Suppressive Effects of Flavonoids on Macrophage-Associated Adipocyte Inflammation in a Differentiated Murine Preadipocyte 3T3-L1 Cells Co-Cultured with a Murine Macrophage RAW264.7 Cells
Abstract
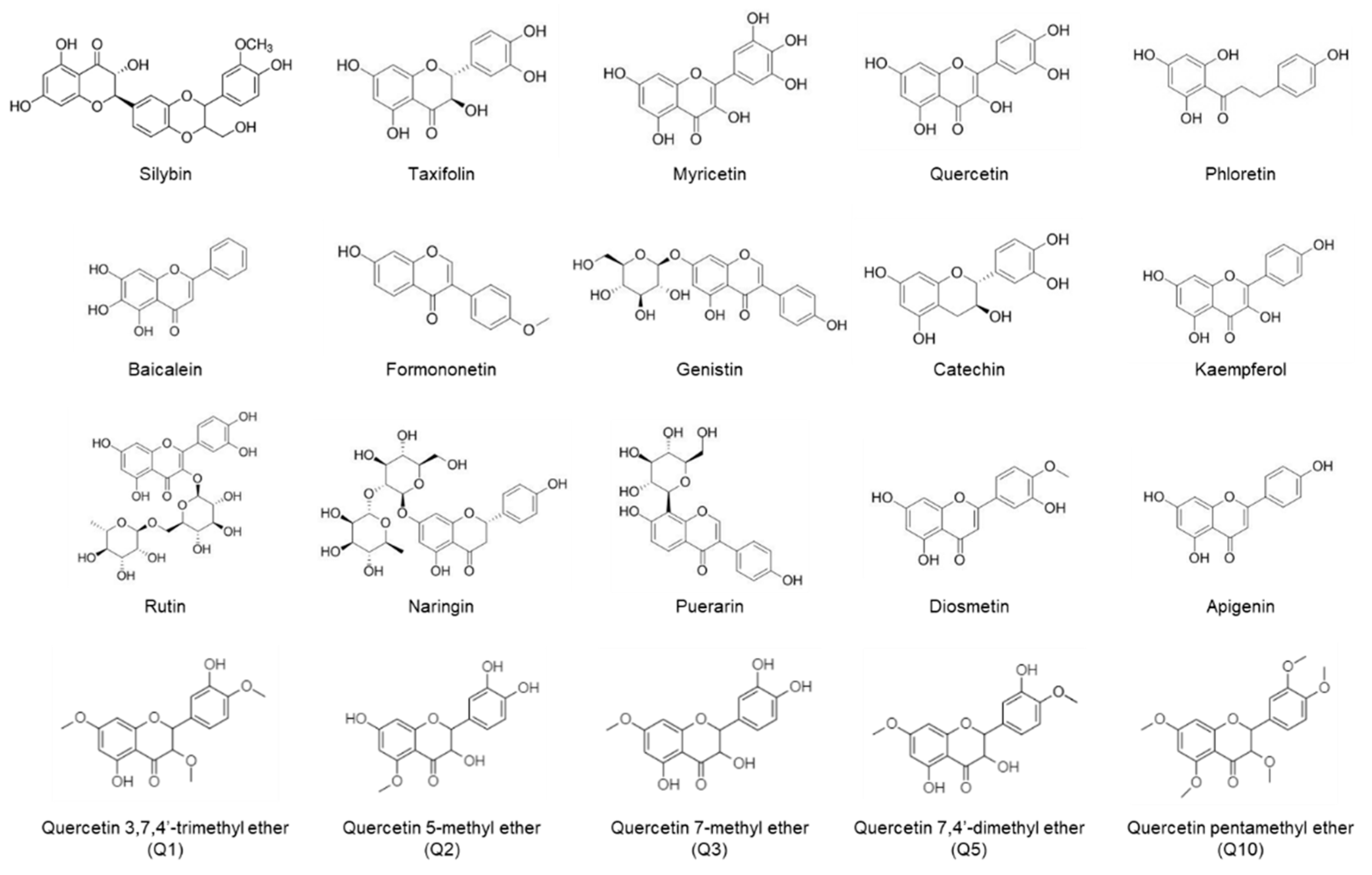
1. Introduction
2. Results
2.1. Inhibitory Effects of Flavonoids on Adipogenesis and Lipid Accumulation in Co-Cultures of 3T3-L1 and RAW264.7 Cells
2.2. Inhibitory Effects of Flavonoids on the Production of IL-6 and MCP-1 in Co-Cultures of 3T3-L1 and RAW264.7 Cells
2.3. Inhibitory Effects of Flavonoids on the Adipogenesis- and Inflammation-Associated Proteins in Co-Cultures of 3T3-L1 and RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Adipogenic Differentiation Assay and Co-Culture
4.5. Oil Red O Staining
4.6. Measurement of Proinflammatory Cytokine Levels
4.7. Western Blotting Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Feng, R.-N.; Zhao, C.; Wang, C.; Niu, Y.-C.; Li, K.; Guo, F.-C.; Li, S.-T.; Sun, C.-H.; Li, Y. BMI is strongly associated with hypertension, and waist circumference is strongly associated with type 2 diabetes and dyslipidemia, in northern Chinese adults. J. Epidemiol. 2012, 22, 317–323. [Google Scholar] [CrossRef]
- Unamuno, X.; Gomez-Ambrosi, J.; Rodriguez, A.; Becerril, S.; Fruhbeck, G.; Catalan, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.; Mourot, J.; Gregoire, F.; Remacle, C. The adipose conversion process: Regulation by extracellular and intracellular factors. Reprod. Nutr. Dev. 2000, 40, 325–358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koutnikova, H.; Auwerx, J. Regulation of adipocyte differentiation. Ann. Med. 2001, 33, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.C.; Lane, M.D. Adipose development: From stem cell to adipocyte. Crit. Rev. Biochem. Mol. Biol. 2005, 40, 229–242. [Google Scholar] [CrossRef]
- Tateya, S.; Kim, F.; Tamori, Y. Recent advances in obesity-induced inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 93. [Google Scholar] [CrossRef]
- García-Barrado, M.J.; Iglesias-Osma, M.C.; Pérez-García, E.; Carrero, S.; Blanco, E.J.; Carretero-Hernández, M.; Carretero, J. Role of flavonoids in the interactions among obesity, inflammation, and autophagy. Pharmaceuticals 2020, 13, 342. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Doseff, A.I. The targeted impact of flavones on obesity-induced inflammation and the potential synergistic role in cancer and the gut microbiota. Molecules 2020, 25, 2477. [Google Scholar] [CrossRef]
- Xiao, P.; Yang, Z.; Sun, J.; Tian, J.; Chang, Z.; Li, X.; Zhang, B.; Ye, Y.; Ji, H.; Yu, E. Silymarin inhibits adipogenesis in the adipocytes in grass carp Ctenopharyngodon idellus in vitro and in vivo. Fish Physiol. Biochem. 2017, 43, 1487–1500. [Google Scholar] [CrossRef]
- Kang, J.S.; Jeon, Y.J.; Kim, H.M.; Han, S.H.; Yang, K.-H. Inhibition of inducible nitric-oxide synthase expression by silymarin in lipopolysaccharide-stimulated macrophages. J. Pharmacol. Exp. Ther. 2002, 302, 138–144. [Google Scholar] [CrossRef]
