Allelopathic Effects of Amomum villosum Lour. Volatiles from Different Organs on Selected Plant Species and Soil Microbiota
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of the Volatile Oils from Different Plant Parts
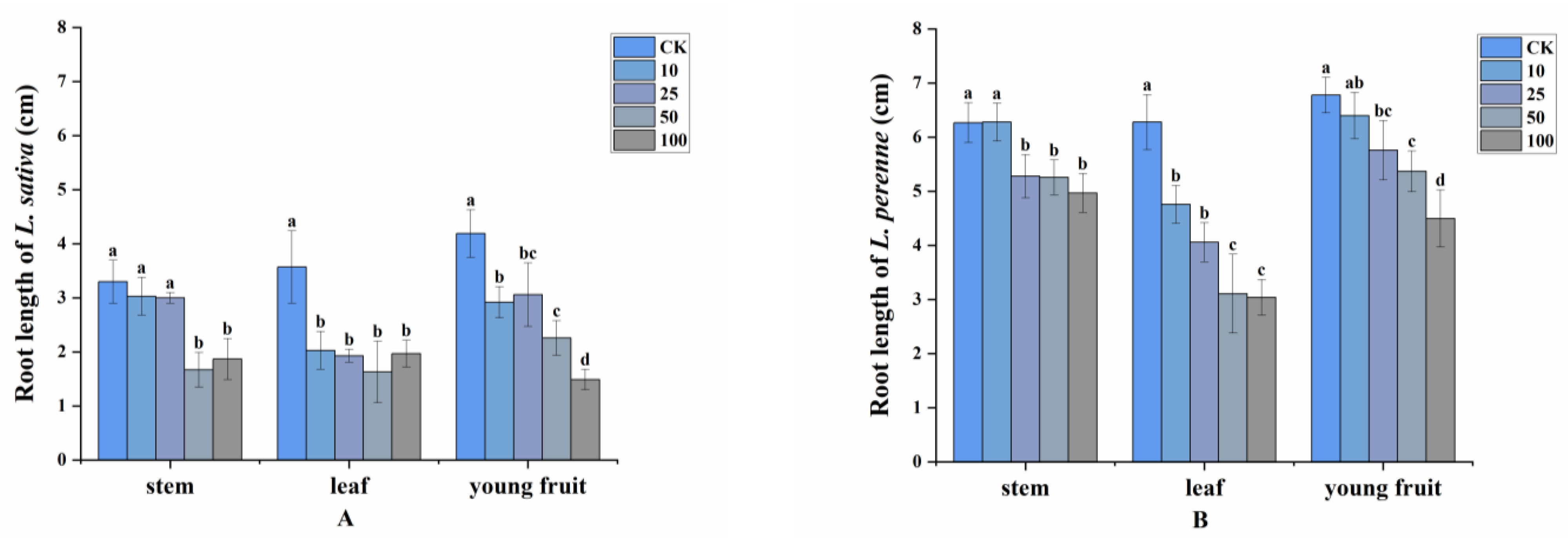
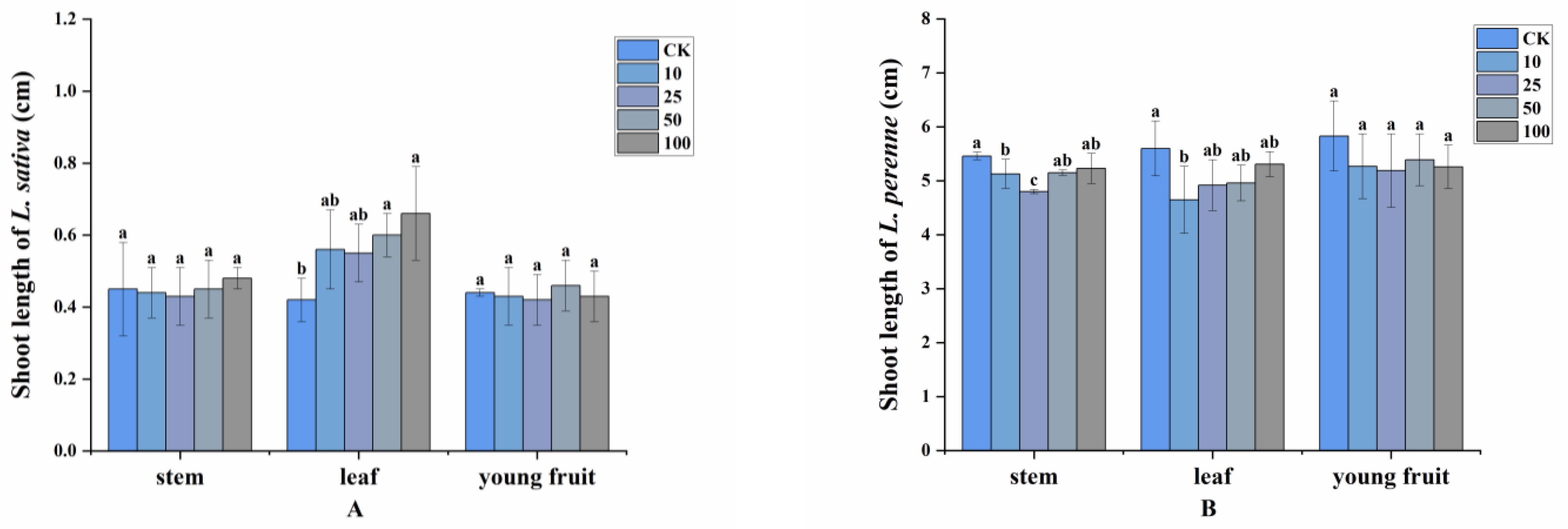
2.2. Allelopathy of Volatile Compounds
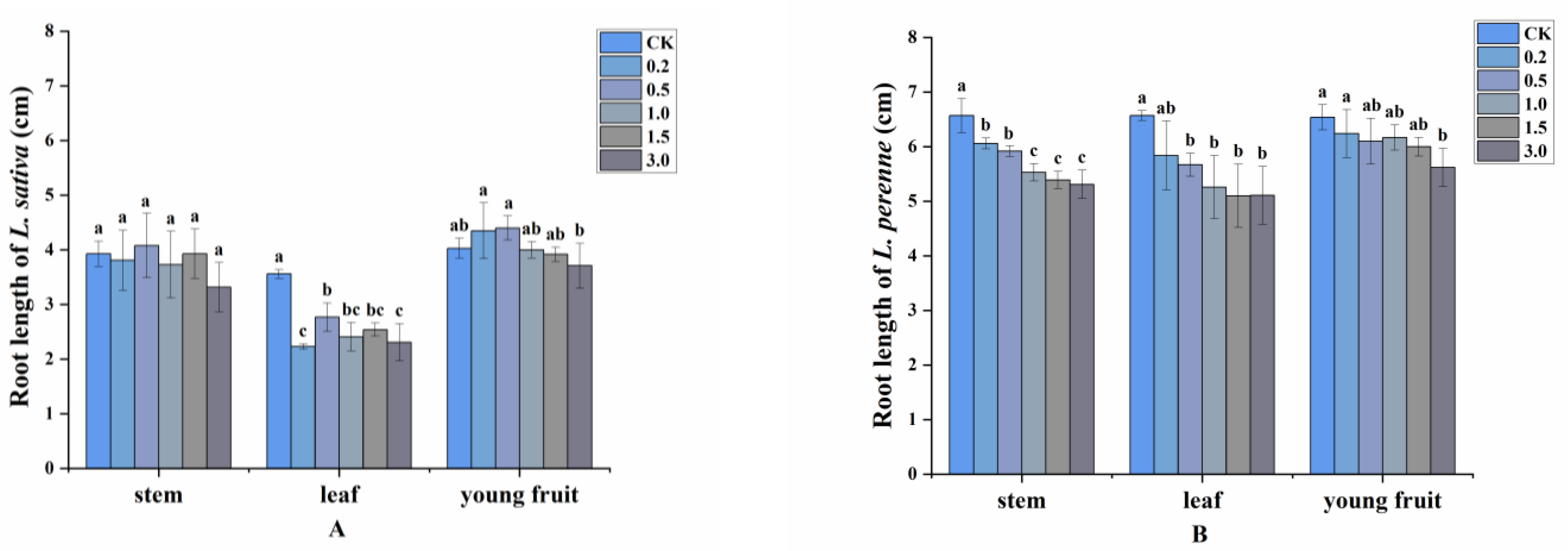
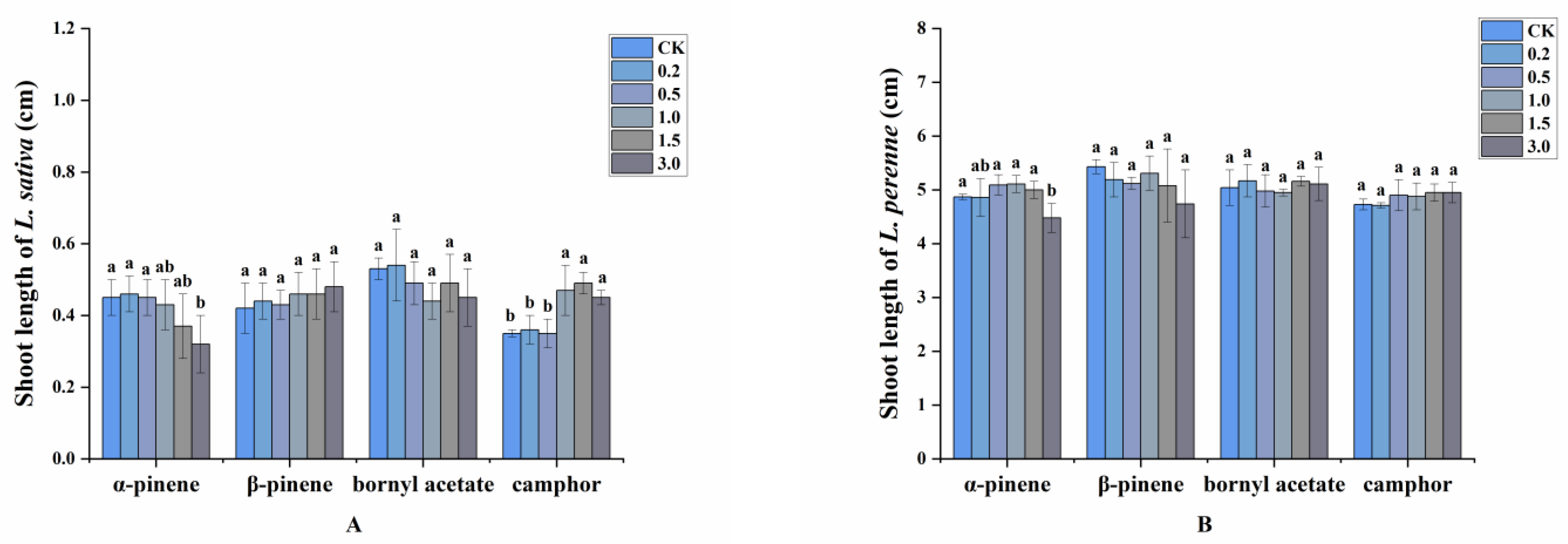
2.3. Allelopathic Effects of the Volatile Oils and Their Main Constituents
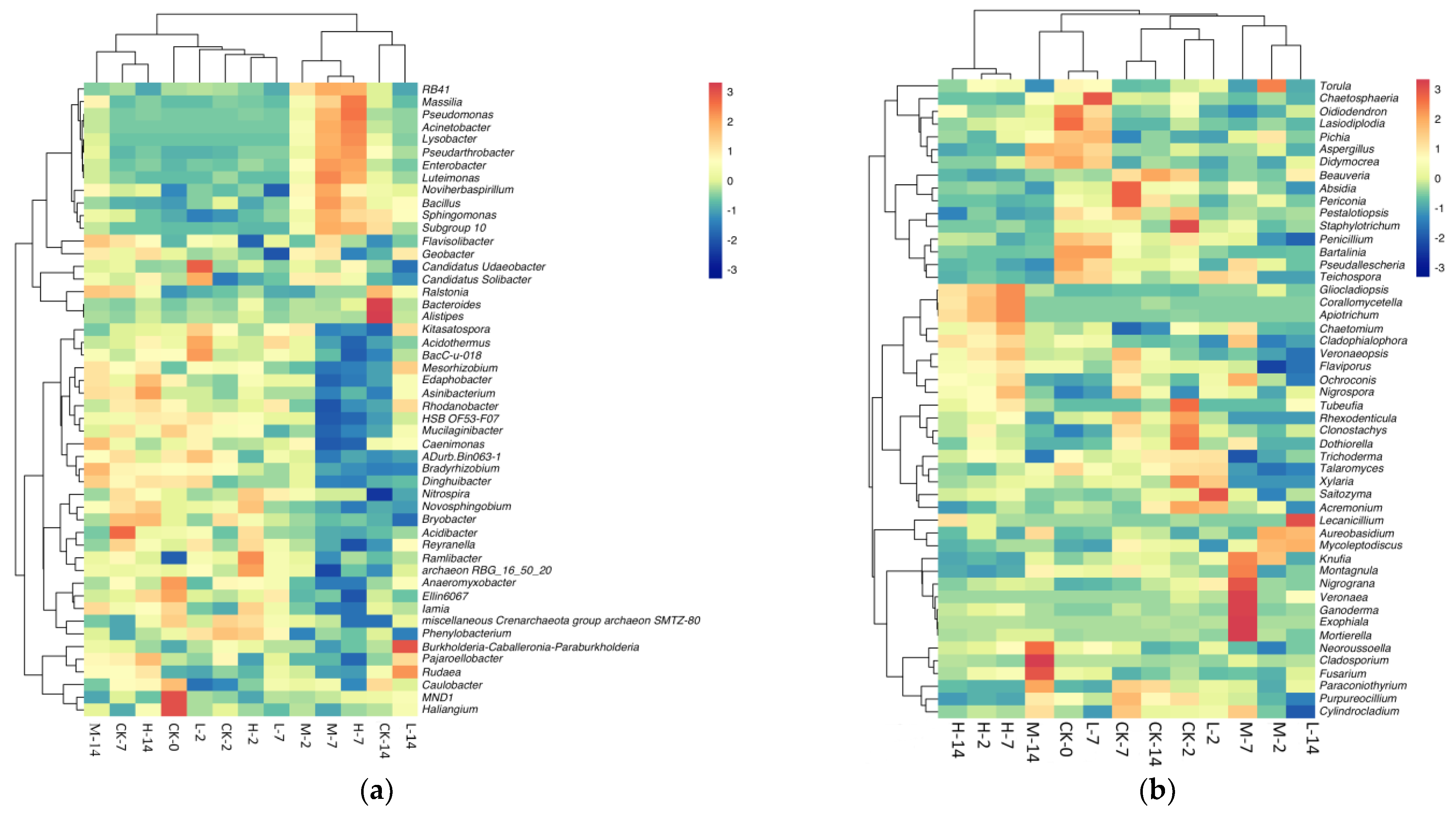
2.4. Effects of Volatile Oil on Soil Microbiome
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Isolation of the Volatile Oils
4.3. Gas Chromatography–Mass Spectrometry Analysis
4.4. Allelopathy of Volatile Compounds
4.5. Allelopathic Effects of the Volatile Oils and Main Compounds
4.6. Effects of the Volatile Oil on Soil Microorganisms
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rice, E.L. Introduction. In Allelopathy, 2nd ed.; Rice, E.L., Ed.; Academic Press: San Diego, CA, USA, 1984; Volume 1, p. 1. ISBN 978-0-12-587055-9. [Google Scholar]
- Inderjit; Keating, K.I. Allelopathy: Principles, Procedures, Processes, and Promises for Biological Control. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: San Diego, CA, USA, 1999; Volume 67, pp. 141–231. [Google Scholar]
- Kessler, A.; Baldwin, I.T. Defensive Function of Herbivore-Induced Plant Volatile Emissions in Nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.B.; Fischer, N.H.; Richardson, D.R.; de la Peña, A. Chemical Inhibition of Fire-Prone Grasses by Fire-Sensitive Shrub,Conradina Canescens. J. Chem Ecol. 1989, 15, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Asensio, D.; Tholl, D.; Wenke, K.; Rosenkranz, M.; Piechulla, B.; Schnitzler, J.P. Biogenic Volatile Emissions from the Soil. Plant Cell Env. 2014, 37, 1866–1891. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.H.; Williamson, G.B.; Weidenhamer, J.D.; Richardson, D.R. In Search of Allelopathy in the Florida Scrub: The Role of Terpenoids. J. Chem. Ecol. 1994, 20, 1355–1380. [Google Scholar] [CrossRef] [PubMed]
