Chemical Survey of Three Species of the Genus Rauhia Traub (Amaryllidaceae)
Abstract
1. Introduction
2. Results and Discussion
2.1. Alkaloid Profiling
2.2. Acetylcholinesterase Inhibition
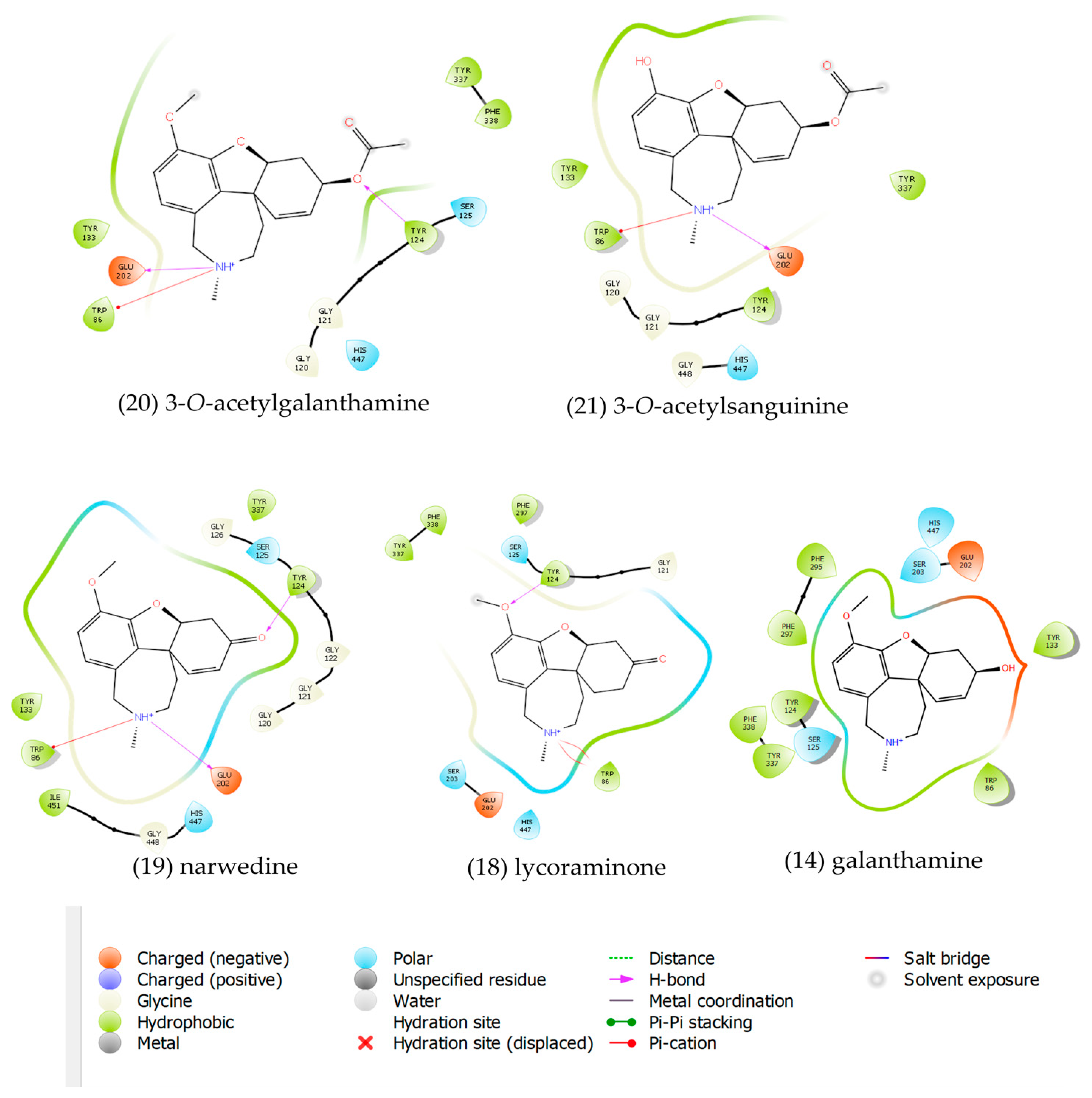
2.3. Molecular Docking
3. Materials and Methods
3.1. Plant Material Voucher
3.2. Extraction
3.3. GC-MS Analysis
3.4. Alkaloid Identification and Quantification
3.5. Enzymatic Assay
3.6. Statistical Analysis
3.7. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization–Biodiversity and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/biodiversity-and-health (accessed on 25 July 2022).
