Cytosolic Sodium Influx in Mesophyll Protoplasts of Arabidopsis thaliana, wt, sos1:1 and nhx1 Differs and Induces Different Calcium Changes
Abstract
1. Introduction
1.1. Sodium Uptake and Transport
1.2. Calcium Transport and Signalling under Salinity
2. Results
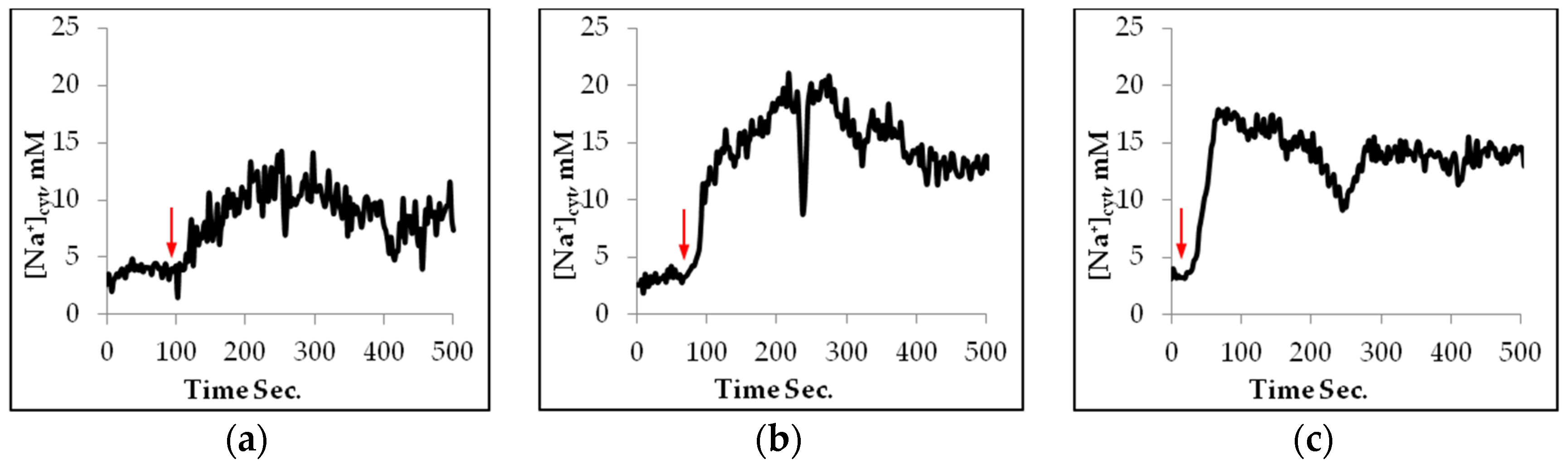
2.1. Sodium Uptake in Mesophyll Protoplasts
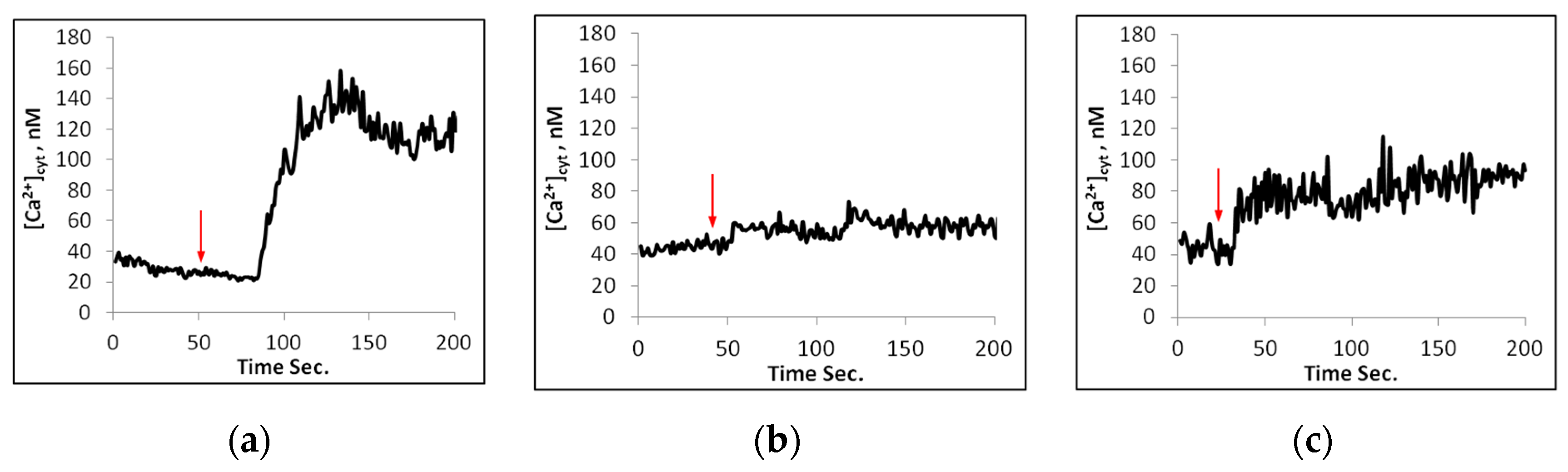
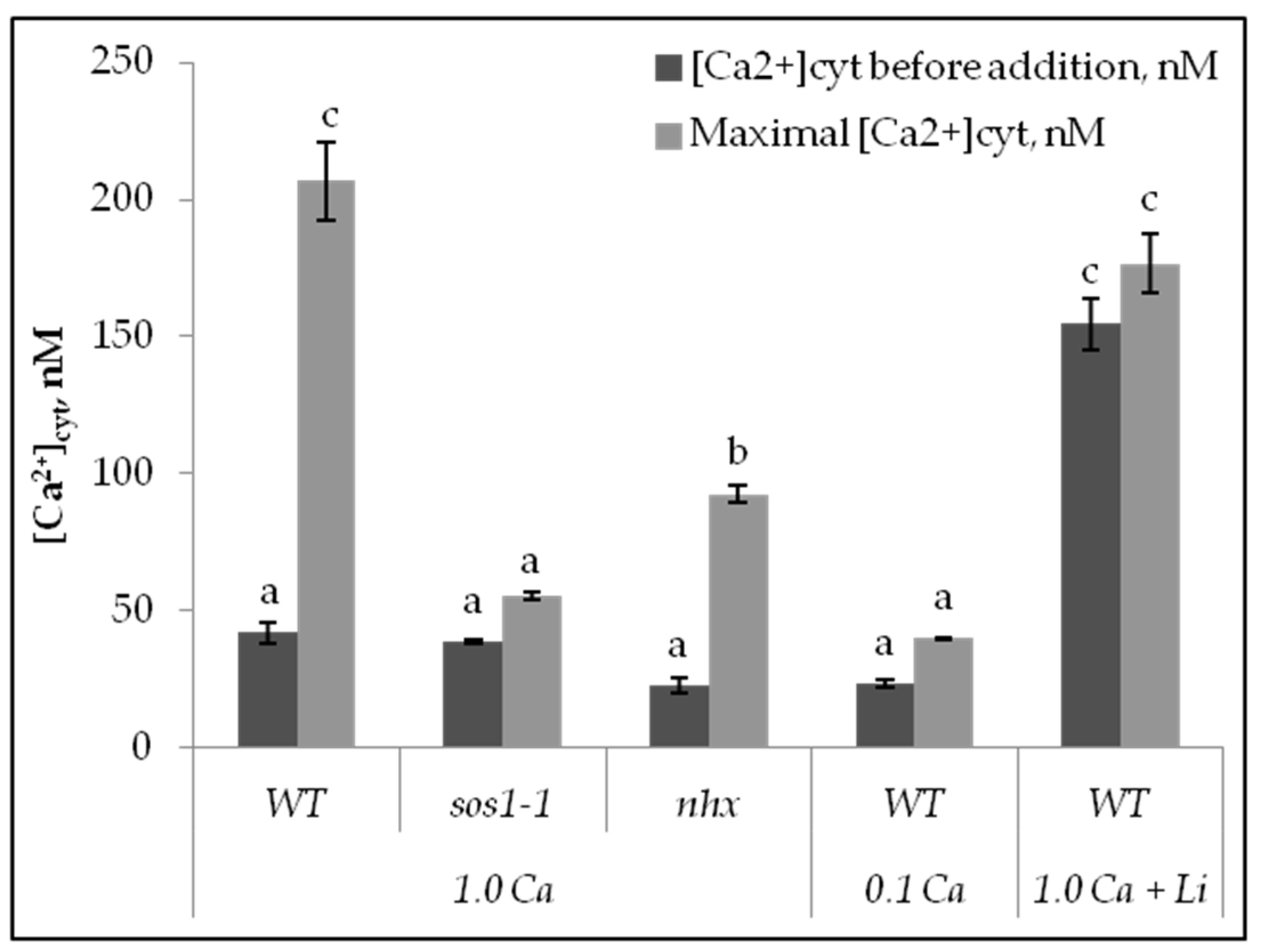
2.2. Calcium Changes in Mesophyll Protoplasts upon NaCl Addition
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Protoplast Isolation
4.3. Dye Loading and Fluorescence Measurements
4.4. Fluorescence Measurements
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the costs? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Keisham, M.; Mukherjee, S.; Bhatla, S. Mechanisms of sodium transport in plants—Progresses and challenges. Int. J. Mol. Sci. 2018, 19, 647. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Guo, Q.; Meng, L.; Han, J.; Mao, P.; Tian, X.; Zheng, M.; Mur, L.A.J. SOS1 is a key systemic regulator of salt secretion and K+/Na+ homeostasis in the recretohalophyte Karelinia caspia. Environ. J. Exp. Bot. 2020, 177, 104098. [Google Scholar] [CrossRef]
- Pardo, J.M.; Cubero, B.; Leidi, E.O.; Quintero, F.J. Alkali cation exchangers: Roles on cellular homeostasis and stress tolerance. J. Exp. Bot. 2006, 57, 1181–1199. [Google Scholar] [CrossRef]
- Apse, M.P.; Blumwald, E. Na+ transport in plants. FEBS Lett. 2007, 581, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Brini, F.; Hanin, M.; Mezghani, I.; Gerald, A.; Berkowitz, G.A.; Masmoudi, K. Overexpression of wheat Na+/H+ antiporter TNHX1 and H+-pyrophosphatase TVP1 improve salt- and drought-stress tolerance in Arabidopsis thaliana plants. J. Exp. Bot. 2007, 98, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.; Beyhlb, D.; Esther Görlich, E.; Al-Rasheid, K.A.S.; Marten, I.; Stierhofd, Y.-D.; Hedrich, R.; Schumacher, K. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc. Natl. Acad. Sci. USA 2010, 107, 3251–3256. [Google Scholar] [CrossRef]
- Andres, Z.; Perez-Hormaeche, J.; Leidi, E.O.; Schlucking, K.; Steinhorst, L.; McLachlan, D.H.; Schumacher, K.; Hetherington, A.M.; Kudla, J.; Cubero, B.; et al. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc. Natl. Acad. Sci. USA 2014, 111, E1806–E1814. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, P.; Zhu, R.; Fu, J.; Su, J.; Zheng, J.; Wang, Z.; Wang, D.; Gong, Q. Autophagy is rapidly induced by salt stress and is required for salt tolerance in Arabidopsis. Front. Plant Sci. 2017, 8, 1459. [Google Scholar] [CrossRef]