- Bozok, F. Herbicidal Activity of Nepeta Flavida Essential Oil. J. Essent. Oil Bear. Plants 2018, 21, 1687–1693. [Google Scholar] [CrossRef]
- Grichi, A.; Nasr, Z.; Khouja, M.L. Phytotoxic Effects of Essential Oil from Eucalyptus Lehmanii against Weeds and Its Possible Use as a Bioherbicide. Bull. Env. Pharm. Life Sci. 2016, 5, 17–23. [Google Scholar]
- Pardavella, I.; Daferera, D.; Tselios, T.; Skiada, P.; Giannakou, I. The Use of Essential Oil and Hydrosol Extracted from Cuminum Cyminum Seeds for the Control of Meloidogyne Incognita and Meloidogyne Javanica. Plants 2020, 10, 46. [Google Scholar] [CrossRef]
- Changbunjong, T.; Boonmasawai, S.; Sungpradit, S.; Weluwanarak, T.; Leesombun, A. Contact and Fumigant Activities of Citrus Aurantium Essential Oil against the Stable Fly Stomoxys Calcitrans (Diptera: Muscidae). Plants 2022, 11, 1122. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, H.P.; Mittal, S.; Batish, D.R.; Kohli, R.K. Phytotoxic Effects of Volatile Oil from Artemisia Scoparia against Weeds and Its Possible Use as a Bioherbicide. Ind. Crops Prod. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Dudai, N.; Poljakoff-Mayber, A.; Mayer, A.M.; Putievsky, E.; Lerner, H.R. Essential Oils as Allelochemicals and Their Potential Use as Bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089. [Google Scholar] [CrossRef]
- Inderjit; Duke, S.O. Ecophysiological Aspects of Allelopathy. Planta 2003, 217, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Ridenour, W.M. Novel Weapons: Invasive Success and the Evolution of Increased Competitive Ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Shao, H.; Huang, X.; Wei, X.; Zhang, C. Phytotoxic Effects and a Phytotoxin from the Invasive Plant Xanthium Italicum Moretti. Molecules 2012, 17, 4037–4046. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Peng, S.; Wei, X.; Zhang, D.; Zhang, C. Potential Allelochemicals from an Invasive Weed Mikania Micrantha HBK. J. Chem. Ecol. 2005, 31, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Vyvyan, J.R. Allelochemicals as Leads for New Herbicides and Agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Poonpaiboonpipat, T.; Krumsri, R.; Kato-Noguchi, H. Allelopathic and Herbicidal Effects of Crude Extract from Chromolaena Odorata (L.) RM King and H. Rob. on Echinochloa crus-galli and Amaranthus viridis. Plants 2021, 10, 1609. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, S.; Saharkhiz, M.J.; Ramezani, F.; Fotokian, M.H. Use of Essential Oils as Bioherbicides. J. Essent. Oil Bear. Plants 2008, 11, 319–327. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxic Effects of Commercial Eucalyptus Citriodora, Lavandula Angustifolia, and Pinus Sylvestris Essential Oils on Weeds, Crops, and Invasive Species. Molecules 2019, 24, 2847. [Google Scholar] [CrossRef]
- Verdeguer, M.; García-Rellán, D.; Boira, H.; Pérez, E.; Gandolfo, S.; Blázquez, M.A. Herbicidal Activity of Peumus Boldus and Drimys Winterii Essential Oils from Chile. Molecules 2011, 16, 403–411. [Google Scholar] [CrossRef]
- Duke, S.O.; Romagni, J.G.; Dayan, F.E. Natural Products as Sources for New Mechanisms of Herbicidal Action. Crop Prot. 2000, 19, 583–589. [Google Scholar] [CrossRef]
- Zhou, S. Cultivation of Amomum Villosum in Tropical Forests. For. Ecol. Manag. 1993, 60, 157–162. [Google Scholar] [CrossRef]
- Sheng-tan, Z.; Zhao-yu, W.; Tie-shan, W.; Miao-xia, L.I.; Jing-ming, L.I.N. Composition and Antimicrobial Activities of Essential Oil of Fructus Amomi. Nat. Prod. Res. Dev. 2011, 23, 462–472. [Google Scholar]
- Wu, X.; Xiao, F.; Zhang, Z.; Li, X.; Xu, Z. Research on the Analgesic Effect and Mechanism of Bornyl Acetate in Volatile Oil from Amomum Villosum. Zhong Yao Cai = Zhongyaocai = J. Chin. Med. Mater. 2005, 28, 505–507. [Google Scholar]
- Wu, X.; Li, X.; Xiao, F.; Zhang, Z.; Xu, Z.; Wang, H. Studies on the Analgesic and Anti-Inflammatory Effect of Bornyl Acetate in Volatile Oil from Amomum Villosum. J. Chin. Med. Mater. 2004, 27, 438–439. [Google Scholar]