- Newman, D.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Feher, M.; Schimidt, J.M. Property distributions: Differences between drugs, natural products, and molecules from combinatorial chemistry. J. Chem. Inf. Comput. Sci. 2003, 43, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-J.; Bao, J.-L.; Chen, X.-P.; Huang, M.; Wang, Y.-T. Alkaloids isolated from natural herbs as the anticancer agents. Evid. Based. Compl. Alt. 2012, 2012, 485042. [Google Scholar] [CrossRef]
- Bastida, J.; Lavilla, R.; Viladomat, F. Chemical and biological aspects of Narcissus alkaloids. In The Alkaloids: Chemistry and Physiology; Cordell, G.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 63, pp. 87–179. [Google Scholar] [CrossRef]
- Meerow, A.W.; Snijman, D.A. Amaryllidaceae. In Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin, Germany, 1998; Volume 3, pp. 83–110. [Google Scholar]
- Konrath, E.L.; Passos, C.D.S.; Klein-Júnior, L.C.; Henriques, A.T. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2013, 65, 1701–1725. [Google Scholar] [CrossRef]
- Traub, H.P. Genus Rauhia and R. peruviana, gen. & sp. nov. Plant Life 1957, 13, 73–75. [Google Scholar]
- Ravenna, P. Contribution to South American Amaryllidaceae II. Plant Life 1969, 25, 55–76. [Google Scholar]
- Traub, H.P. Amaryllid notes, 1966. Plant Life 1966, 22, 11. [Google Scholar]
- Ravenna, P. Contributions to South American Amaryllidaceae VII. Plant Life 1978, 34, 69–91. [Google Scholar]
- Ravenna, P. Contribution to South American Amaryllidaceae VII [VIII]. Plant Life 1981, 37, 57–83. [Google Scholar]
- Ravenna, P. New Rauhia species from northern Peru. Onira 2002, 7, 11–12. [Google Scholar]
- Meerow, A.W.; Nakamura, K. Two new species of Peruvian Amaryllidaceae, an expanded concept of the genus Paramongaia, and taxonomic notes in Stenomesson. Phytotaxa 2019, 416, 184–196. [Google Scholar] [CrossRef]
- Meerow, A.W.; Gardner, E.M.; Nakamura, K. Phylogenomics of the Andean tetraploid clade of the American Amaryllidaceae (subfamily Amaryllidoideae): Unlocking a polyploid generic radiation abetted by continental geodynamics. Front. Plant Sci. 2020, 11, 582422. [Google Scholar] [CrossRef] [PubMed]
- Meerow, A.W.; Guy, C.L.; Li, Q.B.; Yang, S.L. Phylogeny of the American Amaryllidaceae based on nrDNA ITS sequences. Syst. Bot. 2000, 25, 708–726. [Google Scholar] [CrossRef]
- Berkov, S.; Osorio, E.; Viladomat, F.; Bastida, J. Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 83, pp. 113–185. [Google Scholar] [CrossRef]
- Heinrich, M.; Teoh, H.L. Galanthamine from snowdrop—The development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J. Ethnopharmacol. 2004, 92, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Maelicke, A.; Samochocki, M.; Jostock, R.; Fehrenbacher, A.; Ludwig, J.; Albuquerque, E.X.; Zerlin, M. Allosteric sensitization of nicotinic receptors by galanthamine, a new treatment strategy for Alzheimer’s disease. Biol. Psychiatry 2001, 49, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Georgieva, L.; Boriana, S.; Bastida, J. Evaluation of Hippeastrum papilio (Ravenna) Van Scheepen potencial as a new industrial source of galanthamine. Ind. Crops Prod. 2022, 178, 114619. [Google Scholar] [CrossRef]
- Berkov, S.; Bastida, J.; Codina, C.; de Andrade, J.P.; Berbee, R.L.M. Extract of Hippeastrum papilio rich in galanthamine. EP2999480B1, 7 March 2013. Available online: https://patents.google.com/patent/EP2999480B1/en (accessed on 28 July 2022).