- Köster, P.; Wallrad, L.; Edel, K.H.; Faisal, M.; Alatar, A.A.; Kudla, J. The battles of two ions: Ca2+ signalling against Na+ stress. Plant Biol. 2018, 21, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guan, C.; Wang, P.; Ma, Q.; Bao, A.-K.; Zhang, J.-L.; Wan, S.-M. The Effect of AtHKT1;1 or AtSOS1 mutation on the expressions of Na+ or K+ transporter genes and ion homeostasis in Arabidopsis thaliana under salt stress. Int. J. Mol. Sci. 2019, 20, 1085. [Google Scholar] [CrossRef] [PubMed]
- Nayef, M.A.; Celymar, S.; Shabala, L.; Ogura, T.; Chen, Z.; Bose, J.; Maathuis, F.J.M.; Venkataraman, G.; Tanoi, K.; Yu, M.; et al. Changes in expression level of OsHKT1; 5 alters activity of membrane transporters involved in K+ and Ca2+ acquisition and homeostasis in salinized rice roots. Int. J. Mol. Sci. 2020, 21, 4882. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Choi, W.-G.; Gilroy, S.; Morris, R.J. A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPCl cation channel propagates the systemic response to salt stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Park, C.-J.; Shin, R. Calcium channels and transporters: Roles in response to biotic and abiotic stresses. Front. Plant Sci. 2022, 13, 964059. [Google Scholar] [CrossRef]
- Ye, F.; Xu, L.; Li, X.; Zen, W.; Gan, N.; Zhao, C.; Yang, W.; Jiang, Y.; Guo, J. Voltage-gating and cytosolic Ca2+ activation mechanisms of Arabidopsis two-pore channel AtTPC1. Proc. Natl. Acad. Sci. USA 2021, 118, e2113946118. [Google Scholar] [CrossRef]
- Leshem, Y.; Melamed-Book, N.; Cagnac, O.; Ronen, G.; Nishri, Y.; Solomon, M.; Cohen, G.; Levine, A. Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc. Natl. Acad. Sci. USA 2006, 103, 18008–18013. [Google Scholar] [CrossRef]
- Shi, H.; Quintero, F.J.; Pardo, J.M.; Zhu, J.-K. The Putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transports in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Testerink, C. Tuning plant signaling and growth to survive salt. Trends Plant Sci. 2015, 20, 586–594. [Google Scholar] [CrossRef]