- Zhang, T.; Lu, S.H.; Bi, Q.; Liang, L.; Wang, Y.F.; Yang, X.X.; Gu, W.; Yu, J. Volatile Oil from Amomi Fructus Attenuates 5-Fluorouracil-Induced Intestinal Mucositis. Front. Pharmacol. 2017, 8, 786. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, S.; Cao, J.; Pang, X.; Geng, Z.; Wang, Y.; Zhang, Z.; Du, S. Insecticidal and Repellent Activity of Essential Oil from Amomum Villosum Lour. and Its Main Compounds against Two Stored-Product Insects. Int. J. Food Prop. 2018, 21, 2265–2275. [Google Scholar] [CrossRef]
- Liu, H.; Gao, L.; Zheng, Z.; Feng, Z. The Impact of Amomum Villosum Cultivation on Seasonal Rainforest in Xishuangbanna, Southwest China. Biodivers. Conserv. 2006, 15, 2971–2985. [Google Scholar] [CrossRef]
- Dai, N.; Huong, L.; Thang, T.; Ogunwande, I.; Eresanya, O. Chemical Constituents of Essential Oil from the Stem of Amomum Villosum Lour. Trends Phytochem. Res. 2018, 2, 61–64. [Google Scholar]
- Ao, H.; Wang, J.; Chen, L.; Li, S.; Dai, C. Comparison of Volatile Oil between the Fruits of Amomum Villosum Lour. and Amomum Villosum Lour. Var. Xanthioides T. L. Wu et Senjen Based on GC-MS and Chemometric Techniques. Molecules 2019, 24, 1663. [Google Scholar] [CrossRef]
- Dai, D.N.; Huong, L.T.; Thang, T.D.; Ogunwande, I.A. Chemical Composition of Essential Oils of Amomum Villosum Lour. Am. J. Essent. Oil. Nat. Prod. 2016, 4, 08–11. [Google Scholar]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Chao, A.; Yang, M.C.K. Stopping Rules and Estimation for Recapture Debugging with Unequal Failure Rates. Biometrika 1993, 80, 193–201. [Google Scholar] [CrossRef]
- Haegeman, B.; Hamelin, J.; Moriarty, J.; Neal, P.; Dushoff, J.; Weitz, J.S. Robust Estimation of Microbial Diversity in Theory and in Practice. ISME J. 2013, 7, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.J.; Cornell, H.V.; Comita, L.S.; Davies, K.F.; et al. Navigating the Multiple Meanings of β Diversity: A Roadmap for the Practicing Ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef]
- de Melo, S.C.; de Sa, L.E.C.; de Oliveira, H.L.M.; Trettel, J.R.; da Silva, P.S.; Gonçalves, J.E.; Gazim, Z.C.; Magalhães, H.M. Chemical Constitution and Allelopathic Effects of’Curcuma Zedoaria’essential Oil on Lettuce Achenes and Tomato Seeds. Aust. J. Crop Sci. 2017, 11, 906–916. [Google Scholar] [CrossRef]