- Chang, X. Lycoris, the basis of the galanthamine industry in China. Res. Rev. J. Agric. Allied Sci. 2015, 4, 1–8. [Google Scholar]
- Nair, J.J.; Van Staden, J. Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae. Nat. Prod. Commun. 2014, 9, 1193–1210. [Google Scholar] [CrossRef]
- Nair, J.J.; Rárová, L.; Strnad, M.; Bastida, J.; Van Staden, J. Mechanistic insights to the cytotoxicity of Amaryllidaceae alkaloids. Nat. Prod. Commun. 2015, 10, 171–182. [Google Scholar] [CrossRef]
- Kaur, H.; Chahal, S.; Jha, P.; Lekhak, M.M.; Shekhawat, M.S.; Naidoo, D.; Arencibia, A.D.; Ochatt, S.J.; Kumar, V. Harnessing plant biotechnology-based strategies for in vitro galanthamine (GAL) biosynthesis: A potent drug against Alzheimer’s disease. Plant Cell. Tiss. Org. 2022, 149, 81–103. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Garro, A.; Pigni, N.B.; Agüero, M.B.; Roitman, G.; Slanis, A.; Enriz, R.D.; Feresin, G.E.; Bastida, J.; Tapia, A. Cholinesterase-inhibitory effect and in silico analysis of alkaloids from bulbs of Hieronymiella species. Phytomedicine 2018, 39, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Šafratová, M.; Hošt’álková, A.; Hulcová, D.; Breiterová, K.; Hrabcová, V.; Machado, M.; Fontinha, D.; Prudêncio, M.; Kuneš, J.; Chlebek, J.; et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch. Pharm. Res. 2018, 41, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Hulcová, D.; Maříková, J.; Korábečný, J.; Hošťálková, A.; Jun, D.; Kuneš, J.; Chlebek, J.; Opletal, L.; De Simone, A.; Nováková, L.; et al. Amaryllidaceae alkaloids from Narcissus pseudonarcissus L. cv. Dutch Master as potential drugs in treatment of Alzheimer’s disease. Phytochemistry 2019, 165, 112055. [Google Scholar] [CrossRef] [PubMed]
- Cortes, N.; Alvarez, R.; Osorio, E.H.; Alzate, F.; Berkov, S.; Osorio, E. Alkaloid metabolite profiles by GC/MS and acetylcholinesterase inhibitory activities with binding-mode predictions of five Amaryllidaceae plants. J. Pharmaceut. Biomed. 2015, 102, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Cortes, N.; Posada-Duque, R.A.; Alvarez, R.; Alzate, F.; Berkov, S.; Cardona-Gómez, G.P.; Osorio, E. Neuroprotective activity and acetylcholinesterase inhibition of five Amaryllidaceae species: A comparative study. Life Sci. 2015, 122, 42–50. [Google Scholar] [CrossRef]
- Cortes, N.; Sierra, K.; Alzate, F.; Osorio, E.H.; Osorio, E. Alkaloids of Amaryllidaceae as inhibitors of cholinesterases (AChEs and BChEs): An integrated bioguided study. Phytochem. Anal. 2018, 29, 217–227. [Google Scholar] [CrossRef]
- Trujillo-Chacón, L.M.; Alarcón-Enos, J.E.; Céspedes-Acuña, C.L.; Bustamante, L.; Baeza, M.; López, M.G.; Fernández-Mendívil, C.; Cabezas, F.; Pastene—Navarrete, E.R. Neuroprotective activity of isoquinoline alkaloids from Chilean Amaryllidaceae plants against oxidative stress-induced cytotoxicity on human neuroblastoma SH-SY5Y cells and mouse hippocampal slice culture. Food Chem. Toxicol. 2019, 132, 110665. [Google Scholar] [CrossRef]
- Moreno, R.; Tallini, L.R.; Salazar, C.; Osorio, E.H.; Montero, E.; Bastida, J.; Oleas, N.H.; León, K.A. Chemical profiling and cholinesrerase inhibitory activity of five Phaedranassa Herb. (Amaryllidaceae) species from Ecuador. Molecules 2020, 25, 2092. [Google Scholar] [CrossRef]
- Acosta, K.L.; Inca, A.; Tallini, L.R.; Osorio, E.H.; Robles, J.; Bastida, J.; Oleas, N.H. Alkaloids of Phaedranassa dubia (Kunth) J.F. Macbr. and Phaedranassa brevifolia Meerow (Amaryllidaceae) from Ecuador and its cholinesterase-inhibitory activity. S. Afr. J. Bot. 2021, 136, 91–99. [Google Scholar] [CrossRef]
- Tallini, L.R.; Carrasco, A.; Acosta, K.L.; Vinueza, D.; Bastida, J.; Oleas, N.H. Alkaloid profiling and cholinesterase inhibitory potential of Crinum x amabile Donn. (Amaryllidaceae) collected in Ecuador. Plants 2021, 10, 2686. [Google Scholar] [CrossRef]
- Soto-Vásquez, M.R.; Rodríguez-Muñoz, C.A.; Tallini, L.R.; Bastida, J. Alkaloid composition and biological activities of the Amaryllidaceae species Ismene amancaes (Ker Gawl.) Herb. Plants 2022, 11, 1906. [Google Scholar] [CrossRef] [PubMed]
