Abstract
Cork, an anatomic adaptation of the bark of Quercus suber L. through its suberization process, finds its main application in the production of bottle stoppers. Its processing results in a large waste stream of cork fragments, granulates, and dust, which may be susceptible to valorization. The work presented here explored the use of its extracts to inhibit the growth of phytopathogenic microorganisms associated with apple tree diseases. The in vitro antimicrobial activity of cork aqueous ammonia extract was assayed against four fungi, viz. Monilinia fructigena and M. laxa (brown rot), Neofussicoccum parvum (dieback), and Phytophthora cactorum (collar and root rot), and two bacteria, viz. Erwinia amylovora and Pseudomonas syringae pv. syringae, either alone or in combination with chitosan oligomers (COS). Effective concentration values of EC90 in the 675–3450 μg·mL−1 range, depending on the fungal pathogen, were obtained in growth inhibition tests, which were substantially improved for the conjugate complexes (340–801 μg·mL−1) as a result of strong synergism with COS. Similar enhanced behavior was also observed in antibacterial activity assays, with MIC values of 375 and 750 μg·mL−1 for the conjugate complexes against P. syringae pv. syringae and E. amylovora, respectively. This in vitro inhibitory activity was substantially higher than those exhibited by azoxystrobin and fosetyl-Al, which were tested for comparison purposes, and stood out among those reported for other natural compounds in the literature. The observed antimicrobial activity may be mainly attributed to the presence of glycerin and vanillic acid, identified by gas chromatography–mass spectroscopy. In the first step towards in-field application, the COS–Q. suber bark extract conjugate complex was further tested ex situ against P. cactorum on artificially inoculated excised stems of the ‘Garnem’ almond rootstock, achieving high protection at a dose of 3750 μg·mL−1. These results suggest that cork industrial leftovers may, thus, be a promising source of bioactive compounds for integrated pest management.
1. Introduction
The cork oak (Quercus suber L.) is a slow-growing, evergreen tree indigenous to the Mediterranean region [1]. The cork shields stem shoots and buds from outside threats [2]. Its qualities (lightweight, waterproofness, compressibility, superior acoustic insulation, low thermal conductivity, high energy absorption capacity, and resistance to friction, fire, and impact [3]) make it suitable for flooring and insulation applications, and it is also used as natural cork stoppers for bottles [4]. In cork processing, cork fragments, granulates and dust represent a large waste stream [5], which is susceptible to valorization.
The chemical composition of cork is influenced by its geographic origin, temperature and soil conditions, genetic factors, tree size and age, and cork harvesting regime [6]. The polymeric matrix of cork is mainly composed of suberin, lignin, and polysaccharides [7]. Coquet et al. [8] showed that extraction under acid conditions led to diacids (tetracosanedioic, docosanedioic, and eicosanedioic acids) and monoacids (palmitic, stearic, thapsic, tetracosanoic, docosanoic, and eicosanoic acids), while extraction under neutral conditions led to 2,6-heptanediol and long chain alcohols (tetracosanol, docosanol, eicosanol), as well as sterols and triterpens (β-sitosterol, stigmasterol, 3-friedelanol, friedelin, and betulin). Analyses of extractable phenolic compounds have also shown the presence of gallotannins, ellagitannins, dehydrated tergallic-C-glucosides or ellagic acid derivatives, and mongolicain [9]. Other extractives reported in the literature are trans-squalene, camphene, trans-3-pinanone, 1-terpinen-4-ol, vescalagin, castalagin, pyrogallol, glucosan, sitost-4-en-3-one, o-cymene, and quinic acid [10].