- Muramatsu, D.; Uchiyama, H.; Kida, H.; Iwai, A. In vitro anti-inflammatory and anti-lipid accumulation properties of taxifolin-rich extract from the Japanese larch, Larix kaempferi. Heliyon 2020, 6, e05505. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.; An, H.-j.; Lee, J.-d.; Yi, Y. Anti-inflammatory activities of taxifolin from Opuntia humifusa in lipopolysaccharide stimulated RAW 264.7 murine macrophages. J. Appl. Biol. Chem. 2015, 58, 241–246. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.-T.; Yang, X.; You, P.-p.; Zhang, W. Myricetin suppresses differentiation of 3 T3-L1 preadipocytes and enhances lipolysis in adipocytes. Nutr. Res. 2015, 35, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Hu, S.; Su, Z.; Wang, Q.; Meng, G.; Guo, T.; Zhang, J.; Gao, P. Myricetin attenuates LPS-induced inflammation in RAW 264.7 macrophages and mouse models. Future Med. Chem. 2018, 10, 2253–2264. [Google Scholar] [CrossRef]
- Seo, Y.-S.; Kang, O.-H.; Kim, S.-B.; Mun, S.-H.; Kang, D.-H.; Yang, D.-W.; Choi, J.-G.; Lee, Y.-M.; Kang, D.-K.; Lee, H.-S. Quercetin prevents adipogenesis by regulation of transcriptional factors and lipases in OP9 cells. Int. J. Mol. Med. 2015, 35, 1779–1785. [Google Scholar]
- Mu, M.M.; Chakravortty, D.; Sugiyama, T.; Koide, N.; Takahashi, K.; Mori, I.; Yoshida, T.; Yokochi, T. The inhibitory action of quercetin on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells. J. Endotoxin Res. 2001, 7, 431–438. [Google Scholar] [CrossRef]
- Seo, M.-J.; Choi, H.-S.; Jeon, H.-J.; Woo, M.-S.; Lee, B.-Y. Baicalein inhibits lipid accumulation by regulating early adipogenesis and m-TOR signaling. Food Chem. Toxicol. 2014, 67, 57–64. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, H.-J.; Lee, J.Y.; Kim, D.-H.; Kang, M.S.; Park, W. Anti-inflammatory effect of baicalein on polyinosinic–polycytidylic acid-induced RAW 264.7 mouse macrophages. Viruses 2018, 10, 224. [Google Scholar] [CrossRef]
- Gautam, J.; Khedgikar, V.; Kushwaha, P.; Choudhary, D.; Nagar, G.K.; Dev, K.; Dixit, P.; Singh, D.; Maurya, R.; Trivedi, R. Formononetin, an isoflavone, activates AMP-activated protein kinase/β-catenin signalling to inhibit adipogenesis and rescues C57BL/6 mice from high-fat diet-induced obesity and bone loss. Br. J. Nutr. 2017, 117, 645–661. [Google Scholar] [CrossRef]
- Kim, M.-S.; Park, J.-S.; Chung, Y.C.; Jang, S.; Hyun, C.-G.; Kim, S.-Y. Anti-inflammatory effects of formononetin 7-O-phosphate, a novel biorenovation product, on LPS-Stimulated RAW 264.7 macrophage cells. Molecules 2019, 24, 3910. [Google Scholar] [CrossRef]
- Choi, Y.R.; Shim, J.; Kim, M.J. Genistin: A novel potent anti-adipogenic and anti-lipogenic agent. Molecules 2020, 25, 2042. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zhang, Y.; Yang, Q.; Cheng, S.; Hao, J.; Zhao, X.; Jiang, Z. Genistein suppresses LPS-induced inflammatory response through inhibiting NF-κB following AMP kinase activation in RAW 264.7 macrophages. PLoS ONE 2012, 7, e53101. [Google Scholar] [CrossRef] [PubMed]
