Guettarda crispiflora Vahl Methanol Extract Ameliorates Acute Lung Injury and Gastritis by Suppressing Src Phosphorylation
Abstract
1. Introduction
2. Results
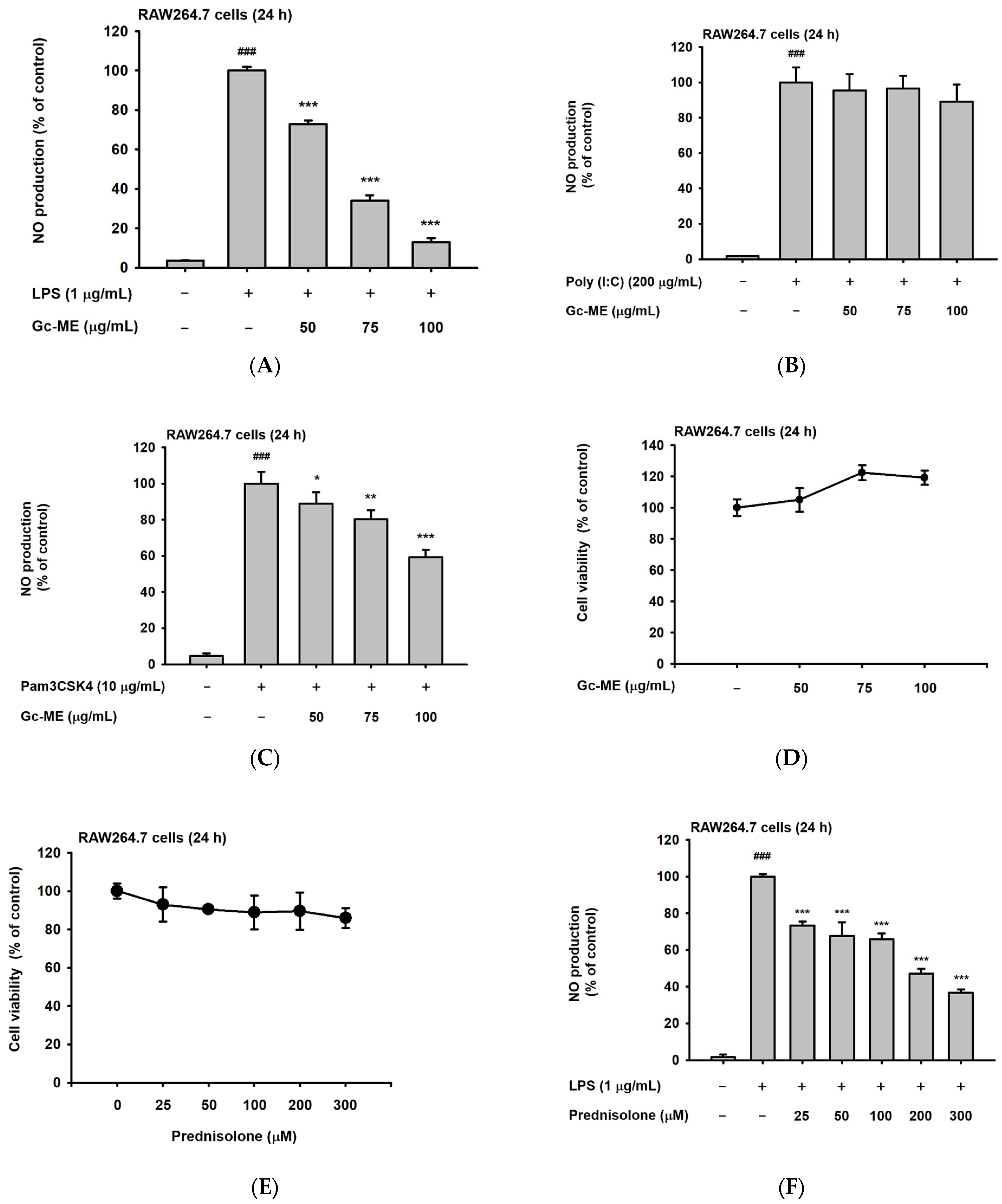
2.1. Effects of Gc-ME on NO Protection
2.2. Effects of Gc-ME on Inflammatory Cytokine mRNA Expression
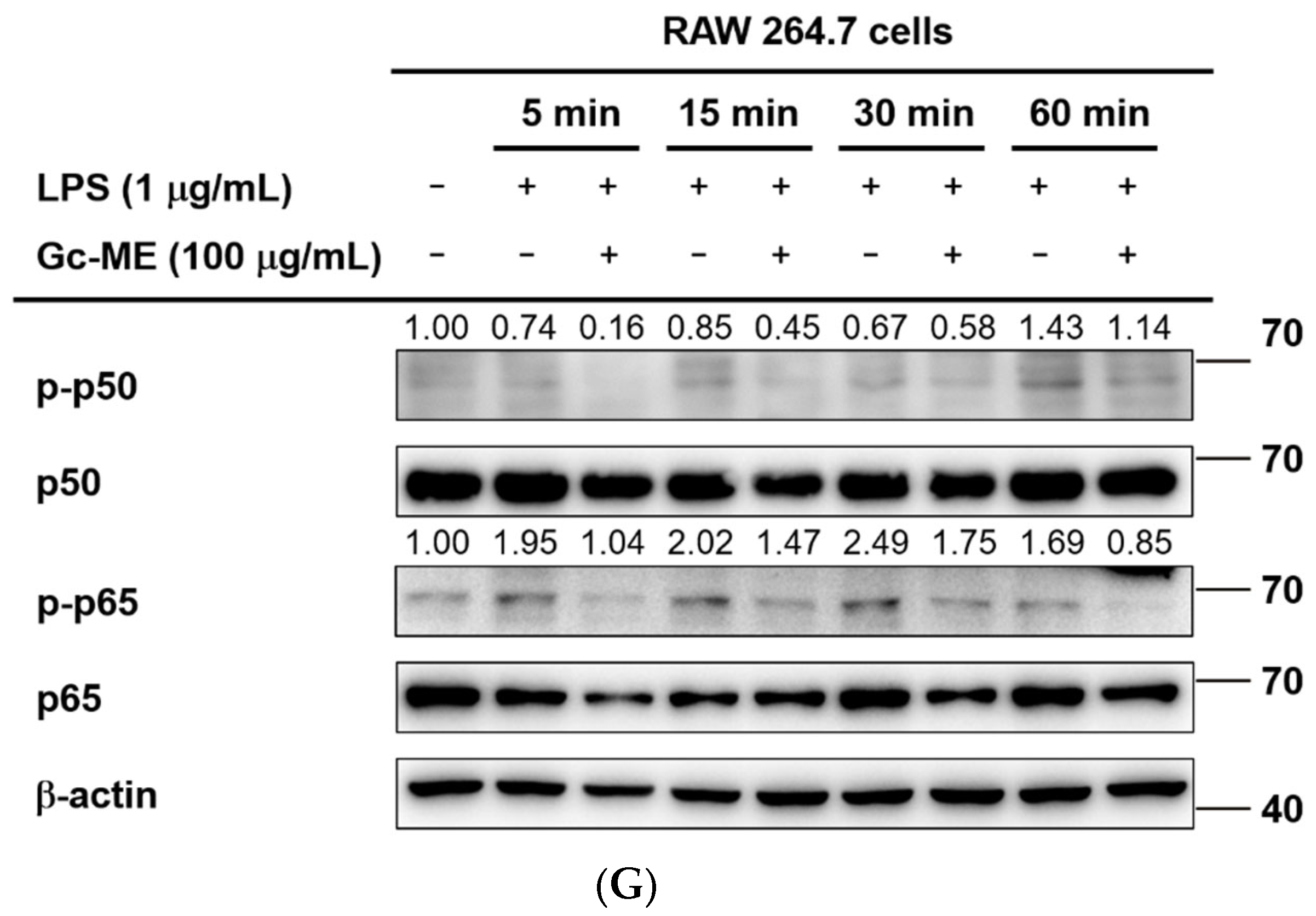
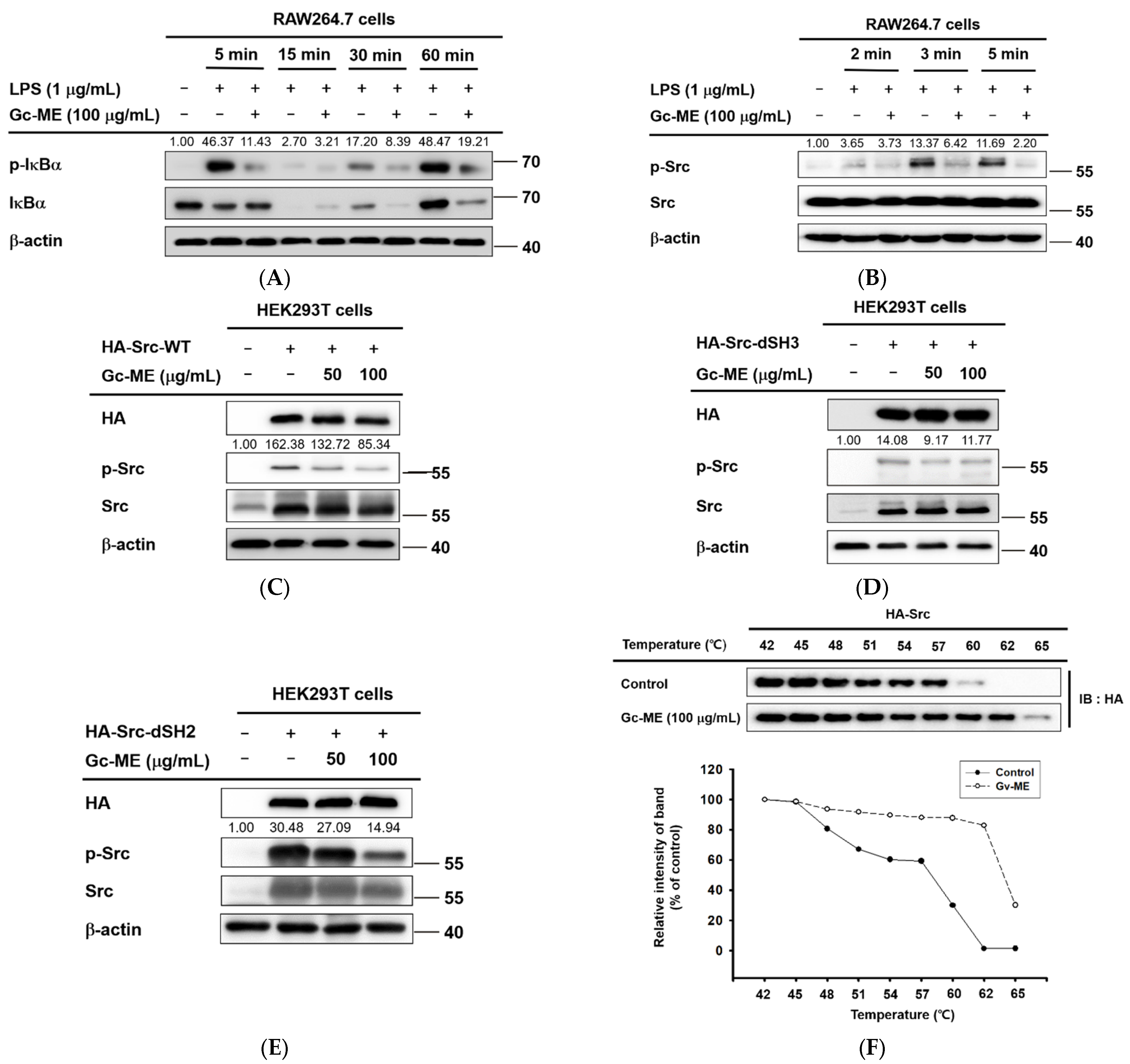
2.3. Effects of Gc-ME on Protein Activation
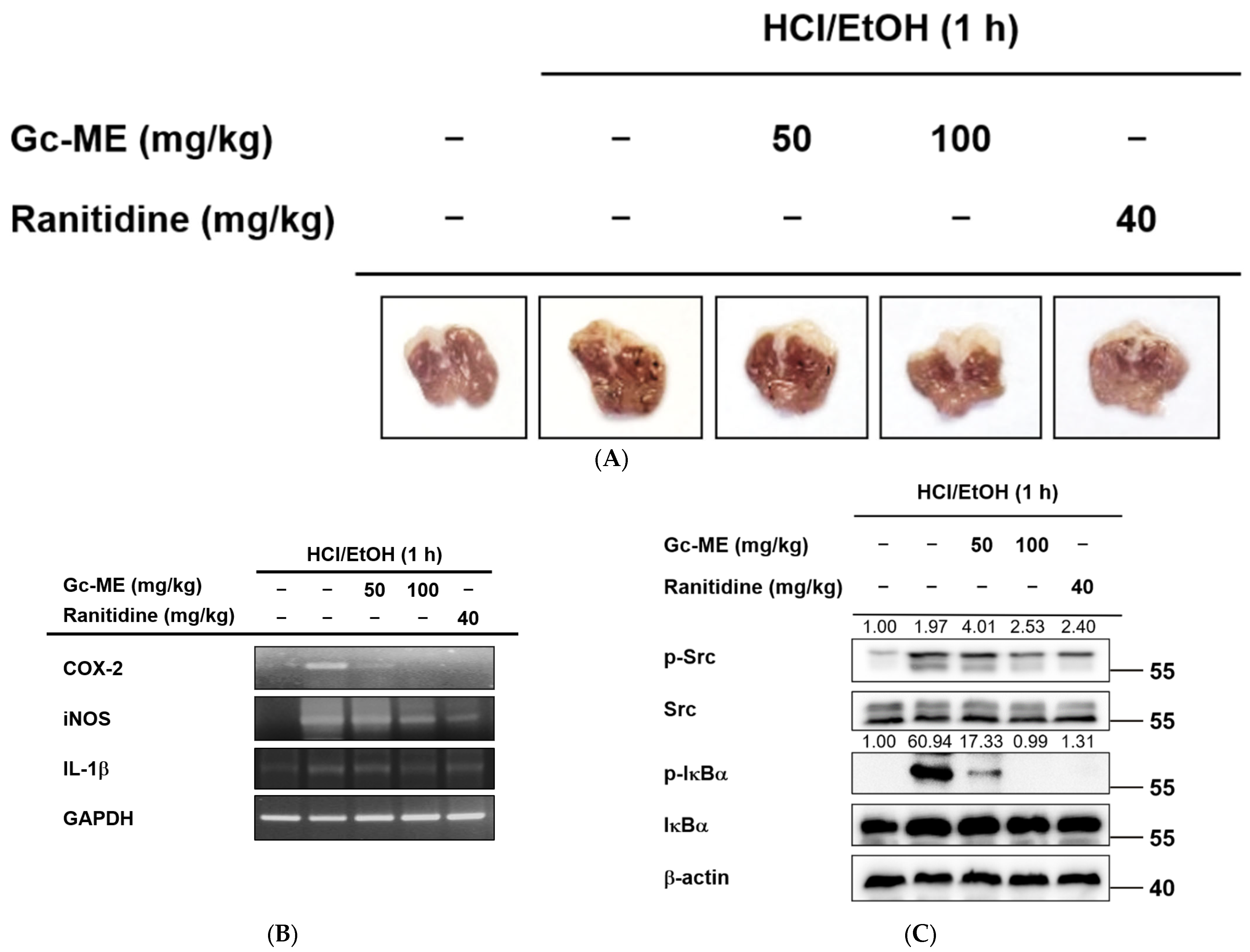
2.4. Anti-Inflammatory Effects of Gc-ME in an HCl/EtOH-Induced Mouse Model of Gastritis
2.5. Gc-ME Ameliorates Acute Lung Injury in an LPS-Induced ALI Model
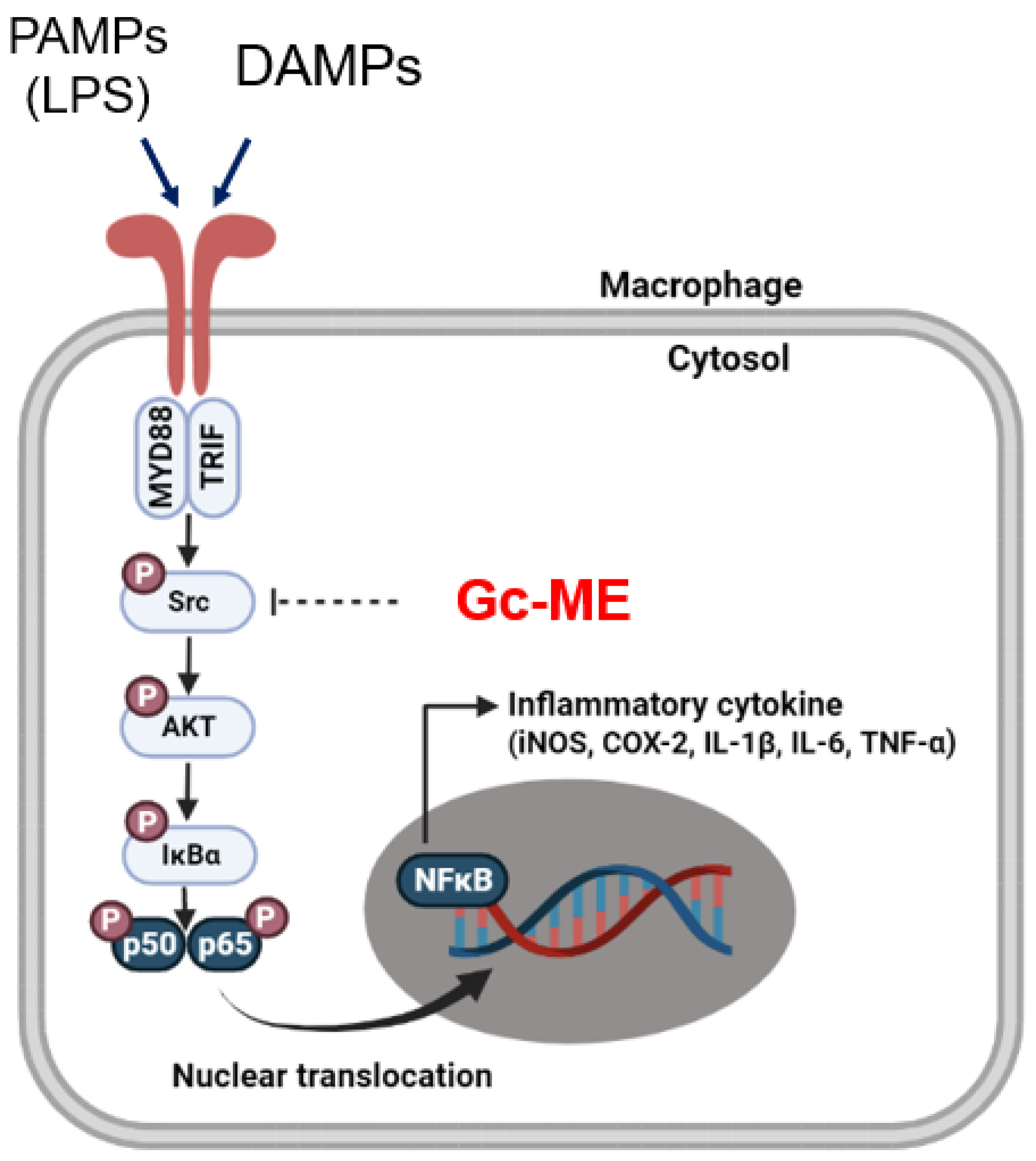
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of G. crispiflora Vahl Extract and Its Use
4.3. Animals
4.4. Cell Culture
4.5. Nitric Oxide (NO) Assay
4.6. Cell Viability Assay
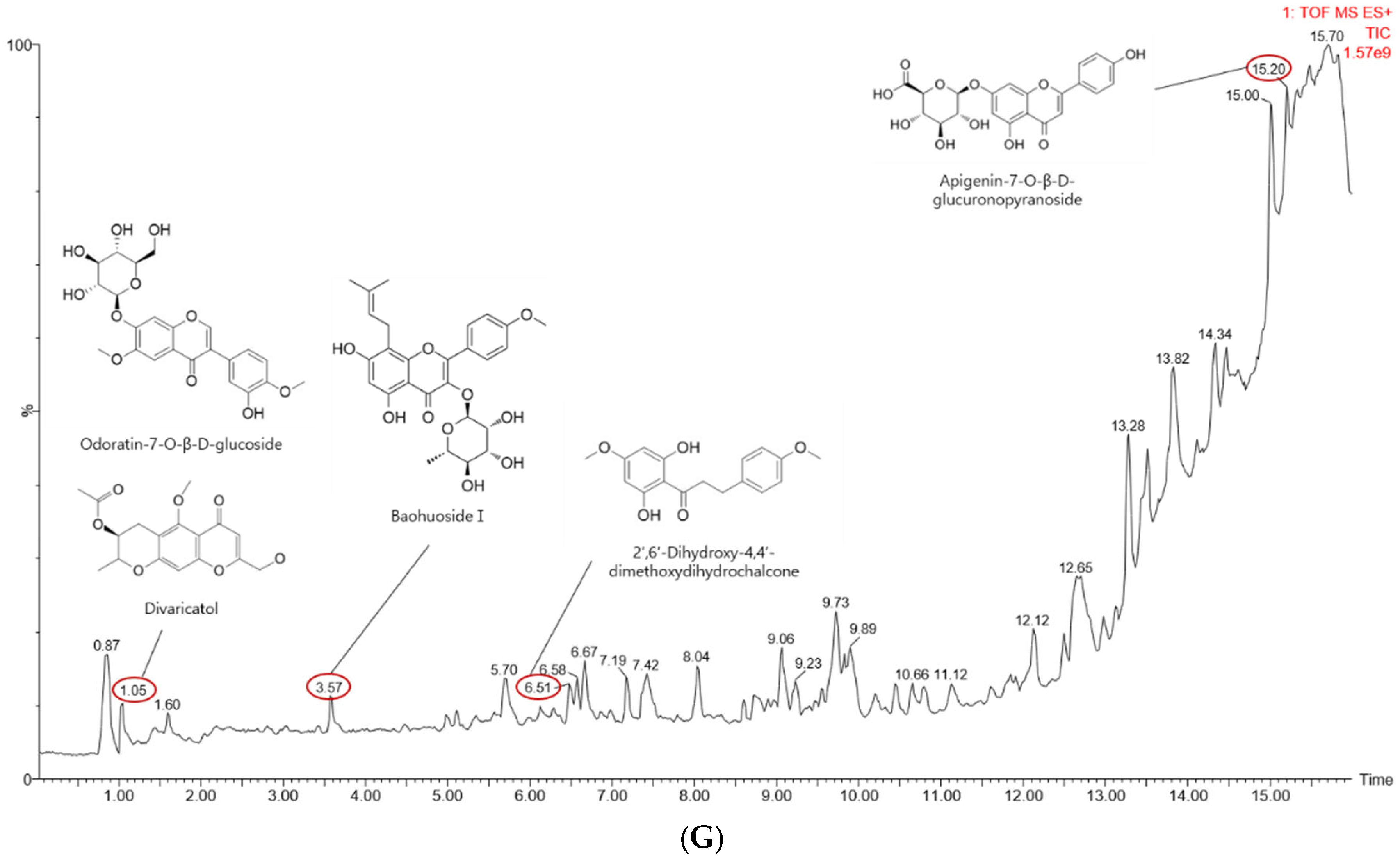
4.7. Liquid Chromatography–Mass Spectrometry (LC-MS)
4.8. Analysis of mRNA Expression Using Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.9. Luciferase Reporter Gene Activity Assay
4.10. Western Blotting Analyses
4.11. In Vivo HCl/EtOH-Induced Acute Gastritis Mouse Model
4.12. In Vivo LPS-Induced ALI Model
4.13. Cellular Thermal Shift Assay (CETSA)
4.14. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.K.; Shin, K.K.; Kim, H.; Hong, Y.H.; Choi, W.; Kwak, Y.S.; Han, C.K.; Hyun, S.H.; Cho, J.Y. Korean Red Ginseng exerts anti-inflammatory and autophagy-promoting activities in aged mice. J. Ginseng Res. 2021, 45, 717–725. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, L.; Kim, M.Y.; Cho, J.Y. The Role of Thymoquinone in Inflammatory Response in Chronic Diseases. Int. J. Mol. Sci. 2022, 23, 10246. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.H.; Li, J.R.; Zheng, T.H.; Yao, F.B.; Gao, B.; Xue, T.C. Neutrophils: Driving inflammation during the development of hepatocellular carcinoma. Cancer Lett. 2021, 522, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ho, L.; Tergaonkar, V. sORF-Encoded MicroPeptides: New players in inflammation, metabolism, and precision medicine. Cancer Lett. 2021, 500, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like Receptor-4 Mediates Lipopolysaccharide-induced Signal Transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef] [PubMed]
- Misako Matsumoto, T.S. TLR3: Interferon induction by double-stranded RNA including poly(I:C). Adv. Drug Deliv. Rev. 2008, 60, 805–812. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, H.G.; Lee, Y.; Yoon, K.; Kim, S.; Kim, J.H.; Cho, J.Y. Isoprenylcysteine carboxyl methyltransferase inhibitors exerts anti-inflammatory activity. Biochem. Pharmacol. 2020, 182, 114219. [Google Scholar] [CrossRef]
- Byeon, S.E.; Yi, Y.-S.; Oh, J.; Yoo, B.C.; Hong, S.; Cho, J.Y. The Role of Src Kinase in Macrophage-Mediated Inflammatory Responses. Mediat. Inflamm. 2012, 2012, 1–18. [Google Scholar] [CrossRef]
- Nagumo, Y.; Kandori, S.; Tanuma, K.; Nitta, S.; Chihara, I.; Shiga, M.; Hoshi, A.; Negoro, H.; Kojima, T.; Mathis, B.J.; et al. PLD1 promotes tumor invasion by regulation of MMP-13 expression via NF-kappaB signaling in bladder cancer. Cancer Lett. 2021, 511, 15–25. [Google Scholar] [CrossRef]
- Tang, G.; Luo, L.; Zhang, J.; Zhai, D.; Huang, D.; Yin, J.; Zhou, Q.; Zhang, Q.; Zheng, G. lncRNA LINC01057 promotes mesenchymal differentiation by activating NF-kappaB signaling in glioblastoma. Cancer Lett. 2021, 498, 152–164. [Google Scholar] [CrossRef]
- Zhu, D.; Zheng, S.; Fang, C.; Guo, X.; Han, D.; Tang, M.; Fu, H.; Jiang, M.; Xie, N.; Nie, Y.; et al. Dysbindin promotes pancreatic ductal adenocarcinoma metastasis by activating NF-κB/MDM2 via miR-342-3p. Cancer Lett. 2020, 477, 107–121. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Huang, L.; Jang, J.; Hong, Y.H.; Kim, H.G.; Chen, H.; Shin, C.Y.; Yoon, J.H.; Manilack, P.; Sounyvong, B.; et al. Callerya atropurpurea suppresses inflammation in vitro and ameliorates gastric injury as well as septic shock in vivo via TLR4/MyD88-dependent cascade. Phytomedicine 2022, 105, 154338. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Matthay, M.A. Acute Lung Injury: Epidemiology, Pathogenesis, and Treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Le, H.; Cho, Y.C.; Cho, S. Methanol extract of Guettarda speciosa Linn. inhibits the production of inflammatory mediators through the inactivation of Syk and JNK in macrophages. Int. J. Mol. Med. 2018, 41, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, J.Y.; Ahn, S.; Won, R.; Kim, S.-J.; Jeong, S.-I.; Lee, J.J.; Kim, J.-I.; Choi, J.-Y.; Joo, M. The methanol extract of Guettarda speciosa Linn. Ameliorates acute lung injury in mice. BMC Complement. Med. Ther. 2020, 20, 40. [Google Scholar] [CrossRef]
- Tan, M.A.; Lagamayo, M.W.D.; Alejandro, G.J.D.; An, S.S.A. Anti-Amyloidogenic and Cyclooxygenase Inhibitory Activity of Guettarda speciosa. Molecules 2019, 24, 4112. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Kim, H.; Shin, K.K.; Kim, H.G.; Jo, M.; Kim, J.K.; Lee, J.S.; Choung, E.S.; Li, W.Y.; Lee, S.W.; Kim, K.-H.; et al. Src/NF-κB-Targeted Anti-Inflammatory Effects of Potentilla glabra var. Mandshurica (Maxim.) Hand.-Mazz. Ethanol Extract. Biomolecules 2020, 10, 648. [Google Scholar] [CrossRef]
- Song, C.; Hong, Y.H.; Park, J.G.; Kim, H.G.; Jeong, D.; Oh, J.; Sung, G.H.; Hossain, M.A.; Taamalli, A.; Kim, J.H.; et al. Suppression of Src and Syk in the NF-kappaB signaling pathway by Olea europaea methanol extract is leading to its anti-inflammatory effects. J. Ethnopharmacol. 2019, 235, 38–46. [Google Scholar] [CrossRef]