- Quintero, F.J.; Martinez-Atienza, J.; Villalta, I.; Jiang, X.; Kim, W.-Y.; Ali, Z.; Fujii, H.; Mendoza, I.; Yun, D.-J.; Zhu, J.-K.; et al. Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc. Natl. Acad. Sci. USA 2011, 108, 2611–2616. [Google Scholar] [CrossRef]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Egea, I.; Pined, B.A.; Ortíz-Atienza, A.; Plasencia, F.A.; Drevensek, S.; García-Sogo, B.; Yuste-Lisbona, F.J.; Barrero-Gil, J.; Atarés, A.; Flores, F.B.; et al. The SlCBL10 Calcineurin B-like protein ensures plant growth under salt stress by regulating Na+ and Ca2+ homeostasis. Plant Physiol. 2018, 176, 1676–1693. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.; Lindberg, S.; Seidel, T.; Golldack, D.; Yemelyanov, V. Sodium sensing induces different changes in free cytosolic calcium concentration and pH in salt-tolerant and salt-sensitive rice (Oryza sativa L.) cultivars. Physiol. Plant. 2007, 130, 99–111. [Google Scholar] [CrossRef]
- Morgan, S.H.; Lindberg, S.; Jha Maity, P.; Geilfus, C.M.; Plieth, C.; Mühling, K.H. Apoplastic and cytosolic Ca2+ and pH dynamics in salt-stressed Vicia faba leaves change in response to calcium. Func. Plant Biol. 2017, 44, 515–524. [Google Scholar] [CrossRef]
- Villalobos-Lo’pez, M.A.; Arroyo-Becerra, A.; Quintero-Jiménez, A.; Iturriaga, G. Biotechnological Advances to Improve Abiotic Stress Tolerance in Crops. Int. J. Mol. Sci. 2022, 23, 12053. [Google Scholar] [CrossRef]
- Ding, L.; Zhu, J.-K. Reduced Na+ uptake in the NaCI-hypersensitive sos1 mutant of Arabidopsis thaliana. Plant Physiol. 1997, 113, 795–799. [Google Scholar] [CrossRef]
- Essah, P.A.; Davenport, R.; Tester, M. Sodium influx and accumulation in Arabidopsis. Plant Physiol. 2003, 133, 307–318. [Google Scholar] [CrossRef]
- Kader, M.A.; Lindberg, S. Uptake of sodium in protoplasts of salt-sensitive and salt-tolerant cultivars of rice, Oryza sativa L. determined by the fluorescent dye SBFI. J. Exp. Bot. 2005, 56, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Ohnuma, A. Comparative Functional Analysis of Class II Potassium Transporters, SvHKT2;1, SvHKT2;2, and HvHKT2;1, on Ionic Transport and Salt Tolerance in Transgenic Arabidopsis. Plants 2020, 9, 786. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Shishova, M.F.; Chirkova, T.V.; Lindberg, S. Anoxia-induced elevation of cytosolic Ca2+ concentration depends on different Ca2+ sources in rice and wheat protoplasts. Planta 2011, 234, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Maathuis, F.J.M. Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. New Phytol. 2007, 175, 387–404. [Google Scholar] [CrossRef] [PubMed]