- Xuan, T.; Quan, N.; Quan, N.; Rayee, R.; Khanh, T.; Tran, H.; Trung, N. Allelopathic Plants: 26. Alpinia zerumbet (Pers.) BL Burtt & RM Sm.(Zingiberaceae). Allelopath. J. 2019, 48, 1–13. [Google Scholar]
- Kumar, N.K.H.; Bharath, N.H.; Shobha, J. Allelopathic Efficacy of Zingiber Officinale Rosc Aqueous Leaf, Stem and Rhizome Extract on Biochemical Parameters of Zea mays L. Res. Plant Biol. 2015, 5, 9–15. [Google Scholar]
- Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Chemical Characterization and Allelopathic Potential of Volatile Oil of Eucalyptus Tereticornis against Amaranthus Viridis. J. Plant Interact. 2011, 6, 297–302. [Google Scholar] [CrossRef][Green Version]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. α-Pinene Inhibits Growth and Induces Oxidative Stress in Roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Chowhan, N.; Singh, H.P.; Batish, D.R.; Kaur, S.; Ahuja, N.; Kohli, R.K. β-Pinene Inhibited Germination and Early Growth Involves Membrane Peroxidation. Protoplasma 2013, 250, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Chowhan, N.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxic Effects of β-Pinene on Early Growth and Associated Biochemical Changes in Rice. Acta Physiol. Plant 2011, 33, 2369–2376. [Google Scholar] [CrossRef]
- Salomé Kpoviessi, D.S.; Gbenou, J.D.; Gbaguidi, F.A.; Ahoussi, L.; Accrombessi, G.C.; Moudachirou, M.; Quetin-Leclercq, J. Justicia anselliana (Nees) T. Anders Essential Oils Compounds and Allelopathic Effects on Cowpea Vigna unguiculata (L.) Walp Plant. J. Essent. Oil Res. 2009, 21, 83–88. [Google Scholar] [CrossRef][Green Version]
- Barney, J.N.; Hay, A.G.; Weston, L.A. Isolation and Characterization of Allelopathic Volatiles from Mugwort (Artemisia vulgaris). J. Chem. Ecol. 2005, 31, 247–265. [Google Scholar] [CrossRef]
- Jiang, C.-Y.; Zhou, S.-X.; Toshmatov, Z.; Mei, Y.; Jin, G.-Z.; Han, C.-X.; Zhang, C.; Shao, H. Chemical Composition and Phytotoxic Activity of the Essential Oil of Artemisia Sieversiana Growing in Xinjiang, China. Nat. Prod. Res. 2022, 36, 2434–2439. [Google Scholar] [CrossRef]
