Technol. 2018, 249, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Yoshida, A.; Akizuki, S.; Kishi, M.; Toda, T. Microalgae cultivation using undiluted anaerobic digestate by introducing aerobic nitrification–desulfurization treatment. Water Sci. Technol. 2020, 82, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Akizuki, S.; Kishi, M.; Cuevas-Rodríguez, G.; Toda, T. Effects of different light conditions on ammonium removal in a consortium of microalgae and partial nitrifying granules. Water Res. 2020, 171, 115445. [Google Scholar] [CrossRef]
- Fernandes, F.; Silkina, A.; Gayo-Peláez, J.I.; Kapoore, R.V.; de la Broise, D.; Llewellyn, C.A. Microalgae cultivation on nutrient rich digestate: The importance of strain and digestate tailoring under pH control. Appl. Sci. 2022, 12, 5429. [Google Scholar] [CrossRef]
- Khatiwada, J.R.; Guo, H.; Shrestha, S.; Chio, C.; Chen, X.; Mokale Kognou, A.L.; Qin, W. Cultivation of microalgae in unsterile malting effluent for biomass production and lipid productivity improvement. Fermentation 2022, 8, 186. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, L.; Qi, Y. Enhancing the productivity of microalgae cultivated in wastewater toward biofuel production: A critical review. Appl. Energy 2015, 137, 282–291. [Google Scholar] [CrossRef]
- Pei, H.; Jiang, L.; Hou, Q.; Yu, Z. Toward facilitating microalgae cope with effluent from anaerobic digestion of kitchen waste: The art of agricultural phytohormones. Biotechnol. Biofuels 2017, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for biogas upgrading to biomethane: A review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Ziganshin, A.M. Anaerobic digestion of chicken manure in the presence of magnetite, granular activated carbon, and biochar: Operation of anaerobic reactors and microbial community structure. Microorganisms 2022, 10, 1422. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Impact of granular activated carbon on anaerobic process and microbial community structure during mesophilic and thermophilic anaerobic digestion of chicken manure. Sustainability 2022, 14, 447. [Google Scholar] [CrossRef]
- Bartoli, A.; Hamelin, L.; Rozakis, S.; Borzęcka, M.; Brandão, M. Coupling economic and GHG emission accounting models to evaluate the sustainability of biogas policies. Renew. Sustain. Energy Rev. 2019, 106, 133–148. [Google Scholar] [CrossRef]
- Tambone, F.; Orzi, V.; D’Imporzano, G.; Adani, F. Solid and liquid fractionation of digestate: Mass balance, chemical characterization, and agronomic and environmental value. Bioresour. Technol. 2017, 243, 1251–1256. [Google Scholar] [CrossRef]
- Zhu, L.; Yan, C.; Li, Z. Microalgal cultivation with biogas slurry for biofuel production. Bioresour. Technol. 2016, 220, 629–636. [Google Scholar] [CrossRef]
- Silkina, A.; Ginnever, N.E.; Fernandes, F.; Fuentes-Grünewald, C. Large-scale waste bio-remediation using microalgae cultivation as a platform. Energies 2019, 12, 2772. [Google Scholar] [CrossRef] [Green Version]
- Bauer, L.; Ranglová, K.; Masojídek, J.; Drosg, B.; Meixner, K. Digestate as sustainable nutrient source for microalgae—Challenges and prospects. Appl. Sci. 2021, 11, 1056. [Google Scholar] [CrossRef]
- Zuliani, L.; Frison, N.; Jelic, A.; Fatone, F.; Bolzonella, D.; Ballottari, M. Microalgae cultivation on anaerobic digestate of municipal wastewater, sewage sludge and agro-waste. Int. J. Mol. Sci. 2016, 17, 1692. [Google Scholar] [CrossRef] [Green Version]