- El Mahi, H.; Péres-Hormaeche, J.; De Luca, A.; Villlalta, I.; Espartero, J.; Gámes-Arjona, F.; Fernández, J.L.; Bundó, M.; Mendoza, I.; Mieulet, D.; et al. A Critical Role of Sodium Flux via the Plasma Membrane Na+/H+ Exchanger SOS1 in the Salt Tolerance of Rice. Plant Physiol. 2019, 180, 1046–1065. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Kader, A.; Lindberg, S. Uptake of sodium in quince, wheat and sugar beet protoplasts determined by the fluorescent sodium-binding benzofuran isophthalate dye. J. Plant Physiol. 2005, 162, 421–428. [Google Scholar] [CrossRef]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Calcium signal. Plant J. 1997, 12, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Knight, M.R.; Trewavas, A.J.; Sattelmacher, B.; Plieth, C. Self-reporting Arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol. 2004, 134, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.H.; Geilfus, C.M.; Lindberg, S.; Mühling, K.H. The leaf ion homeostasis and PM H+-ATPase activity in Vicia faba L. change after extra calcium and potassium supply under salinity. Plant Physiol. Biochem. 2014, 82, 244–253. [Google Scholar] [CrossRef]
- Allen, G.J.; Muir, S.R.; Sanders, D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science 1995, 268, 735–737. [Google Scholar] [CrossRef]
- Parre, E.; Ghars, M.A.; Leprince, A.-S.; Thiery, L.; Lefebvre, D.; Bordenave, M.; Richard, L.; Mazars, C.; Abdelly, C.; Savouré, A. Calcium signaling via phospholipase C is essential for proline accumulation upon ionic but not nonionic hyperosmotic stresses in Arabidopsis. Plant Physiol. 2007, 144, 503–512. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Creelman, R.A.; Zhu, J.-K. From laboratory to field. Using information from Arabidopsis to engineer salt, cold, and drought tolerance in crops. Plant Physiol. 2004, 135, 615–621. [Google Scholar] [CrossRef]
- Shabala, L.; Cuin, T.A.; Newman, I.A.; Shabala, S. Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 2005, 222, 1041–1050. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, X.; Pei, Z.-M. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef]
- Steinhorst, L.; Kudla, J. How plants perceive salt. Nature 2019, 572, 318–320. [Google Scholar] [CrossRef]