- Najar, B.; Ferri, B.; Cioni, P.L.; Pistelli, L. Volatile Emission and Essential Oil Composition of Sambucus nigra L. Organs during Different Developmental Stages. Plant Biosyst. -Int. J. Deal. All Asp. Plant Biol. 2021, 155, 721–729. [Google Scholar] [CrossRef]
- Vokou, D.; Liotiri, S. Stimulation of Soil Microbial Activity by Essential Oils. Chemoecology 1999, 9, 41–45. [Google Scholar] [CrossRef]
- Jouini, A.; Verdeguer, M.; Pinton, S.; Araniti, F.; Palazzolo, E.; Badalucco, L.; Laudicina, V.A. Potential Effects of Essential Oils Extracted from Mediterranean Aromatic Plants on Target Weeds and Soil Microorganisms. Plants 2020, 9, 1289. [Google Scholar] [CrossRef]
- McBride, S.G.; Osburn, E.D.; Lucas, J.M.; Simpson, J.S.; Brown, T.; Barrett, J.E.; Strickland, M.S. Volatile and Dissolved Organic Carbon Sources Have Distinct Effects on Microbial Activity, Nitrogen Content, and Bacterial Communities in Soil. Microb Ecol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kayanifard, M.; Mohsenzadeh, S. Allelopathic Analysis of Four Ecotypes of Ajowan. Iran. J. Sci. Technol. Trans. A Sci. 2017, 41, 971–978. [Google Scholar] [CrossRef]
- Jurová, J.; Matoušková, M.; Wajs-Bonikowska, A.; Kalemba, D.; Renčo, M.; Sedlák, V.; Gogal’ová, Z.; Poráčová, J.; Šalamún, P.; Grul’ová, D. Potential Phytotoxic Effect of Essential Oil of Non-Native Species Impatiens Parviflora DC. Plants 2019, 8, 241. [Google Scholar] [CrossRef]
- Kong, Q.; Zhou, L.; Wang, X.; Luo, S.; Li, J.; Xiao, H.; Zhang, X.; Xiang, T.; Feng, S.; Chen, T. Chemical Composition and Allelopathic Effect of Essential Oil of Litsea Pungens. Agronomy 2021, 11, 1115. [Google Scholar] [CrossRef]
- Bozok, F.; Cenet, M.; Sezer, G.; Ulukanli, Z. Essential Oil and Bioherbicidal Potential of the Aerial Parts of Nepeta nuda Subsp. albiflora (Lamiaceae). J. Essent. Oil Bear. Plants 2017, 20, 148–154. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume 4, p. 233. [Google Scholar]
