Myrrh and Chamomile Flower Extract Inhibit Mediator Release from IgE-stimulated Mast-Cell-Like RBL-2H3 Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Plant Extract Characterization
2.2. Influence on Cell viability
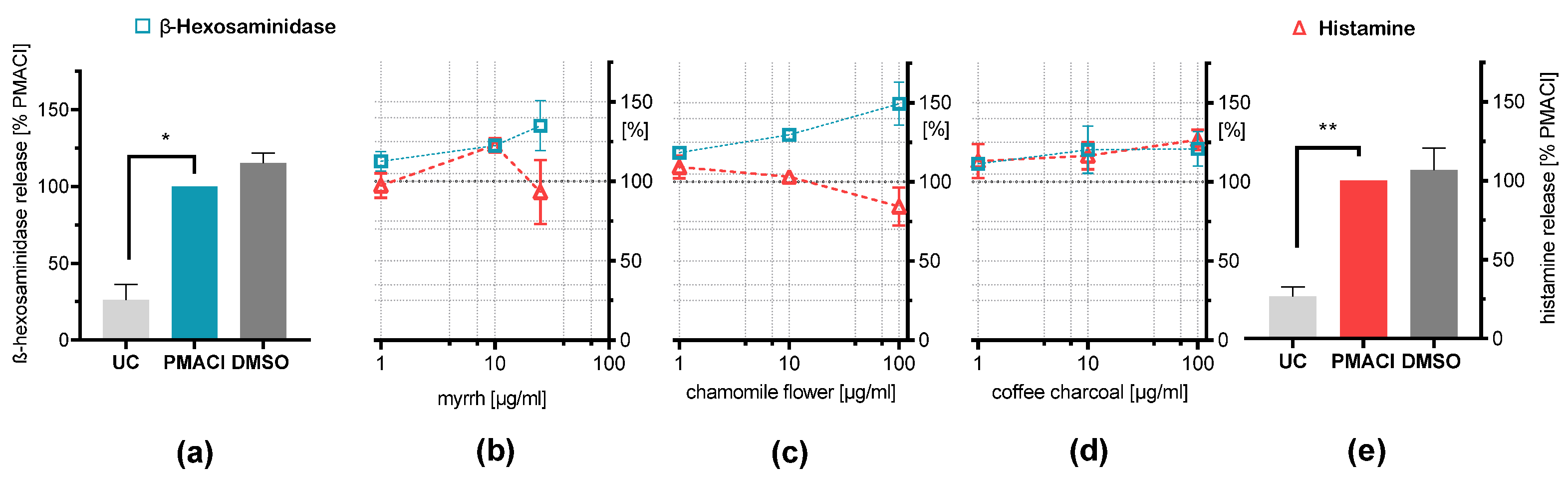
2.3. Influence on Chemical Induced Mediator Release
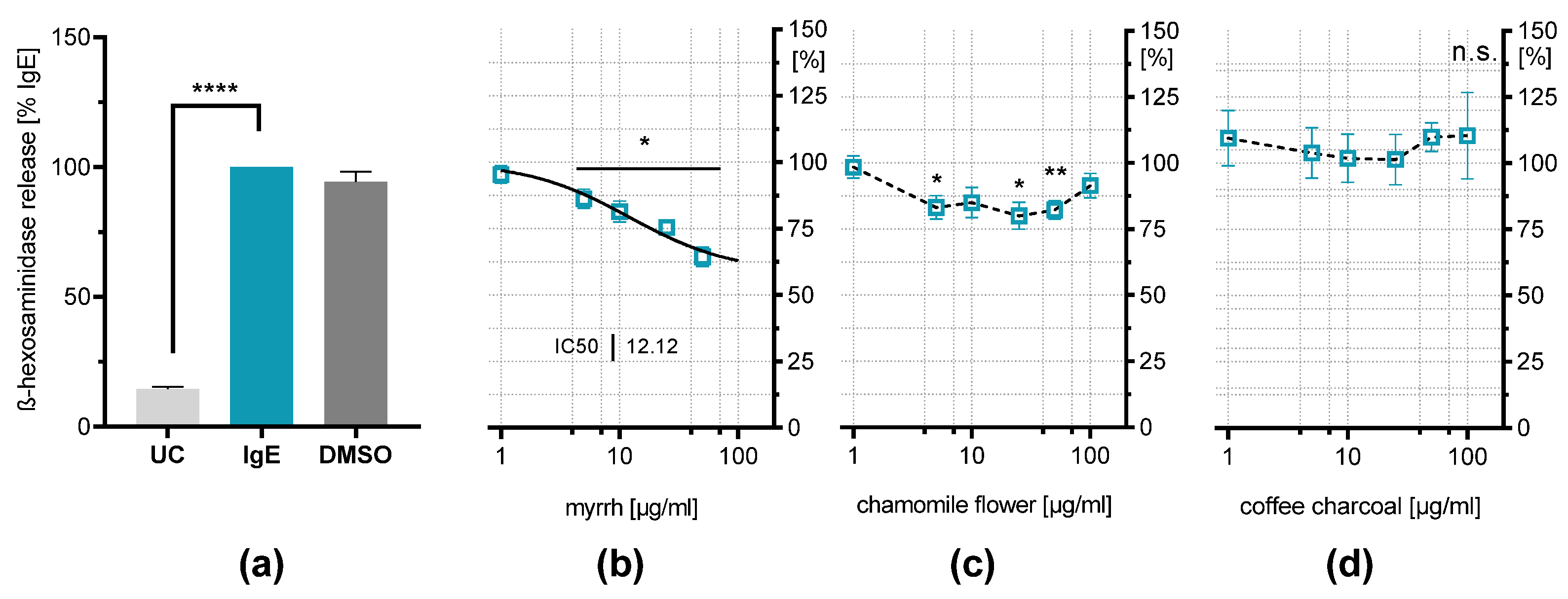
2.4. Influence on IgE-Induced β-Hexosaminidase Release
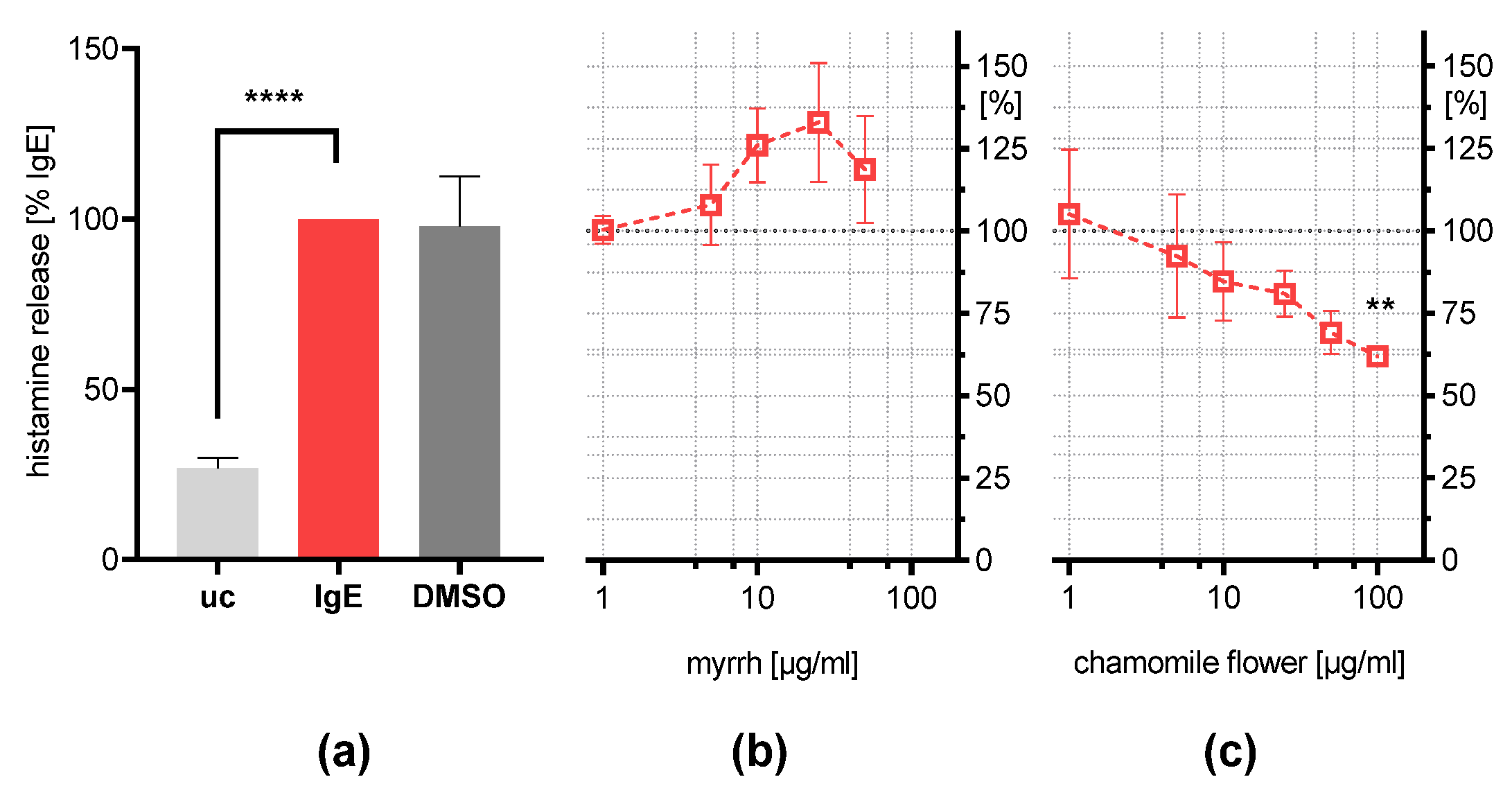
2.5. Influence on IgE-Induced Histamine Release
3. Materials and Methods
3.1. Chemicals
3.2. Cell Culture
3.3. Plant Material
3.4. Quantification of β-Hexosaminidase Release
3.5. Quantification of Histamine Release
3.6. Influence on Cell Viability
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [PubMed]
- Simrén, M.; Brazier, J.; Coremans, G.; Dapoigny, M.; Müller-Lissner, S.A.; Pace, F.; Smout, A.J.P.M.; Stockbrügger, R.W.; Vatn, M.H.; Whorwell, P.J. Quality of life and illness costs in irritable bowel syndrome. Digestion 2004, 69, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Vicario, M.; Santos, J. The role of mast cells in functional GI disorders. Gut 2016, 65, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Matricon, J.; Meleine, M.; Gelot, A.; Piche, T.; Dapoigny, M.; Muller, E.; Ardid, D. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 36, 1009–1031. [Google Scholar] [CrossRef]
- Santos, J.; Saperas, E.; Nogueiras, C.; Mourelle, M.; Antolín, M.; Cadahia, A.; Malagelada, J.-R. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology 1998, 114, 640–648. [Google Scholar] [CrossRef]
- Hardcastle, J.; Hardcastle, P.T. The secretory actions of histamine in rat small intestine. J. Physiol. 1987, 388, 521–532. [Google Scholar] [CrossRef]
- Russell, D.A. Mast cells in the regulation of intestinal electrolyte transport. Am. J. Physiol. 1986, 251, G253–G262. [Google Scholar] [CrossRef]
- Mall, M.; Gonska, T.; Thomas, J.; Hirtz, S.; Schreiber, R.; Kunzelmann, K. Activation of ion secretion via proteinase-activated receptor-2 in human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G200–G210. [Google Scholar] [CrossRef]