- Jeong, S.G.; Kim, S.; Kim, H.G.; Kim, E.; Jeong, D.; Kim, J.H.; Yang, W.S.; Oh, J.; Sung, G.H.; Hossain, M.A.; et al. Mycetia cauliflora methanol extract exerts anti-inflammatory activity by directly targeting PDK1 in the NF-kappaB pathway. J. Ethnopharmacol. 2019, 231, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Zhu, J.; Chang, Q.; Zhou, H.; Shi, Z.; Min, L.; Cai, Y.; Guan, H. Alpha, 2’-dihydroxy-4,4’-dimethoxydihydrochalcone inhibits cell proliferation, invasion, and migration in gastric cancer in part via autophagy. Biomed. Pharmacother. 2018, 98, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shu, L.; Zhang, Z.; Tan, X.; Sun, E.; Jin, X.; Chen, Y.; Jia, X. Reactive oxygen species-mediated mitochondrial pathway is involved in Baohuoside I-induced apoptosis in human non-small cell lung cancer. Chem. -Biol. Interact. 2012, 199, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Ping, G.; Geng, L.; Seow, W.K.; Thong, Y.H. Immunopharmacology and toxicology of the plant flavonoid baohuoside-1 in mice. Int. J. Immunopharmacol. 1994, 16, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, E.; Hasegawa, T.; Matsushita, T.; Fujimoto, H.; Ishibashi, M.; Yamazaki, M. Analgesic Components of Saposhnikovia Root (Saposhnikovia divaricata). Chem. Pharm. Bull. 2001, 49, 154–160. [Google Scholar] [CrossRef]
- Nam, K.-W.; Je, K.-H.; Lee, J.H.; Han, H.J.; Lee, H.J.; Kang, S.K.; Mar, W. Inhibition of COX-2 activity and proinflammatory cytokines (TNF-α and IL-1β) production by water-soluble sub-fractionated parts from bee (Apis mellifera) venom. Arch. Pharmacal Res. 2003, 26, 383–388. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Schwenger, P.; Alpert, D.; Skolnik, E.Y.; Vilcek, J. Activation of p38 Mitogen-Activated Protein Kinase by Sodium Salicylate Leads to Inhibition of Tumor Necrosis Factor-Induced IκBα Phosphorylation and Degradation. Mol. Cell. Biol. 1998, 18, 78–84. [Google Scholar] [CrossRef]
- Yi, Y.S.; Kim, H.G.; Kim, J.H.; Yang, W.S.; Kim, E.; Jeong, D.; Park, J.G.; Aziz, N.; Kim, S.; Parameswaran, N.; et al. Syk-MyD88 Axis Is a Critical Determinant of Inflammatory-Response in Activated Macrophages. Front. Immunol. 2021, 12, 767366. [Google Scholar] [CrossRef]