- Premkumar, A.; Lindberg, S.; Lager, I.; Rasmussen, U.; Schulz, A. Arabidopsis PLDs with C2-domain function distinctively in hypoxia signaling. Physiol. Plant. 2019, 107, 90–110. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, L.; Lindberg, S.; Vitagliano, C. Cytoplasmic free calcium dynamics in single tomato (Lycopersicon esculentum L) protoplasts subjected to chilling temperatures. Physiol. Plant. 1999, 105, 239–245. [Google Scholar] [CrossRef]
- Poenie, M.; Alderton, J.; Steinhardt, R.; Tsien, R. Calcium rises abruptly and briefly throughout the cell cycle and onset of anaphase. Science 1986, 233, 886. [Google Scholar] [CrossRef] [PubMed]
- Bright, G.R.; Fisher, G.W.; Rogowska, J.; Taylor, D.L. Fluorescence ratio imaging microscopy: Temporal and spatial measurements of cytoplasmic pH. J. Cell Biol. 1987, 104, 1019–1033. [Google Scholar] [CrossRef]
- Tsien, R.Y.; Poenie, M. Fluorescence ratio imaging: A new window into intracellular ionic signalling. Trends Biochem. Sci. 1986, 11, 450–455. [Google Scholar] [CrossRef]
- Lindberg, S.; Premkumar, A.; Rasmussen, U.; Schulz, A.; Lager, I. Phospholipases AtPLDζ1 and AtPLDζ2 function differently in hypoxia. Physiol. Plant. 2018, 162, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Schulze, C.; Sticht, H.; Meyerhoff, P.; Dietrich, P. Differential contribution of EF-hands to the Ca2+-dependent activation in the plant two-pore channel TPC1. Plant J. 2011, 68, 424–432. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, S.H.; Kader, M.A.; Lindberg, S. Cytosolic Sodium Influx in Mesophyll Protoplasts of Arabidopsis thaliana, wt, sos1:1 and nhx1 Differs and Induces Different Calcium Changes. Plants 2022, 11, 3439. https://doi.org/10.3390/plants11243439
Morgan SH, Kader MA, Lindberg S. Cytosolic Sodium Influx in Mesophyll Protoplasts of Arabidopsis thaliana, wt, sos1:1 and nhx1 Differs and Induces Different Calcium Changes. Plants. 2022; 11(24):3439. https://doi.org/10.3390/plants11243439
Chicago/Turabian StyleMorgan, Sherif H., Md Abdul Kader, and Sylvia Lindberg. 2022. "Cytosolic Sodium Influx in Mesophyll Protoplasts of Arabidopsis thaliana, wt, sos1:1 and nhx1 Differs and Induces Different Calcium Changes" Plants 11, no. 24: 3439. https://doi.org/10.3390/plants11243439
APA StyleMorgan, S. H., Kader, M. A., & Lindberg, S. (2022). Cytosolic Sodium Influx in Mesophyll Protoplasts of Arabidopsis thaliana, wt, sos1:1 and nhx1 Differs and Induces Different Calcium Changes. Plants, 11(24), 3439. https://doi.org/10.3390/plants11243439