- Martínez, C.; Lobo, B.; Pigrau, M.; Ramos, L.; González-Castro, A.M.; Alonso, C.; Guilarte, M.; Guilá, M.; de Torres, I.; Azpiroz, F.; et al. Diarrhoea-predominant irritable bowel syndrome: An organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut 2013, 62, 1160–1168. [Google Scholar] [CrossRef]
- Bueno, L. Protease activated receptor 2: A new target for IBS treatment. Eur. Rev. Med. Pharmacol. Sci. 2008, 12 (Suppl. 1), 95–102. [Google Scholar]
- Barbara, G.; Wang, B.; Stanghellini, V.; de Giorgio, R.; Cremon, C.; Di Nardo, G.; Trevisani, M.; Campi, B.; Geppetti, P.; Tonini, M.; et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 2007, 132, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Buhner, S.; Li, Q.; Vignali, S.; Barbara, G.; de Giorgio, R.; Stanghellini, V.; Cremon, C.; Zeller, F.; Langer, R.; Daniel, H.; et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 2009, 137, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; La, J.H.; Schwartz, E.S.; Gebhart, G.F. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1085–G1098. [Google Scholar] [CrossRef] [PubMed]
- Klooker, T.K.; Braak, B.; Koopman, K.E.; Welting, O.; Wouters, M.M.; van der Heide, S.; Schemann, M.; Bischoff, S.C.; van den Wijngaard, R.M.; Boeckxstaens, G.E. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010, 59, 1213–1221. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhou, H.; Gu, W.; Wang, X.; Yang, J. Clinical efficacy and safety of ketotifen in treating irritable bowel syndrome with diarrhea. Eur. J. Gastroenterol. Hepatol. 2020, 32, 706–712. [Google Scholar] [CrossRef]
- Nellesen, D.; Yee, K.; Chawla, A.; Lewis, B.E.; Carson, R.T. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J. Manag. Care Pharm. JMCP 2013, 19, 755–764. [Google Scholar] [CrossRef]
- Layer, P.; Andresen, V.; Pehl, C.H.A.; Bischoff, S.C.; Claßen, M.; Enck, P.; Frieling, T.; Haag, S.; Holtmann, G.; Karaus, M.; et al. S3-Leitlinie Reizdarmsyndrom: Definition, Pathophysiologie, Diagnostik und Therapie. Gemeinsame Leitlinie der Deutschen Gesellschaft für Verdauungs- und Stoffwechselkrankheiten (DGVS) und der Deutschen Gesellschaft für Neurogastroenterologie und Motilität (DGNM) [Irritable bowel syndrome: German consensus guidelines on definition, pathophysiology and management]. Z Gastroenterol. 2011, 49, 237–293. [Google Scholar]