- Furuyashiki, T.; Nagayasu, H.; Aoki, Y.; Bessho, H.; Hashimoto, T.; Kanazawa, K.; Ashida, H. Tea catechin suppresses adipocyte differentiation accompanied by down-regulation of PPARγ2 and C/EBPα in 3T3-L1 cells. Biosci. Biotechnol. Biochem. 2004, 68, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Sunil, M.; Sunitha, V.; Santhakumaran, P.; Mohan, M.C.; Jose, M.S.; Radhakrishnan, E.; Mathew, J. Protective effect of (+)–catechin against lipopolysaccharide-induced inflammatory response in RAW 264.7 cells through downregulation of NF-κB and p38 MAPK. Inflammopharmacology 2021, 29, 1139–1155. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Choi, H.-S.; Seo, M.-J.; Jeon, H.-J.; Kim, K.-J.; Lee, B.-Y. Kaempferol suppresses lipid accumulation by inhibiting early adipogenesis in 3T3-L1 cells and zebrafish. Food Funct. 2015, 6, 2824–2833. [Google Scholar] [CrossRef]
- Palacz-Wrobel, M.; Borkowska, P.; Paul-Samojedny, M.; Kowalczyk, M.; Fila-Danilow, A.; Suchanek-Raif, R.; Kowalski, J. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomed. Pharmacother. 2017, 93, 1205–1212. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.; Cheng, W.; Cao, H.; Zhang, P.; Qin, L. Puerarin promotes osteogenesis and inhibits adipogenesis in vitro. Chin. Med. 2013, 8, 1–12. [Google Scholar] [CrossRef]
- Chang, Y.; Guo, A.; Jing, Y.; Lin, J.; Sun, Y.; Kong, L.; Zheng, H.; Deng, Y. Immunomodulatory activity of puerarin in RAW264. 7 macrophages and cyclophosphamide-induced immunosuppression mice. Immunopharmacol. Immunotoxicol. 2021, 43, 223–229. [Google Scholar] [CrossRef]
- Choi, I.; Park, Y.; Choi, H.; Lee, E.H. Anti-adipogenic activity of rutin in 3T3-L1 cells and mice fed with high-fat diet. Biofactors 2006, 26, 273–281. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Yang, M.; Liu, M. Rutin prevents inflammation induced by lipopolysaccharide in RAW 264.7 cells via conquering the TLR4-MyD88-TRAF6-NF-κB signalling pathway. J. Pharm. Pharmacol. 2021, 73, 110–117. [Google Scholar] [CrossRef]
- Dayarathne, L.A.; Ranaweera, S.S.; Natraj, P.; Rajan, P.; Lee, Y.J.; Han, C.-H. Restoration of the adipogenic gene expression by naringenin and naringin in 3T3-L1 adipocytes. J. Vet. Sci. 2021, 22, e55. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.-i.; Shouji, A.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Ujibe, M.; Obara, Y.; Nakahata, N.; Ishikawa, M. Inhibitory effect of naringin on lipopolysaccharide (LPS)-induced endotoxin shock in mice and nitric oxide production in RAW 264.7 macrophages. Life Sci. 2006, 78, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Fujimori, K. Antiadipogenic effect of dietary apigenin through activation of AMPK in 3T3-L1 cells. J. Agric. Food Chem. 2011, 59, 13346–13352. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, Y.; Li, J. Research progress of cell co-culture method. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2016, 28, 765–768. [Google Scholar]
- Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Inhibitory effect of diosmetin on inflammation and lipolysis in coculture of adipocytes and macrophages. J. Food Biochem. 2020, 44, e13261. [Google Scholar] [CrossRef] [PubMed]
- Oliyarnyk, O.; Malinska, H.; Markova, I.; Trnovska, J.; Skop, V.; Kazdova, L.; Vecera, R.; Pravenec, M. Comparative effect of silymarin and silybin treatment on inflammation and oxidative stress in transgenic spontaneously hypertensive rats overexpressing human C-reactive protein. Atherosclerosis 2016, 252, e220. [Google Scholar] [CrossRef]