- Lee, Y.G.; Chain, B.M.; Cho, J.Y. Distinct role of spleen tyrosine kinase in the early phosphorylation of inhibitor of kappaB alpha via activation of the phosphoinositide-3-kinase and Akt pathways. Int. J. Biochem. Cell Biol. 2009, 41, 811–821. [Google Scholar] [CrossRef]
- Yang, F.; Duan, M.; Zheng, F.; Yu, L.; Wang, Y.; Wang, G.; Lin, J.; Han, S.; Gan, D.; Meng, Z.; et al. Fas signaling in adipocytes promotes low-grade inflammation and lung metastasis of colorectal cancer through interaction with Bmx. Cancer Lett. 2021, 522, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Sun, Z.; Zhan, H. Macrophages in pancreatic cancer: An immunometabolic perspective. Cancer Lett. 2021, 498, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.M.; Delgobo, M.; Agnes, J.P.; das Neves, R.N.; Falchetti, M.; Casagrande, T.; Garcia, A.P.V.; Vieira, T.C.; Somensi, N.; Bruxel, M.A.; et al. COX-2 promotes mammary adipose tissue inflammation, local estrogen biosynthesis, and carcinogenesis in high-sugar/fat diet treated mice. Cancer Lett. 2021, 502, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Rahmawati, L.; Park, S.H.; Kim, D.S.; Lee, H.P.; Aziz, N.; Lee, C.Y.; Kim, S.A.; Jang, S.G.; Kim, D.S.; Cho, J.Y. Anti-Inflammatory Activities of the Ethanol Extract of Prasiola japonica, an Edible Freshwater Green Algae, and Its Various Solvent Fractions in LPS-Induced Macrophages and Carrageenan-Induced Paw Edema via the AP-1 Pathway. Molecules 2021, 27, 194. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Chou, H.Y.; Chiu, Y.C.; Cheng, A.N.; Fan, C.C.; Chang, Y.N.; Chen, C.H.; Jiang, S.S.; Chen, N.J.; Lee, A.Y. Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. 2020, 474, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.; De Simone, F.; Pizza, C.; Conti, C.; Stein, M.L. Plant metabolites. Structure and in vitro antiviral activity of quinovic acid glycosides from Uncaria tomentosa and Guettarda platypoda. J. Nat. Prod. 1989, 52, 679–685. [Google Scholar] [CrossRef]