- Albrecht, U.; Müller, V.; Schneider, B.; Stange, R. Efficacy and safety of a herbal medicinal product containing myrrh, chamomile and coffee charcoal for the treatment of gastrointestinal disorders: A non-interventional study. BMJ Open Gastroenterol. 2014, 1, e000015. [Google Scholar] [CrossRef]
- Vissiennon, C.; Goos, K.-H.; Goos, O.; Nieber, K. Antispasmodic effects of myrrh due to calcium antagonistic effects in inflamed rat small intestinal preparations. Planta Med. 2015, 81, 116–122. [Google Scholar] [CrossRef]
- Vissiennon, C.; Hammoud, D.; Rodewald, S.; Fester, K.; Goos, K.H.; Nieber, K.; Arnhold, J. Chamomile Flower, Myrrh, and Coffee Charcoal, Components of a Traditional Herbal Medicinal Product, Diminish Proinflammatory Activation in Human Macrophages. Planta Med. 2017, 83, 846–854. [Google Scholar] [CrossRef]
- Weber, L.; Kuck, K.; Jürgenliemk, G.; Heilmann, J.; Lipowicz, B.; Vissiennon, C. Anti-Inflammatory and Barrier-Stabilising Effects of Myrrh, Coffee Charcoal and Chamomile Flower Extract in a Co-Culture Cell Model of the Intestinal Mucosa. Biomolecules 2020, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Hammoud Mahdi, D.; Jankuhn, S.; Lipowicz, B.; Vissiennon, C. Bioactive Plant Compounds in Coffee Charcoal (Coffeae carbo) Extract Inhibit Cytokine Release from Activated Human THP-1 Macrophages. Molecules 2019, 24, 4263. [Google Scholar] [CrossRef] [PubMed]
- Passante, E.; Frankish, N. The RBL-2H3 cell line: Its provenance and suitability as a model for the mast cell. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2009, 58, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.H.; Kim, J.H.; Kinet, J.P. Calcium mobilization via sphingosine kinase in signalling by the Fc epsilon RI antigen receptor. Nature 1996, 380, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Ludowyke, R.I.; Scurr, L.L.; McNally, C.M. Calcium ionophore-induced secretion from mast cells correlates with myosin light chain phosphorylation by protein kinase C. J. Immunol. 1996, 157, 5130–5138. [Google Scholar] [PubMed]