- Tallini, L.R.; Bastida, J.; Cortes, N.; Osorio, E.H.; Theoduloz, C.; Schmeda-Hirschmann, G. Cholinesterase inhibition activity, alkaloid profiling, and molecular docking of Chilean Rhodophiala (Amaryllidaceae). Molecules 2018, 23, 1532. [Google Scholar] [CrossRef] [PubMed]
- Moraga-Nicolás, F.; Jara, C.; Godoy, R.; Iturriaga-Vásquez, P.; Venthur, H.; Quiroz, A.; Becerra, J.; Mutis, A.; Hormazábal, E. Rhodolirium andicola: A new renewable source of alkaloids with acetylcholinesterase inhibitory activity, a study from nature to molecular docking. Rev. Bras. Farmacogn. 2018, 28, 34–43. [Google Scholar] [CrossRef]
- Fernández-Galleguillos, C.; Romero-Parra, J.; Puerta, A.; Padrón, J.M.; Simirgiotis, M.J. Alkaloid profiling, anti-enzymatic and antiproliferative activity of the endemic Chilean Amaryllidaceae Phycella cyrtanthoides. Metabolites 2022, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Del Rojas-Vera, J.C.; Buitrago-Díaz, A.A.; Possamai, L.M.; Timmers, L.F.S.M.; Tallini, L.R.; Bastida, J. Alkaloid profile and cholinesterase inhibition activity of five species of Amaryllidaceae family collected from Mérida state-Venezuela. S. Afri. J. Bot. 2021, 136, 126–136. [Google Scholar] [CrossRef]
- De Andrade, J.P.; Giordani, R.B.; Torras-Claveria, L.; Pigni, N.B.; Berkov, S.; Font-Bardia, M.; Calvet, T.; Konrath, E.; Bueno, K.; Sachett, L.G.; et al. The Brazilian Amaryllidaceae as a source of aceylcholinesterase inhibitoy alkaloids. Phytochem. Rev. 2016, 15, 147–160. [Google Scholar] [CrossRef]
- Gasca, C.A.; Moreira, N.C.S.; de Almeida, F.C.; Gomes, J.V.D.; Castillo, W.O.; Fagg, C.W.; Magalhaes, P.O.; Fonseca-Bazzo, Y.M.; Sakamoo-Hojo, E.; de Medeiros, Y.K.; et al. Aceylcholinesterase inhibitory activity, anti-inflammaory, and neuroprotective potential of Hippeastrum psittacinum (Ker Gawl.) Herb (Amaryllidaceae). Food Chem. Toxicol. 2000, 145, 111703. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Pigni, N.B.; Andujar, S.A.; Roitman, G.; Suvire, F.D.; Enriz, R.D.; Tapia, A.; Basida, J.; Feresin, G.E. Alkaloids from Hippeastrum argentinum and their cholinesterase-inhibitory activities: An in vitro and in silico study. J. Nat. Prod. 2016, 79, 1241–1248. [Google Scholar] [CrossRef]
- Zaragoza-Puchol, D.; Ortiz, J.E.; Orden, A.A.; Sanchez, M.; Palermo, J.; Tapia, A.; Bastida, J.; Feresin, G.E. Alkaloids analysis of Habranthus cardanasianus (Amaryllidaceae), anti-cholinesterase activity and biomass production by propagation strategies. Molecules 2021, 26, 192. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Berkov, S.; Pigni, N.B.; Theoduloz, C.; Roitman, G.; Tapia, A.; Bastida, J.; Feresin, G.E. Wild Argentinian Amaryllidaceae, a new renewable source of the acetylcholinesterase inhibitor galanthamine and other alkaloids. Molecules 2012, 17, 13473–13482. [Google Scholar] [CrossRef]
- García, N.; Meerow, A.W.; Arroyo-Leuenberger, S.; Oliveira, R.S.; Dutilh, J.H.; Soltis, P.S.; Judd, W.S. Generic classification of Amaryllidaceae tribe Hippeastreae. Taxon 2019, 68, 481–498. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Gary, E.N.; Shiomi, K.; Rosenberry, T.L. Structures of human acetylcholinesterase bound to dihydrotanshinone I and territrem B show peripheral site flexibility. ACS Med. Chem. Lett. 2013, 4, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Sierra, K.; de Andrade, J.P.; Tallini, L.R.; Osorio, E.H.; Yañéz, O.; Osorio, M.I.; Oleas, N.H.; García-Beltrán, O.; de Borges, W.S.; Bastida, J.; et al. In vitro and in silico analysis of galanthine from Zephyranthes carinata as an inhibitor of acetylcholinesterase. Biomed. Pharmacother. 2022, 150, 113016. [Google Scholar] [CrossRef] [PubMed]
- Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Daffodils as potential crops of galanthamine. Assessment of more than 100 ornamental varieties for their alkaloid content and acetylcholinesterase inhibitory activity. Ind. Crops Prod. 2013, 43, 237–244. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Schrödinger Release 2022-3: Maestro; Schrödinger, Inc.: New York, NY, USA, 2021.