Recent studies report that cork oak has high antimicrobial activity; Borrero et al. [11] showed that cork compost inhibited the growth of Fusarium spp., Pythium aphanidermatum (Edson) Fitzpatrick, Rhizoctonia solani Kühn, and Botrytis cinerea Pers. A suberin film extracted from cork showed bactericidal action against Staphylococcus aureus NCTC8325 and Escherichia coli (Migula) Castellani & Chalmers [12]. A methanolic leaf and stem extract exhibited inhibitory activity against Bacillus subtilis (Ehrenberg) Cohn, Streptococcus pneumoniae (Klein) Chester, E. coli, and S. aureus, and against Aspergillus niger Tiegh., Penicillium sp. and Fusarium oxysporum Schlecht. fungal strains that was reportedly better than those of other species of the genus Quercus [13].
Despite cork’s well-known and varied qualities, little attention has been paid to its potential use as a biorational against phytopathogens in fruticulture, in particular, to protect plants that belong to the Rosaceae family. Fungi of the genus Monilinia, especially Monilinia fructigena (Pers.) Honey and Monilinia laxa (Aderh. & Ruhland) Honey, cause brown rot disease in stone and pome fruits. In susceptible cultivars, these taxa spread to both young shoots and flowering buds, causing twig cankers and wilting of growing shoots, as well as fruit rot. Monilinia laxa causes significant losses of stone fruit in the field and after harvest [14,15]. Neofussicoccum parvum (Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips causes fruit rot, cankers, and dieback [16]. As a result of climate change, it is becoming an emerging disease of Rosaceae plant species, which enhances the necessity for learning about its pathogenicity, particularly concerning apple varieties of significant economic value [17]. As for the oomycete Phytophthora cactorum (Lebert & Cohn) J. Schröt., it causes fruit rot, starting with wilt and eventually destroying the tissues, in apple, apricot, citrus, plum, and strawberry crops. Regarding bacterial diseases, fire blight, caused by Erwinia amylovora (Burrill) Winslow et al., is a serious global threat to the production of apples and pears [18], and the genus Pseudomonas is among the ten most widespread bacterial plant diseases worldwide. Specifically, Pseudomonas syringae pv. syringae van Hall causes bacterial leaf spot and cankers and can affect species from Fabaceae, Cruciferae, Solanaceae, and Rosaceae families [19].
Different cultural methods, chemical fungicides in the orchard, treatments on mummified fruits, and post-harvest storage at low temperatures can be used to manage diseases of apple trees [20]. However, regarding chemical fungicides, it should be noted that a gradual withdrawal of some substances is taking place, due to concerns about their detrimental effects on the environment and human health, the persistent threat of the emergence of resistance, and the emergence of new virulence alleles [21]. As an alternative, the European Union, through Regulation (EU) 2019/1009, Council Regulation (EC) 834/2007, Commission Regulation (EC) 889/2008, and Article 14 of Directive 2009/128/EC, encourages the use of formulations based on natural products in an integrated pest management context.
In line with the latter approach, this work proposes the valorization of an aqueous ammonia extract of Q. suber bark as an antimicrobial agent for the protection of crops of the Rosaceae family, and, in particular, of Malus domestica Borkh. The main constituents were identified by gas chromatography–mass spectroscopy (GC–MS). The antimicrobial activity of the extract, both alone and as part of conjugated complexes with chitosan oligomers (COS), as well as the activity of its main bioactive compounds against the aforementioned phytopathogens, were tested in vitro. Finally, to test the preventive potential of the extract, an ex situ study was carried out on excised stems of a rootstock accession susceptible to P. cactorum.
2. Results
2.1. Identification of Phytochemicals by GC–MS
The GC–MS analysis of the aqueous ammonia extract of Q. suber bark revealed the presence of a hundred chemicals (Figure S1, Table S1). Out of these, glycerin (7.7%), 4-hydroxy-3-[[1,3-dihydroxy-2-propoxy]methyl]-1H-pyrazole-5-carboxamide (4.4%), 2-azabicyclo[2.2.1]heptane (3.6%), 4-hydroxy-3-methoxy-benzoic acid (or vanillic acid) (3.3%), benzoic acid (2.7%), 4-hydroxy-benzoic acid (1.6%), azelaic acid (2.6%), 1-decene (2.3%), cyclopentadecane (2.2%), and α-amino-γ-butyrolactone (1.9%) were the main natural constituents, while N-hydroxycarbamic acid, 2-(propoxycarbonylamino) ethyl ester, and 3D-10D cyclosiloxanes were found to be contaminants, given that the former is a product from reactions that involve both monoethanolamine (MEA) and methyl diethanolamine (MDEA) degradation products [22] (and MEA is considered as a potential atmospheric pollutant, since it is a benchmark and widely utilized solvent in a leading CO2 capture technology [23]), and the latter originates from septum and column bleed [24,25]. The chemical structure of the former is presented in Figure 1.