- Watanabe, J.; Shinmoto, H.; Tsushida, T. Coumarin and flavone derivatives from estragon and thyme as inhibitors of chemical mediator release from RBL-2H3 Cells. Biosci. Biotechnol. Biochem. 2005, 69, 1–6. [Google Scholar] [CrossRef]
- Mastuda, H. Structural requirements of flavonoids for inhibition of antigen-Induced degranulation, TNF-α and IL-4 production from RBL-2H3 cells. Bioorganic Med. Chem. 2002, 10, 3123–3128. [Google Scholar] [CrossRef]
- Park, C.-H.; Min, S.-Y.; Yu, H.-W.; Kim, K.; Kim, S.; Lee, H.-J.; Kim, J.-H.; Park, Y.-J. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. PTR 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Chirumbolo, S. Hormesis, resveratrol and plant-derived polyphenols: Some comments. Hum. Exp. Toxicol. 2011, 30, 2027–2030. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. U-Shaped Dose-Responses in Biology, Toxicology, and Public Health. Annu. Rev. Public Health 2001, 22, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Trovato, A.; Cavallaro, M.; Mancuso, C.; Di Rienzo, L.; Condorelli, D.; de Lorenzo, A.; Calabrese, E.J. The hormetic role of dietary antioxidants in free radical-related diseases. Curr. Pharm. Des. 2010, 16, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Che, D.N.; Cho, B.O.; Kang, H.J.; Kim, J.; Jang, S.I. Commiphora myrrha inhibits itch-associated histamine and IL-31 production in stimulated mast cells. Exp. Ther. Med. 2019, 18, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-H.; Lee, S.; Son, H.-Y.; Park, S.-B.; Kim, M.-S.; Choi, E.-J.; Singh, T.S.K.; Ha, J.-H.; Lee, M.-G.; Kim, J.-E.; et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharmacal Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef]
- Raposo, G.; Tenza, D.; Mecheri, S.; Peronet, R.; Bonnerot, C.; Desaymard, C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol. Biol. Cell 1997, 8, 2631–2645. [Google Scholar] [CrossRef]