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckinan genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1999, 19, 1639–1662. [Google Scholar] [CrossRef]
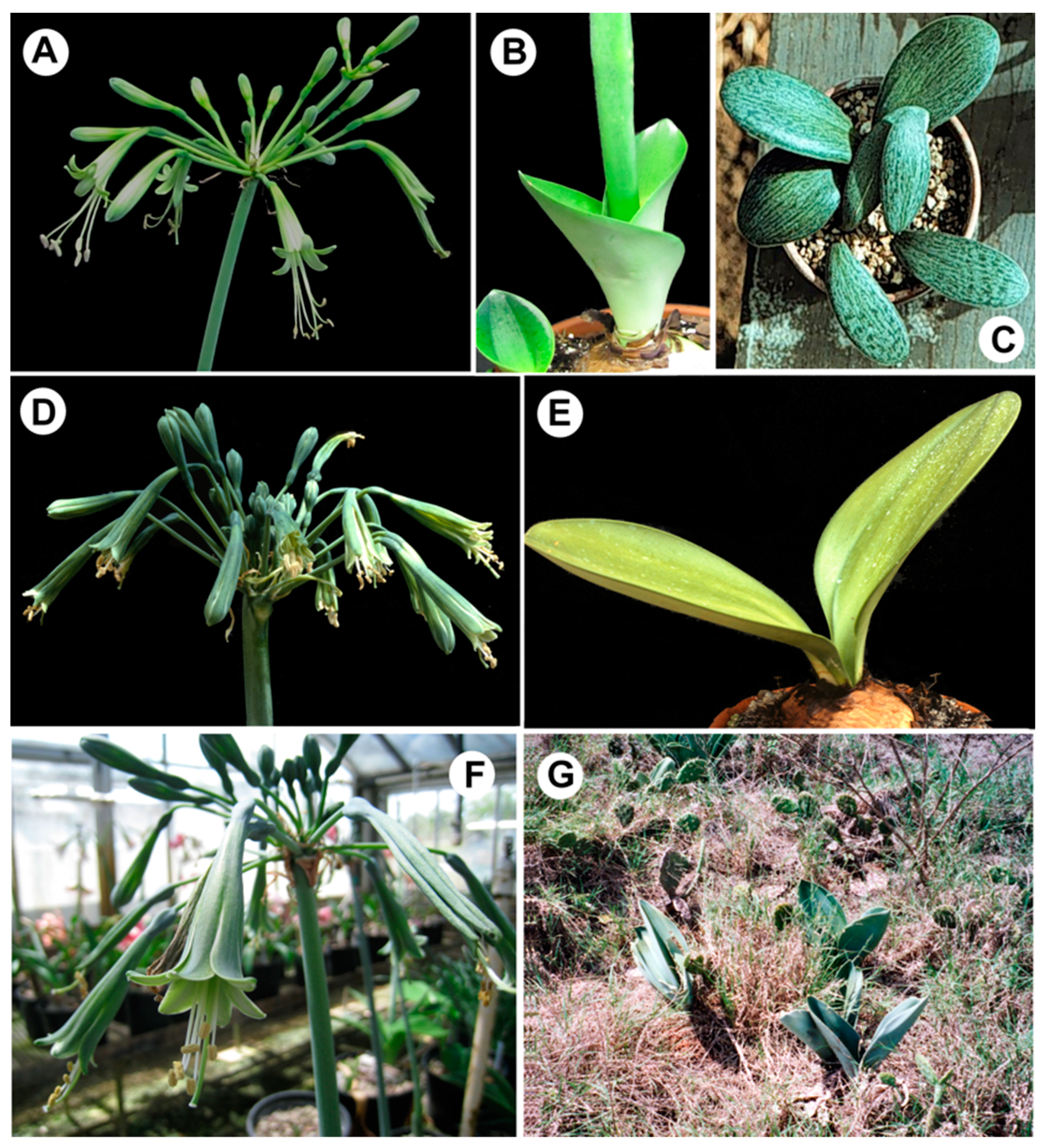
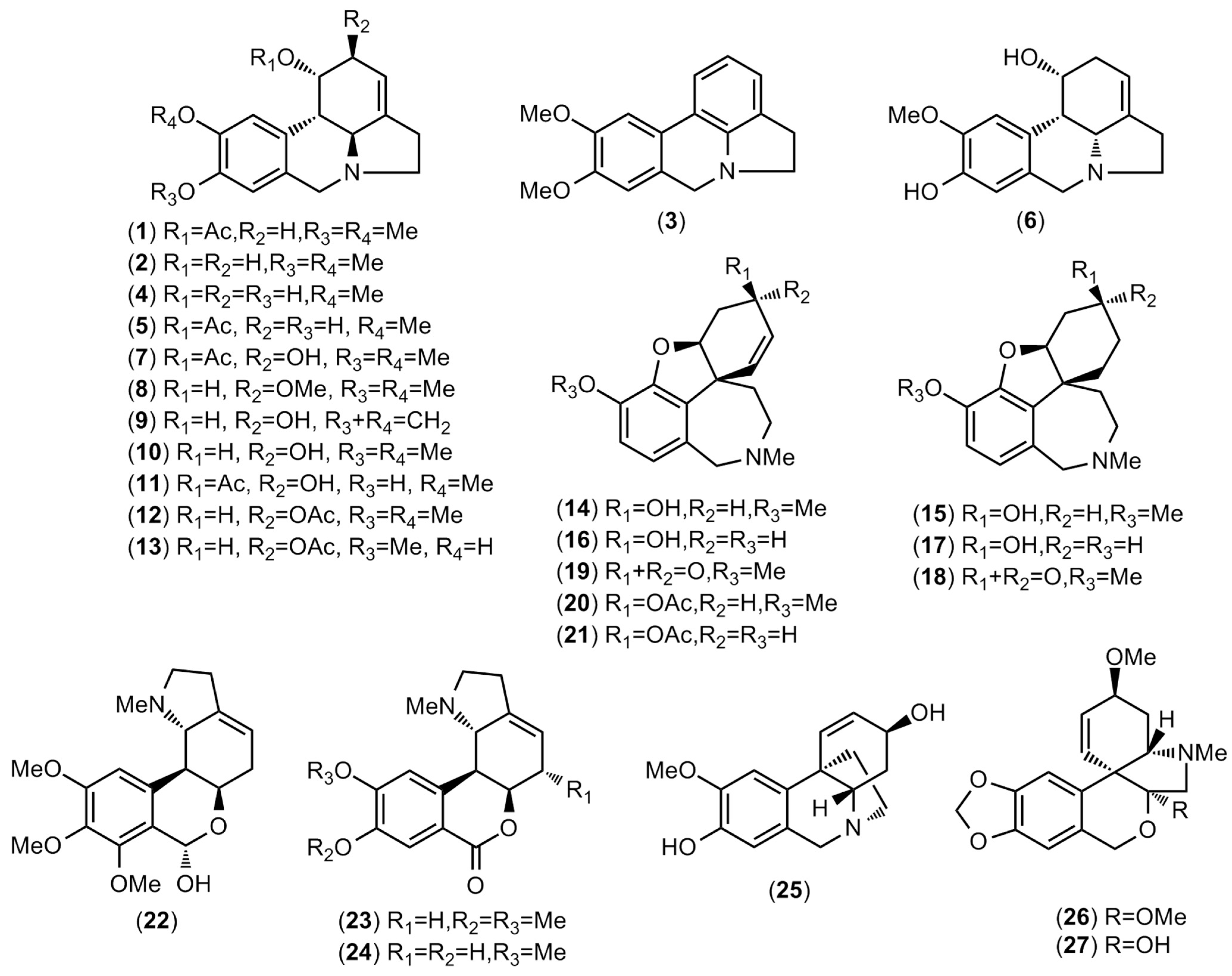
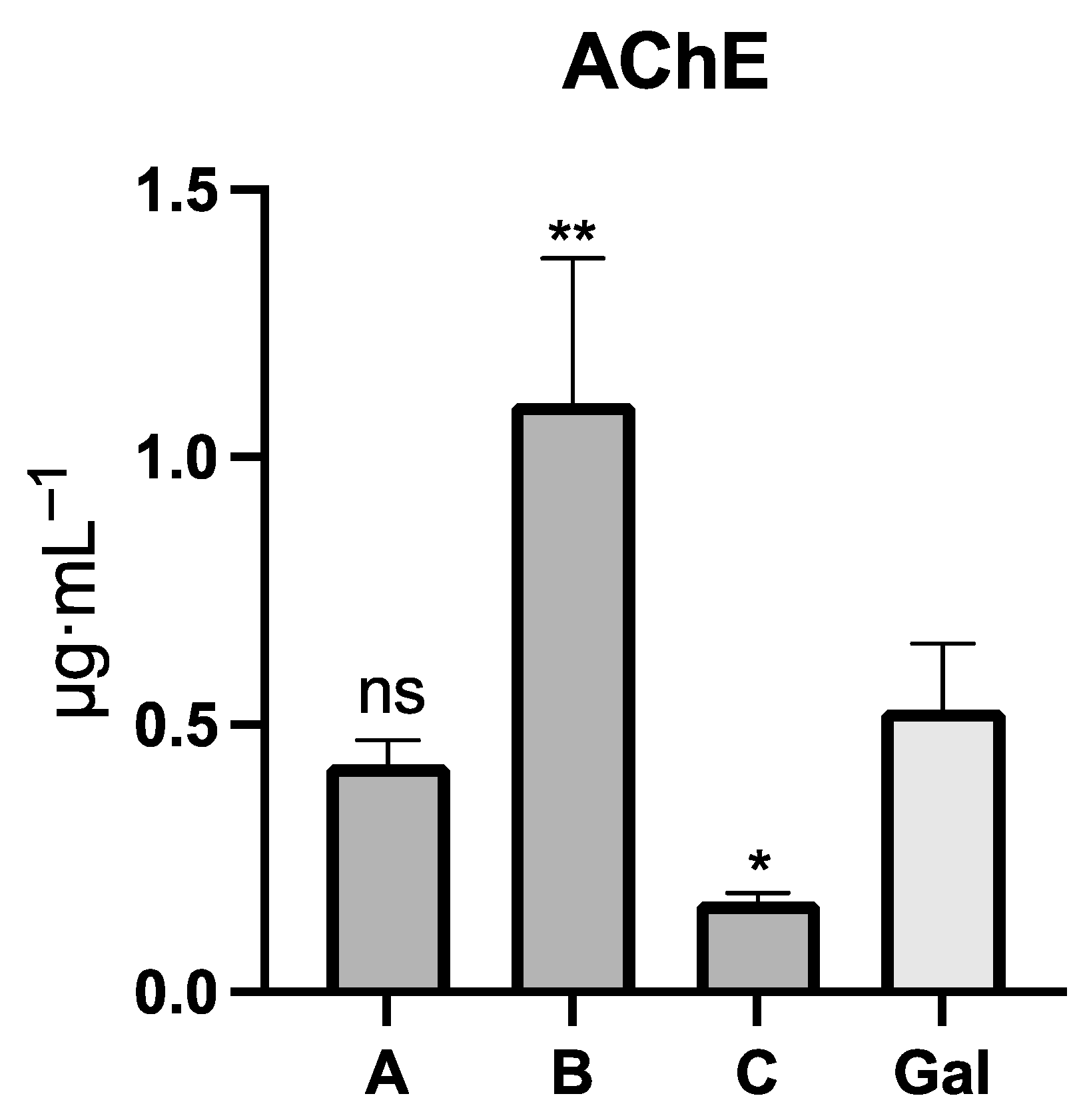
| Alkaloid | [M]+ | m/z | RI | A 1 | A 2 | B 1 | B 2 | C 1 | C 2 |
|---|---|---|---|---|---|---|---|---|---|
| Lycorine-type | 317.5 | 314.5 | |||||||
| 1-O-acetylpluviine (1) | 329 (80) | 268 (85), 242 (100) | 2598.0 | - | - | 10.1 | 0.1 | - | - |
| pluviine (2) | 287 (78) | 286 (52), 268 (55), 243 (61), 242 (100) | 2608.2 | 16.1 | 1.4 | 69.2 | 11.9 | - | - |
| assoanine (3) | 267 (57) | 266 (100), 250 (28), 222 (12), 180 (13) | 2622.3 | 21.6 | 3.3 | 24.0 | 2.9 | - | - |
| norpluviine (4) | 273 (80) | 254 (60), 228 (100) | 2635.7 | - | - | 10.3 | 0.3 | - | - |
| 1-O-acetylnorpluviine (5) | 315 (80) | 254 (90), 228 (100) | 2641.5 | - | - | 16.0 | 2.4 | - | - |
| kirkine (6) | 273 (<1) | 253 (55), 252 (100), 237 (21), 209 (22) | 2642.2 | 13.9 | 1.4 | - | - | - | - |
| 1-O-acetyl-9-O-methylpseudolycorine (7) | 345 (30) | 284 (25), 242 (100) | 2769.3 | - | - | 10.5 | 0.3 | - | - |
| galanthine (8) | 317 (20) | 298 (10), 268 (15), 242 (100), 228 (5) | 2775.9 | 21.6 | 3.3 | - | - | - | - |
| lycorine (9) | 287 (30) | 268 (27), 250 (15), 226 (100), 147 (15) | 2789.3 | - | - | 10.1 | 0.1 | - | - |