- Pulgarin, A.; Kapeller, A.G.; Tarik, M.; Egloff, S.; Mariotto, M.; Ludwig, C.; Refardt, D. Cultivation of microalgae at high-density with pretreated liquid digestate as a nitrogen source: Fate of nitrogen and improvements on growth limitations. J. Clean. Prod. 2021, 324, 129238. [Google Scholar] [CrossRef]
- Kisielewska, M.; Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Quattrocelli, P.; Bordiean, A. Effects of liquid digestate treatment on sustainable microalgae biomass production. Bioenergy Res. 2021, 15, 357–370. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Assessment of Chlorella sorokiniana growth in anaerobic digester effluent. Plants 2021, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Growth characteristics of Chlorella sorokiniana in a photobioreactor during the utilization of different forms of nitrogen at various temperatures. Plants 2022, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, H.W.; Bold, H.C. Trichosarcina polymorpha Gen. et Sp. Nov. J. Phycol. 1965, 1, 34–38. [Google Scholar] [CrossRef]
- Schagerl, M.; Siedler, R.; Konopáčová, E.; Ali, S.S. Estimating biomass and vitality of microalgae for monitoring cultures: A roadmap for reliable measurements. Cells 2022, 11, 2455. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kuo, E.W.; Nagarajan, D.; Ho, S.H.; Dong, C.D.; Lee, D.J.; Chang, J.S. Cultivating Chlorella sorokiniana AK-1 with swine wastewater for simultaneous wastewater treatment and algal biomass production. Bioresour. Technol. 2020, 302, 122814. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Liu, K.; Nasr, L.K.; Byers, N.; Rosenberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Bouwer, E.J. Bioprospecting of microalgae for integrated biomass production and phytoremediation of unsterilized wastewater and anaerobic digestion centrate. Appl. Microbiol. Biotechnol. 2015, 99, 6139–6154. [Google Scholar] [CrossRef]
- Kobayashi, N.; Noel, E.A.; Barnes, A.; Watson, A.; Rosenberg, J.N.; Erickson, G.; Oyler, G.A. Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour. Technol. 2013, 150, 377–386. [Google Scholar] [CrossRef]
- Franchino, M.; Comino, E.; Bona, F.; Riggio, V.A. Growth of three microalgae strains and nutrient removal from an agro-zootechnical digestate. Chemosphere 2013, 92, 738–744. [Google Scholar] [CrossRef]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, X.; Zou, G.; Zhou, T.; Liu, Y.; Ruan, R. Cultivation of Chlorella vulgaris in manure-free piggery wastewater with high-strength ammonium for nutrients removal and biomass production: Effect of ammonium concentration, carbon/nitrogen ratio and pH. Bioresour. Technol. 2019, 273, 203–211. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, Y.; Liu, T.; Yuan, M.; Liu, G.; Fang, J.; Yang, B. Exploration of microalgal species for nutrient removal from anaerobically digested swine wastewater and potential lipids production. Microorganisms 2021, 9, 2469. [Google Scholar] [CrossRef]
- Wang, Y.; Tibbetts, S.; McGinn, P. Microalgae as sources of high-quality protein for human food and protein supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef]
- Sydney, E.B.; da Silva, T.E.; Tokarski, A.; Novak, A.C.; de Carvalho, J.C.; Woiciecohwski, A.L.; Larroche, C.; Soccol, C.R. Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl. Energy 2011, 88, 3291–3294. [Google Scholar] [CrossRef]