- Capasso, A.; Balderrama, L.; Sivila, S.C.; De Tommasi, N.; Sorrentino, L.; Pizza, C. Phytochemical and pharmacological studies of Guettarda acreana. Planta Med. 1998, 64, 348–352. [Google Scholar] [CrossRef]
- Kim, S.A.; Lee, C.Y.; Mitra, A.; Kim, H.; Woo, B.Y.; Hong, Y.D.; Noh, J.K.; Yi, D.K.; Kim, H.G.; Cho, J.Y. Anti-Inflammatory Effects of Huberia peruviana Cogn. Methanol Extract by Inhibiting Src Activity in the NF-kappaB Pathway. Plants 2021, 10, 2335. [Google Scholar] [CrossRef]
- Min, Y.S.; Yim, S.H.; Bai, K.L.; Choi, H.J.; Jeong, J.H.; Song, H.J.; Park, S.Y.; Ham, I.; Whang, W.K.; Sohn, U.D. The effects of apigenin-7-O-beta-D-glucuronopyranoside on reflux oesophagitis and gastritis in rats. Auton. Autacoid Pharmacol. 2005, 25, 85–91. [Google Scholar] [CrossRef]
- Kim, S.A.; Oh, J.; Choi, S.R.; Lee, C.H.; Lee, B.H.; Lee, M.N.; Hossain, M.A.; Kim, J.H.; Lee, S.; Cho, J.Y. Anti-Gastritis and Anti-Lung Injury Effects of Pine Tree Ethanol Extract Targeting Both NF-kappaB and AP-1 Pathways. Molecules 2021, 26, 6275. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; He, N.; Wang, Z.; Fu, X.; Aung, L.H.H.; Liu, Y.; Li, M.; Cho, J.Y.; Yang, Y.; Yu, T. Functional roles and mechanisms of ginsenosides from Panax ginseng in atherosclerosis. J. Ginseng Res. 2021, 45, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Vonkeman, H.E.; van de Laar, M.A. Nonsteroidal anti-inflammatory drugs: Adverse effects and their prevention. Semin. Arthritis Rheum. 2010, 39, 294–312. [Google Scholar] [CrossRef]