| 9-O-methylpseudolycorine (10) | 303 (33) | 302 (22), 284 (14), 243 (78), 242 (100) | 2830.1 | 11.4 | 1.1 | 17.9 | 2.4 | - | - |
| sternbergine (11) | 331 (41) | 270 (32), 252 (14), 229 (72), 228 (100) | 2844.1 | 25.8 | 17.4 | 10.8 | 0.6 | - | - |
| 2-O-acetyl-9-O-methylpseudolycorine (12) | 345 (30) | 284 (100), 268 (40), 242 (40) | 2907.3 | 168.9 | 32.5 | 135.6 | 16.6 | - | - |
| 2-O-acetylpseudolycorine (13) | 331 (30) | 270 (100), 254 (75), 228 (80) | 2945.1 | 38.2 | 15.4 | - | - | - | - |
| Galanthamine-type | 10.0 | 10.8 | 278.7 | ||||||
| galanthamine (14) | 287 (94) | 286 (100), 270 (25), 244 (42), 216 (49) | 2437.0 | - | - | - | - | 103.6 | 48.3 |
| lycoramine (15) | 289 (78) | 288 (100), 232 (14), 202 (22), 187 (18) | 2459.4 | 10.0 | 0.1 | 10.8 | 0.4 | 73.1 | 10.3 |
| sanguinine (16) | 273 (100) | 272 (81), 256 (23), 230 (16), 202 (44) | 2476.2 | - | - | - | - | 21.7 | 5.3 |
| O-demethyllycoramine (17) | 275 (67) | 274 (100), 218 (8), 174 (13), 173 (17) | 2487.6 | - | - | - | - | 23.4 | 5.9 |
| lycoraminone (18) | 287 (68) | 286 (100), 244 (5), 218 (17), 202 (23) | 2491.6 | - | - | - | - | 10.3 | 0.3 |
| narwedine (19) | 285 (86) | 284 (100), 216 (25), 199 (24), 174 (43) | 2517.5 | - | - | - | - | 17.3 | 1.8 |
| 3-O-acetylgalanthamine (20) | 329 (34) | 328 (31), 270 (100), 216 (31), 165 (17) | 2577.2 | - | - | - | - | 13.0 | 0.9 |
| 3-O-acetylsanguinine (21) | 315 (46) | 256 (100), 255 (59), 254 (40), 212 (29) | 2584.6 | - | - | - | - | 16.3 | 1.4 |
| Homolycorine-type | 57.0 | 42.0 | |||||||