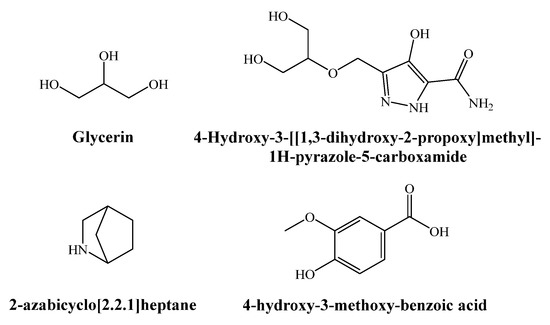
Figure 1.
Main phytochemicals identified in the aqueous ammonia extract of Quercus suber bark.
2.2. In Vitro Antimicrobial Activity Assessment
2.2.1. Antifungal and Anti-Oomycete Activity
The results of the antifungal/anti-oomycete susceptibility test are summarized in Figure 2. For all the assayed products, an increase in concentration led to a decrease in the radial growth of the mycelium, resulting in statistically significant differences. The aqueous ammonia extract of Q. suber showed antifungal activity comparable to that of COS, with minimum inhibitory concentrations of 1500 μg·mL−1 against taxa of the genus Monilinia and 750 μg·mL−1 against P. cactorum. However, the Q. suber extract did not inhibit mycelial growth in N. parvum, with a percentage inhibition of less than 50% for the highest concentration tested (1500 μg·mL−1). Concerning the main constituents of the extract, viz. glycerin, vanillic acid, and 2-azabicyclo[2.2.1]heptane (4-hydroxy-3-[[1,3-dihydroxy-2-propoxy]methyl]-1H-pyrazole-5-carboxamide could not be tested due to its unavailability from chemical suppliers), they presented better or similar inhibition values compared with those obtained by the extract. Specifically, glycerin and vanillic acid completely inhibited mycelial growth, with MICs ranging from 375 to 1500 μg·mL−1, whereas 2-azabicyclo[2.2.1]heptane was unable to fully inhibit the growth of any of the pathogens tested. The formation of conjugate complexes led to an improvement in terms of antifungal activity, as the COS–Q. suber extract resulted in complete inhibition at concentrations in the 375–1000 μg·mL−1 range, while full inhibition was observed at concentrations in the 250–1000 μg·mL−1 range for COS–glycerin and COS–vanillic acid (i.e., at doses lower than that observed for the COS–2-azabicyclo[2.2.1]heptane conjugate complex, with an MIC of 1500 μg·mL−1 against all pathogens). This improvement is more clearly observed in the effective concentration values summarized in Table 1.
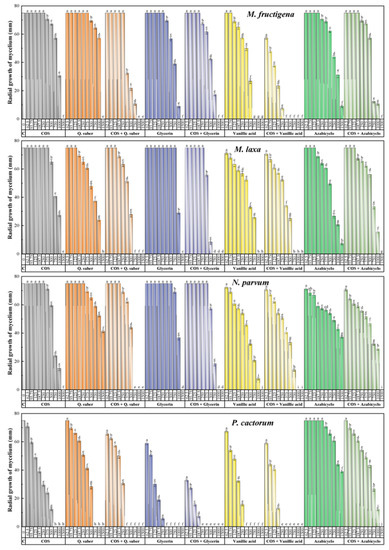
Figure 2.