- Lee, J.O.; Kim, J.H.; Kim, S.; Kim, M.Y.; Hong, Y.H.; Kim, H.G.; Cho, J.Y. Gastroprotective effects of the nonsaponin fraction of Korean Red Ginseng through cyclooxygenase-1 upregulation. J. Ginseng Res. 2020, 44, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Choi, E.; Hong, Y.H.; Kim, H.; Jang, Y.J.; Lee, J.S.; Choung, E.S.; Woo, B.Y.; Hong, Y.D.; Lee, S.; et al. Syk/NF-kappaB-targeted anti-inflammatory activity of Melicope accedens (Blume) T.G. Hartley methanol extract. J. Ethnopharmacol. 2021, 271, 113887. [Google Scholar] [CrossRef]
- Hong, Y.H.; Aziz, N.; Park, J.G.; Lee, D.; Kim, J.K.; Kim, S.A.; Choi, W.; Lee, C.Y.; Lee, H.P.; Huyen Trang, H.T.; et al. The EEF1AKMT3/MAP2K7/TP53 axis suppresses tumor invasiveness and metastasis in gastric cancer. Cancer Lett. 2022, 544, 215803. [Google Scholar] [CrossRef]
- Ren, D.; Sun, Y.; Zhang, D.; Li, D.; Liu, Z.; Jin, X.; Wu, H. SGLT2 promotes pancreatic cancer progression by activating the Hippo signaling pathway via the hnRNPK-YAP1 axis. Cancer Lett. 2021, 519, 277–288. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, H.G.; Kim, E.; Han, S.Y.; Aziz, N.; Yi, Y.S.; Kim, S.; Lee, Y.; Yoo, B.C.; Han, J.W.; et al. Isoprenylcysteine Carboxyl Methyltransferase and Its Substrate Ras Are Critical Players Regulating TLR-Mediated Inflammatory Responses. Cells 2020, 9, 1216. [Google Scholar] [CrossRef]
- Mitra, A.; Rahmawati, L.; Lee, H.P.; Kim, S.A.; Han, C.K.; Hyun, S.H.; Cho, J.Y. Korean Red Ginseng water extract inhibits cadmium-induced lung injury via suppressing MAPK/ERK1/2/AP-1 pathway. J. Ginseng Res. 2022, 46, 690–699. [Google Scholar] [CrossRef]
- Lee, J.O.; Hwang, S.H.; Shen, T.; Kim, J.H.; You, L.; Hu, W.; Cho, J.Y. Enhancement of skin barrier and hydration-related molecules by protopanaxatriol in human keratinocytes. J. Ginseng Res. 2021, 45, 354–360. [Google Scholar] [CrossRef]
- Song, C.; Kim, M.Y.; Cho, J.Y. Olea europaea Suppresses Inflammation by Targeting TAK1-Mediated MAP Kinase Activation. Molecules 2021, 26, 1540. [Google Scholar] [CrossRef] [PubMed]



| Gene (Type) | Direction | Sequences (5′ to 3′) |
|---|---|---|
| COX-2 | Forward | TCACGTGGAGTCCGCTTTAC |