| nerinine (22) | 347 (<1) | 110 (8), 109 (100), 108 (18) | 2511.4 | 11.0 | 0.2 | 18.8 | 1.6 | - | - |
| homolycorine (23) | 315 (<1) | 110 (11), 109 (100), 108 (30) | 2785.4 | 19.2 | 2.5 | 10.0 | 0.1 | - | - |
| 8-O-demethylhomolycorine (24) | 301 (<1) | 110 (23), 109 (100), 108 (53) | 2847.6 | 26.8 | 4.1 | 13.2 | 1.3 | - | - |
| Haemanthamine -type | 11.0 | ||||||||
| 8-O-demethylmaritidine (25) | 273 (100) | 230 (24), 202 (27), 201 (93), 189 (60) | 2549.8 | - | - | 11.0 | 0.4 | - | - |
| Pretazettine-type | 11.7 | 9.9 | |||||||
| O-methyltazettine (26) | 345 (30) | 330 (30), 314 (25), 261 (100), 239 (25) | 2643.2 | - | - | - | - | 9.9 | 0.2 |
| tazettine (27) | 331 (24) | 316 (13), 298 (20), 247 (100), 70 (26) | 2686.1 | - | - | 11.7 | 1.2 | - | - |
| Unidentified | 24.8 | 11.2 | |||||||
| UI 1 (HLY type) (28) | 329 (<1) | 221 (<1), 109 (100) | 2510.8 | - | - | 11.2 | 1.1 | - | - |
| UI 2 (HLY type) (29) | 330 (<1) | 221 (<1), 109 (100) | 2555.9 | 13.4 | 1.2 | - | - | - | - |
| UI 3 (30) | 325 (40) | 282 (100), 266 (10), 139 (60) | 2989.5 | 11.4 | 0.3 | - | - | - | - |
| Total: | 409.3 | 401.2 | 288.6 |
| alkaloid | 4EY5 | 4EY6 | 4EY7 | 4M0E | 4M0F |
|---|---|---|---|---|---|
| 3-O-acetylgalanthamine (20) | −9.08 | −9.77 | −11.25 | −8.57 | −9.93 |
| 3-O-acetylsanguinine (21) | −8.75 | −9.76 | −10.55 | −8.42 | −10.11 |
| narwedine (19) | −9.15 | −9.70 | −10.41 | −8.69 | −9.72 |
| lycoraminone (18) | −9.70 | −9.48 | −9.37 | −9.10 | −9.25 |