- Tang, J.S.; Jiang, C.Y.; Liu, Y.; Zhang, X.Y.; Shao, H.; Zhang, C. Allelopathic Potential of Volatile Organic Compounds Released by Xanthium sibiricum Patrin Ex Widder. Allelopathy J. 2019, 47, 233–242. [Google Scholar] [CrossRef]
- Zhou, S.; Zokir, T.; Mei, Y.; Lei, L.; Shi, K.; Zou, T.; Zhang, C.; Shao, H. Allelopathic Effect of Serphidium kaschgaricum (Krasch.) Poljak. Volatiles on Selected Species. Plants 2021, 10, 495. [Google Scholar] [CrossRef]
- Robertson-Albertyn, S.; Alegria Terrazas, R.; Balbirnie, K.; Blank, M.; Janiak, A.; Szarejko, I.; Chmielewska, B.; Karcz, J.; Morris, J.; Hedley, P.E.; et al. Root Hair Mutations Displace the Barley Rhizosphere Microbiota. Front. Plant Sci. 2017, 8, 1094. [Google Scholar] [CrossRef]
- Alegria Terrazas, R.; Balbirnie-Cumming, K.; Morris, J.; Hedley, P.E.; Russell, J.; Paterson, E.; Baggs, E.M.; Fridman, E.; Bulgarelli, D. A Footprint of Plant Eco-Geographic Adaptation on the Composition of the Barley Rhizosphere Bacterial Microbiota. Sci. Rep. 2020, 10, 12916. [Google Scholar] [CrossRef]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-Coverage ITS Primers for the DNA-Based Identification of Ascomycetes and Basidiomycetes in Environmental Samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]



| Samples | Oil (mg/mL) | Days | Observed Species | Chao1 Index | Shannon Index | |||
|---|---|---|---|---|---|---|---|---|
| Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | |||
| CK-0 | - | 0 | 1461 ± 211 ab | 135 ± 42 bc | 1513.94 ± 219.21 ab | 149.72 ± 53.23 bcd | 5.63 ± 0.43 a | 3.45 ± 0.14 ab |
| CK-2 | 0 | 2 | 1313 ± 89 ab | 127 ± 30 bc | 1349.57 ± 87.86 ab | 146.13 ± 47.40 bcd | 5.49 ± 0.42 a | 2.83 ± 0.35 abc |
| L-2 | 0.5 | 2 | 1472 ± 229 ab | 111 ± 23 bc | 1527.52 ± 235.31 ab | 127.54 ± 31.80 bcd | 5.37 ± 0.83 a | 3.16 ± 0.22 abc |
| M-2 | 1.5 | 2 | 1263 ± 144 ab | 73 ± 8 c | 1295.95 ± 151.30 ab | 78.72 ± 13.43 d | 5.70 ± 0.08 a | 2.46 ± 0.42 abc |
| H-2 | 3.0 | 2 | 1359 ± 230 ab | 153 ± 43 b | 1414.59 ± 246.21 ab | 175.80 ± 53.76 b | 5.59 ± 0.18 a | 3.01 ± 0.84 abc |