Inhibition of the radial growth of the mycelium in in vitro tests performed in PDA medium with different concentrations (in the 62.5–1500 µg·mL−1 range) of chitosan oligomers (COS), Q. suber bark extract, three of its main phytochemical constituents, and their respective conjugated complexes. The same letters for the above concentrations mean that they are not significantly different at p < 0.05. Error bars represent standard deviations. Azabicyclo = 2-azabicyclo[2.2.1]heptane.

Table 1.
Effective concentrations (expressed in µg·mL−1) against M. fructigena, M. laxa, N. parvum, and P. cactorum of chitosan oligomers (COS), the aqueous ammonia extract of Q. suber bark, and three of its main constituents, alone and upon conjugation with COS.
To quantify the synergistic behavior observed for the conjugate complexes, synergy factors [13] were calculated according to the Wadley method (Table 2). Synergism (i.e., SFs > 1) was detected in all cases except for COS–2-azabicyclo[2.2.1]heptane against P. cactorum.

Table 2.
Synergy factors for the conjugate complexes estimated according to Wadley’s method.
For comparison and effectiveness purposes, the results of the experiments conducted with three conventional synthetic fungicides are presented in Table 3. The highest inhibition rates were recorded for a dithiocarbamate (mancozeb), demonstrating full inhibition of the mycelial growth of the four phytopathogens at one-tenth of the recommended dose (i.e., at 150 μg·mL−1). The organophosphorus fungicide (fosetyl-Al) fully inhibited the growth of P. cactorum at the recommended dose (i.e., 2000 μg·mL−1), but required a higher concentration to achieve full inhibition of the other three pathogens. Concerning the strobilurin fungicide (azoxystrobin), it was the least effective; it did not fully inhibit the growth of M. laxa, M. fructigena, or N. parvum at ten times the recommended dose (625 mg·mL−1), although it inhibited the growth of P. cactorum at 62.5 mg·mL−1.

Table 3.
Radial growth of mycelium of M. laxa, M. fructigena, N. parvum, and P. cactorum in in vitro assays performed on a PDA medium with different concentrations (the recommended dose, a tenth of the recommended dose, and ten times the recommended dose) of three commercial synthetic fungicides.
2.2.2. In Vitro Antibacterial Assessment
Table 4 provides a summary of the antibacterial activity results against the two Gram-negative bacterial plant pathogens. In the case of the quarantine species E. amylovora, Q. suber bark extract was more effective than COS, with MIC values of 1000 and 1500 μg·mL−1, respectively. Two of its main constituents, glycerin, and vanillic acid, were more effective than the extract, with MIC values of 500 and 750 μg·mL−1, respectively. Regarding the activity against P. syringae pv. syringae, the aqueous ammonia extract, glycerin, and vanillic acid inhibited bacterial growth at 750 μg·mL−1, while a concentration of 1000 μg·mL−1 was required for COS conjugates. As for 2-azabicyclo[2.2.1]heptane, it did not prevent bacterial growth at the maximum dose assayed of 1500 μg·mL−1.

Table 4.
Antibacterial activity against E. amylovora and P. syringae pv. syringae of chitosan oligomers (COS), the aqueous ammonia extract of Q. suber bark, and its main constituents, alone and upon conjugation with COS. Positive and negative signs indicate the presence/absence of bacterial growth.
As previously discussed for the antifungal/anti-oomycete activity, the formation of conjugated complexes with COS resulted in a synergistic effect, enhancing the antibacterial activity. In the case of P. syringae pv. syringae, the Q. suber–COS conjugate complex fully inhibited bacterial growth at a concentration of 375 μg·mL−1, although a higher concentration of 750 μg·mL−1 was required against E. amylovora. Regarding the conjugates of the extract constituents, COS–glycerin was the most effective against both bacteria, with MIC values of 375 μg·mL−1; followed by COS–vanillic acid, with MIC values of 500 μg·mL−1. Interestingly, the COS–azabicyclo conjugate resulted in full inhibition at 750 μg·mL−1, suggesting strong synergism, which may be tentatively ascribed to solubility enhancement.