| lycoramine (15) | −8.84 | −9.08 | −8.87 | −8.64 | −8.41 |
| O-demethyllycoramine (17) | −8.74 | −9.08 | −8.91 | −8.66 | −8.40 |
| sanguinine (16) | −8.13 | −8.54 | −9.14 | −8.50 | −9.12 |
| galanthamine (14) | −8.59 | −8.75 | −9.83 | −7.90 | −8.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tallini, L.R.; Osorio, E.H.; Berkov, S.; Torras-Claveria, L.; Rodríguez-Escobar, M.L.; Viladomat, F.; Meerow, A.W.; Bastida, J. Chemical Survey of Three Species of the Genus Rauhia Traub (Amaryllidaceae). Plants 2022, 11, 3549. https://doi.org/10.3390/plants11243549
Tallini LR, Osorio EH, Berkov S, Torras-Claveria L, Rodríguez-Escobar ML, Viladomat F, Meerow AW, Bastida J. Chemical Survey of Three Species of the Genus Rauhia Traub (Amaryllidaceae). Plants. 2022; 11(24):3549. https://doi.org/10.3390/plants11243549
Chicago/Turabian StyleTallini, Luciana R., Edison H. Osorio, Strahil Berkov, Laura Torras-Claveria, María L. Rodríguez-Escobar, Francesc Viladomat, Alan W. Meerow, and Jaume Bastida. 2022. "Chemical Survey of Three Species of the Genus Rauhia Traub (Amaryllidaceae)" Plants 11, no. 24: 3549. https://doi.org/10.3390/plants11243549
APA StyleTallini, L. R., Osorio, E. H., Berkov, S., Torras-Claveria, L., Rodríguez-Escobar, M. L., Viladomat, F., Meerow, A. W., & Bastida, J. (2022). Chemical Survey of Three Species of the Genus Rauhia Traub (Amaryllidaceae). Plants, 11(24), 3549. https://doi.org/10.3390/plants11243549