| Reverse | TTCGACAGGAAGGGGATGTT | |
| iNOS | Forward | TGCCAGGGTCACAACTTTACA |
| Reverse | ACCCCAAGCAAGACTTGGAC | |
| IL-1β | Forward | CAGGATGAGGACATGAGCACC |
| Reverse | CTCTGCAGACTCAAACTCCAC | |
| IL-6 | Forward | GCCTTCTTGGGACTGATGG |
| Reverse | TGGAAATTGGGGTAGGAAGGAC | |
| GAPDH | Forward | GAAGGTCGGTGTGAACGGAT |
| Reverse | AGTGATGGCATGGACTGTGG |
| Parameters | Scoring | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| None | 1 to 5 | >5 |
| None | 1 to 5 | >5 |
| None | 1 | >1 |
| None | 1 | >1 |
| <2× | 2×–4× | >4× |
| Scoring = [(20 × A) + (14 × B) + (7 × C) + (2 × D)]/(number of fields × 100) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kim, J.W.; Lee, C.Y.; Oh, J.; Hwang, S.H.; Jo, M.; Kim, S.A.; Choi, W.; Noh, J.K.; Yi, D.-K.; et al. Guettarda crispiflora Vahl Methanol Extract Ameliorates Acute Lung Injury and Gastritis by Suppressing Src Phosphorylation. Plants 2022, 11, 3560. https://doi.org/10.3390/plants11243560
Lee D, Kim JW, Lee CY, Oh J, Hwang SH, Jo M, Kim SA, Choi W, Noh JK, Yi D-K, et al. Guettarda crispiflora Vahl Methanol Extract Ameliorates Acute Lung Injury and Gastritis by Suppressing Src Phosphorylation. Plants. 2022; 11(24):3560. https://doi.org/10.3390/plants11243560
Chicago/Turabian StyleLee, Dahae, Ji Won Kim, Chae Young Lee, Jieun Oh, So Hyun Hwang, Minkyeong Jo, Seung A Kim, Wooram Choi, Jin Kyoung Noh, Dong-Keun Yi, and et al. 2022. "Guettarda crispiflora Vahl Methanol Extract Ameliorates Acute Lung Injury and Gastritis by Suppressing Src Phosphorylation" Plants 11, no. 24: 3560. https://doi.org/10.3390/plants11243560
APA StyleLee, D., Kim, J. W., Lee, C. Y., Oh, J., Hwang, S. H., Jo, M., Kim, S. A., Choi, W., Noh, J. K., Yi, D.-K., Song, M., Kim, H. G., & Cho, J. Y. (2022). Guettarda crispiflora Vahl Methanol Extract Ameliorates Acute Lung Injury and Gastritis by Suppressing Src Phosphorylation. Plants, 11(24), 3560. https://doi.org/10.3390/plants11243560