- Moon, T.C.; Befus, A.D.; Kulka, M. Mast cell mediators: Their differential release and the secretory pathways involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef]
- Baram, D.; Adachi, R.; Medalia, O.; Tuvim, M.; Dickey, B.F.; Mekori, Y.A.; Sagi-Eisenberg, R. Synaptotagmin II negatively regulates Ca2+-triggered exocytosis of lysosomes in mast cells. J. Exp. Med. 1999, 189, 1649–1658. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Bueno, L.; Fioramonti, J. Protease-activated receptor 2 and gut permeability: A review. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2008, 20, 580–587. [Google Scholar] [CrossRef]
- Rosenthal, R.; Luettig, J.; Hering, N.A.; Krug, S.M.; Albrecht, U.; Fromm, M.; Schulzke, J.-D. Myrrh exerts barrier-stabilising and -protective effects in HT-29/B6 and Caco-2 intestinal epithelial cells. Int. J. Color. Dis. 2017, 32, 623–634. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Radinger, M.; Gilfillan, A.M. Measuring mast cell mediator release. Curr. Protoc. Immunol. 2010, 91, 7–38. [Google Scholar] [CrossRef] [PubMed]

| Peak | RT (min) | Peak Height (mAU) | Molecular Weight | Compound |
|---|---|---|---|---|
| 1 | 42.182 | 73.6 | 73.6 | (Iso)ferulic acid O-glucoside |
| 2 | 50.317 | 163.4 | 163.4 | (Iso)ferulic acid O-glucosyl ester |
| 3 | 57.433 | 524.6 | 524.6 | Apigenin-7-O-Glucoside |
| 4 | 60.533 | 65.0 | 65.0 | Apigenin-glucosyl-monoacetate |
| 5 | 62.467 | 84.4 | 84.4 | Apigenin-7-O-malonylglucoside |
| 6 | 66.317 | 149.9 | 149.9 | Apigenin-glucosyl-monoacetate |
| 7 | 70.483 | 49.7 | 49.7 | Apigenin |
| Peak | RT (min) | λ max (nm) | m/z | Ion | Compound |
|---|---|---|---|---|---|
| 1 | 4.1 | 200, 263 | 138.12 | [M + H]+ | Trigonelline |
| 2 | 32.778 | 244, 325 | 352.96 | [M − H]− | Neochlorogenic acid (3-caffeoylquinic acid) |
| 706.84 | [2M − H]− | ||||
| 3 | 39.743 | 244, 325 | 190.92 | [quinic acid − H]− | Chlorogenic acid (5-caffeoylquinic acid) |
| 352.98 | [M − H]− | ||||
| 707.13 | [2M − H]− | ||||
| 4 | 40.093 | 218, 272 | 138.12 | [M + H − OCNCH3]+ | Caffeine |
| 163.08 | [M + H − CH3OH]+ | ||||
| 195.08 | [M + H]+ | ||||