| CK-7 | 0 | 7 | 1749 ± 413 a | 107 ± 13 bc | 1825.86 ± 443.70 a | 122.28 ± 12.89 bcd | 5.70 ± 0.34 a | 3.10 ± 0.38 abc |
| L-7 | 0.5 | 7 | 1438 ± 242 ab | 104 ± 22 bc | 1490.16 ± 239.13 ab | 115.49 ± 34.19 bcd | 5.75 ± 0.20 a | 2.60 ± 0.67 abc |
| M-7 | 1.5 | 7 | 1326 ± 513 ab | 132 ± 18 bc | 1358.64 ± 540.61 ab | 156.43 ± 36.37 bcd | 5.22 ± 1.04 a | 2.83 ± 0.64 abc |
| H-7 | 3.0 | 7 | 1499 ± 362 ab | 136 ± 38 bc | 1554.74 ± 390.70 ab | 164.57 ± 49.86 bc | 5.55 ± 0.48 a | 2.24 ± 0.91 bc |
| CK-14 | 0 | 14 | 1363 ± 121 ab | 80 ± 11 c | 1401.94 ± 116.97 ab | 84.71 ± 11.54 cd | 5.79 ± 0.11 a | 2.39 ± 0.37 abc |
| L-14 | 0.5 | 14 | 1027 ± 200 b | 128 ± 14 bc | 1048.92 ± 216.46 b | 146.10 ± 19.68 bcd | 4.82 ± 0.72 a | 3.11 ± 1.22 abc |
| M-14 | 1.5 | 14 | 1332 ± 114 ab | 70 ± 20 c | 1369.43 ± 121.93 ab | 76.80 ± 15.63 d | 5.48 ± 0.30 a | 2.01 ± 1.25 c |
| H-14 | 3.0 | 14 | 1364 ± 389 ab | 228 ± 82 a | 1409.17 ± 402.55 ab | 258.08 ± 84.25 a | 5.59 ± 0.30 a | 3.60 ± 0.24 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, X.; Wang, Y.; Zhao, H.; Li, G.; Wang, Y.; Li, G.; Zhang, L.; Gao, W. Allelopathic Effects of Amomum villosum Lour. Volatiles from Different Organs on Selected Plant Species and Soil Microbiota. Plants 2022, 11, 3550. https://doi.org/10.3390/plants11243550
Zuo X, Wang Y, Zhao H, Li G, Wang Y, Li G, Zhang L, Gao W. Allelopathic Effects of Amomum villosum Lour. Volatiles from Different Organs on Selected Plant Species and Soil Microbiota. Plants. 2022; 11(24):3550. https://doi.org/10.3390/plants11243550
Chicago/Turabian StyleZuo, Xiang, Yanqian Wang, Hongyou Zhao, Guang Li, Yanfang Wang, Ge Li, Lixia Zhang, and Weiwei Gao. 2022. "Allelopathic Effects of Amomum villosum Lour. Volatiles from Different Organs on Selected Plant Species and Soil Microbiota" Plants 11, no. 24: 3550. https://doi.org/10.3390/plants11243550
APA StyleZuo, X., Wang, Y., Zhao, H., Li, G., Wang, Y., Li, G., Zhang, L., & Gao, W. (2022). Allelopathic Effects of Amomum villosum Lour. Volatiles from Different Organs on Selected Plant Species and Soil Microbiota. Plants, 11(24), 3550. https://doi.org/10.3390/plants11243550