- Tay, K.-C.; Tan, L.T.-H.; Chan, C.K.; Hong, S.L.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Formononetin: A review of its anticancer potentials and mechanisms. Front. Pharmacol. 2019, 10, 820. [Google Scholar] [CrossRef]
- Huang, W.C.; Chang, W.T.; Wu, S.J.; Xu, P.Y.; Ting, N.C.; Liou, C.J. Phloretin and phlorizin promote lipolysis and inhibit inflammation in mouse 3 T 3-L 1 cells and in macrophage-adipocyte co-cultures. Mol. Nutr. Food Res. 2013, 57, 1803–1813. [Google Scholar] [CrossRef]
- Choi, J.-H.; Park, S.-E.; Yeo, S.-H.; Kim, S. Anti-inflammatory and cytotoxicity effects of Cudrania tricuspidata fruits vinegar in a co-culture system with RAW264. 7 macrophages and 3T3-L1 adipocytes. Foods 2020, 9, 1232. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.-H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPalpha induces adipogenesis through PPARgamma: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef]
- Ka, S.O.; Kim, K.A.; Kwon, K.B.; Park, J.W.; Park, B.H. Silibinin attenuates adipogenesis in 3T3-L1 preadipocytes through a potential upregulation of the insig pathway. Int. J. Mol. Med. 2009, 23, 633–637. [Google Scholar] [PubMed]
- Andersen, C.; Kotowska, D.; Tortzen, C.G.; Kristiansen, K.; Nielsen, J.; Petersen, R.K. 2-(2-Bromophenyl)-formononetin and 2-heptyl-formononetin are PPARγ partial agonists and reduce lipid accumulation in 3T3-L1 adipocytes. Bioorganic. Med. Chem. 2014, 22, 6105–6111. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.L.; Maia, B.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, biosynthesis and chemical ecology. Flavonoids-Biosynth. Hum. Health 2017, 13, 78–94. [Google Scholar]
- Tapas, A.; Sakarkar, D.; Kakde, R. A review of flavonoids as nutraceuticals. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Lagana, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Hong, S.; Jung, K.; Choi, S.; Kang, K.S. Suppressive Effects of Flavonoids on Macrophage-Associated Adipocyte Inflammation in a Differentiated Murine Preadipocyte 3T3-L1 Cells Co-Cultured with a Murine Macrophage RAW264.7 Cells. Plants 2022, 11, 3552. https://doi.org/10.3390/plants11243552
Lee D, Hong S, Jung K, Choi S, Kang KS. Suppressive Effects of Flavonoids on Macrophage-Associated Adipocyte Inflammation in a Differentiated Murine Preadipocyte 3T3-L1 Cells Co-Cultured with a Murine Macrophage RAW264.7 Cells. Plants. 2022; 11(24):3552. https://doi.org/10.3390/plants11243552
Chicago/Turabian StyleLee, Dahae, Sukyong Hong, Kiwon Jung, Sungyoul Choi, and Ki Sung Kang. 2022. "Suppressive Effects of Flavonoids on Macrophage-Associated Adipocyte Inflammation in a Differentiated Murine Preadipocyte 3T3-L1 Cells Co-Cultured with a Murine Macrophage RAW264.7 Cells" Plants 11, no. 24: 3552. https://doi.org/10.3390/plants11243552
APA StyleLee, D., Hong, S., Jung, K., Choi, S., & Kang, K. S. (2022). Suppressive Effects of Flavonoids on Macrophage-Associated Adipocyte Inflammation in a Differentiated Murine Preadipocyte 3T3-L1 Cells Co-Cultured with a Murine Macrophage RAW264.7 Cells. Plants, 11(24), 3552. https://doi.org/10.3390/plants11243552