- Apandi, N.; Mohamed, R.; Latiffi, N.; Rozlan, N.; Al-Gheethi, A.A.S. Protein and lipid content of microalgae Scenedesmus sp. biomass grown in wet market wastewater. MATEC Web Conf. 2017, 103, 06011. [Google Scholar] [CrossRef] [Green Version]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Comparison of the photoautotrophic growth regimes of Chlorella sorokiniana AM-02 in a photobioreactor for enhanced biomass productivity. Biology 2020, 9, 169. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Influence of granular activated carbon on anaerobic co-digestion of sugar beet pulp and distillers grains with solubles. Processes 2020, 8, 1226. [Google Scholar] [CrossRef]
- Lizzul, A.M.; Lekuona-Amundarain, A.; Purton, S.; Campos, L.C. Characterization of Chlorella sorokiniana, UTEX 1230. Biology 2018, 7, 25. [Google Scholar] [CrossRef]



| Parameter | Total Solids, % | Volatile Solids, % | Volatile Organic Acids, g L−1 | NH4+, g L−1 | PO43– mg L−1 | SO42– mg L−1 | pH |
|---|---|---|---|---|---|---|---|
| Value | 5.5 ± 0.14 | 3.7 ± 0.11 | 0.37 ± 0.05 | 1.12 ± 0.07 | 74.1 ± 4.1 | 5.3 ± 1.1 | 7.97 ± 0.04 |
| Strain | Treatment | Final Dry Weight (g L−1) | Biomass Productivity (g L−1 Day−1) | Volatile Solids (g L−1) | Final Cell Concentration (×106 Cells mL−1) | Maximum Carotenoids (mg L−1) |
|---|---|---|---|---|---|---|
| C. sorokiniana EZ-07 | 10% ADE | 1.90 ± 0.14 c,d | 0.28 ± 0.02 a,b | 1.81 ± 0.08 c,d | 270.5 ± 18.2 b | 7.3 ± 0.5 c |
| 20% ADE | 2.81 ± 0.10 a,b | 0.30 ± 0.02 a,b | 2.62 ± 0.12 a,b | 380.1 ± 24.2 a | 12.0 ± 0.3 b | |
| C. vulgaris SB-M4 | 10% ADE | 2.13 ± 0.13 b,c,d | 0.27 ± 0.02 b | 2.05 ± 0.10 b,c | 91.3 ± 10.3 c,d | 8.0 ± 0.9 c |
| 20% ADE | 3.26 ± 0.18 a | 0.26 ± 0.01 b | 2.92 ± 0.14 a | 132 ± 8.4 c | 18.5 ± 1.4 a | |
| T. obliquus EZ-K8 | 10% ADE | 2.33 ± 0.15 b,c | 0.27 ± 0.02 b | 2.20 ± 0.12 b,c | 109.1 ± 9.4 c,d | 6.6 ± 0.3 c |
| 20% ADE | 1.07 ± 0.09 e | 0.08 ± 0.01 d | 0.99 ± 0.05 e | 72.1 ± 5.3 c,d | 7.3 ± 0.7 c | |
| Scenedesmaceae sp. EZ-B1 | 10% ADE | 2.31 ± 0.12 b,c | 0.35 ± 0.02 a | 2.21 ± 0.08 b,c | 67.3 ± 7.3 c,d | 7.9 ± 1.0 c |
| 20% ADE | 1.46 ± 0.14 d,e | 0.15 ± 0.01 c | 1.37 ± 0.07 d,e | 50.1 ± 4.2 d | 8.3 ± 0.8 c | |
| Neochloris sp. EE-K3 | 10% ADE | 2.17 ± 0.14 b,c | 0.24 ± 0.02 b | 2.09 ± 0.12 b,c | 64.5 ± 8.2 d | 6.8 ± 0.4 c |
| 20% ADE | ND | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziganshina, E.E.; Bulynina, S.S.; Yureva, K.A.; Ziganshin, A.M. Growth Parameters of Various Green Microalgae Species in Effluent from Biogas Reactors: The Importance of Effluent Concentration. Plants 2022, 11, 3583. https://doi.org/10.3390/plants11243583
Ziganshina EE, Bulynina SS, Yureva KA, Ziganshin AM. Growth Parameters of Various Green Microalgae Species in Effluent from Biogas Reactors: The Importance of Effluent Concentration. Plants. 2022; 11(24):3583. https://doi.org/10.3390/plants11243583
Chicago/Turabian StyleZiganshina, Elvira E., Svetlana S. Bulynina, Ksenia A. Yureva, and Ayrat M. Ziganshin. 2022. "Growth Parameters of Various Green Microalgae Species in Effluent from Biogas Reactors: The Importance of Effluent Concentration" Plants 11, no. 24: 3583. https://doi.org/10.3390/plants11243583
APA StyleZiganshina, E. E., Bulynina, S. S., Yureva, K. A., & Ziganshin, A. M. (2022). Growth Parameters of Various Green Microalgae Species in Effluent from Biogas Reactors: The Importance of Effluent Concentration. Plants, 11(24), 3583. https://doi.org/10.3390/plants11243583