| 5 | 40.646 | 244, 325 | 190.95 | [quinic acid − H]− | Cryptochlorogenic acid (4-caffeoylquinic acid) |
| 352.96 | [M − H]− | ||||
| 707.13 | [2M − H]− | ||||
| 6 | 44.807 | 238 | 172.95 | [quinic acid − H − H2O]− | Feruloylquinic acid |
| 192.96 | [ferulic acid − H]− | ||||
| 366.99 | [M − H]− | ||||
| 734.89 | [2M − H]− | ||||
| 7 | 46.266 | 235 | 172.93 | [quinic acid – H − H2O]− | Feruloylquinic acid |
| 190.92 | [quinic acid − H]− | ||||
| 366.98 | [M − H]− | ||||
| 735.15 | [2M − H]− |
| Standard | RT (Standard) | m/z (Standard) | RT (Extract) | m/z (Extract) | Molecular Formula |
|---|---|---|---|---|---|
| 1 | 6.11 a | 214.1345 c | 6.11 a | 214.1347 c | C15H18O |
| 2 | 6.045 b | 231.1281 c | 6.042 b | 231.1383 c | C15H18O2 |
| 3 | 5.587 b | 321.1700 c | 5.581 b | 321.1699 c | C18H24O5 |
| 4 | 5.473 b | 231.1379 c | 5.470 b | 231.1383 c | C15H18O2 |
| 5 | 4.402 b | 247.1330 c | 4.383 b | 247.1334 c | C15H18O3 |
| 6 | 4.761 b | 249.1487 c | 4.749 b | 249.1486 c | C15H20O3 |
| 7 | 9.024 b | 329.2491 d | 9.040 b | 329.2487 d | C22H24O2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altenbernd, F.; Schwarz, L.; Lipowicz, B.; Vissiennon, C. Myrrh and Chamomile Flower Extract Inhibit Mediator Release from IgE-stimulated Mast-Cell-Like RBL-2H3 Cells. Plants 2022, 11, 3422. https://doi.org/10.3390/plants11243422
Altenbernd F, Schwarz L, Lipowicz B, Vissiennon C. Myrrh and Chamomile Flower Extract Inhibit Mediator Release from IgE-stimulated Mast-Cell-Like RBL-2H3 Cells. Plants. 2022; 11(24):3422. https://doi.org/10.3390/plants11243422
Chicago/Turabian StyleAltenbernd, Fabian, Lena Schwarz, Bartosz Lipowicz, and Cica Vissiennon. 2022. "Myrrh and Chamomile Flower Extract Inhibit Mediator Release from IgE-stimulated Mast-Cell-Like RBL-2H3 Cells" Plants 11, no. 24: 3422. https://doi.org/10.3390/plants11243422
APA StyleAltenbernd, F., Schwarz, L., Lipowicz, B., & Vissiennon, C. (2022). Myrrh and Chamomile Flower Extract Inhibit Mediator Release from IgE-stimulated Mast-Cell-Like RBL-2H3 Cells. Plants, 11(24), 3422. https://doi.org/10.3390/plants11243422