2.3. Protection of Excised Stems against P. cactorum
Ex situ tests were conducted on excised stems from the ‘Garnem’ rootstock to assess the efficacy of the treatment against the phytopathogen for which the best in vitro results had been obtained, namely P. cactorum (Figure 3). At the lowest assayed dose, i.e., the MIC value obtained in the in vitro tests (375 μg·mL−1), no protection effect was observed, with canker lengths similar to those of the untreated stems (Table 5). At five times the MIC dose (1875 μg·mL−1), large cankers were also registered, but with significant differences compared with the controls. It was necessary to increase the dose up to 10 times the MIC (3750 μg·mL−1) to obtain high and statistically significant protection of the excised stems.
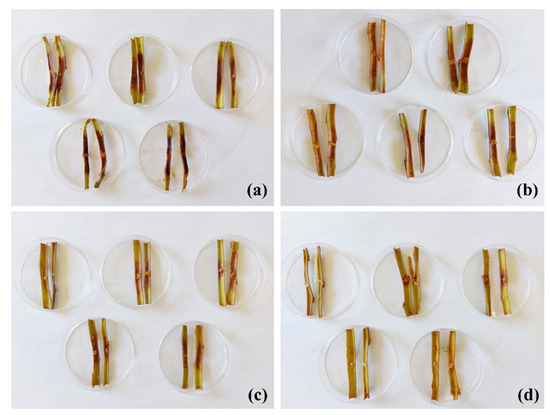
Figure 3.
Canker lengths observed in ‘Garnem’ excised stems artificially inoculated with P. cactorum and treated with the COS–Q. suber bark extract conjugate complex at different concentrations: (a) control, no treatment; (b) MIC = 375 μg·mL−1; (c) MIC × 5 = 1875 μg·mL−1; (d) MIC × 10 = 3750 μg·mL−1. Only half of the replicates per treatment are shown.

Table 5.
Results of the Kruskal–Wallis test, followed by multiple pairwise comparisons using the Conover–Iman procedure for mean lengths of cankers in excised stems after inoculation with P. cactorum. The mean rank values accompanied by the same letters are not significantly different (p-value (one-tailed) < 0.0001, α = 0.05).
3. Discussion
3.1. The Phytochemical Profiles
In general, the main compounds present in cork are terpenes, sterols, saccharides, suberin, lignin, and other phenolic compounds, although their composition may vary as a result of various factors, such as climate, region, age, or part of the tree [2]. To extract phenolic compounds from the bark, polar, organic solvents or hydromethanolic mixtures are mainly used, identifying essentially phenolic acids and aldehydes, coumarins, flavonoids, and tannins [9,26]. Recently, polyphenol recovery in Q. suber bark extracts was considerably increased by microwave-assisted extraction, using different proportions of water and alcohols, mainly obtaining p-coumaric, syringic, and sinapic acids [27]. In addition, a new method has been reported for the extraction of phenolic compounds from cork granulates using a mixture of water with propylene glycol [28]. However, to the best of the authors’ knowledge, this is the first time that an aqueous ammonia solution and ultrasonication have been used for the extraction of bioactive compounds from cork. Previously, our research group used this extraction method with Quercus ilex subsp. ballota (Desf.) Samp. [29] and Uncaria tomentosa L. barks [30].
The obtained hydroxybenzoic acid profile was different from those previously reported [8,10], but exhibited similarities with that of Q. ilex bark [29], i.e., the presence of hydroxyciclopentenones, methylimidazoles, and hexadecanoic and nonanedioic acid/esters. Concerning other phytochemicals, the pyrazole 4-hydroxy-3-[[1,3-dihydroxy-2-propoxy]methyl]-1H-pyrazole-5-carboxamide is analogous to pirazofurin (a C-glycosyl compound that is 4-hydroxy-1H-pyrazole-5-carboxamide in which the hydrogen at position 3 has been replaced by a β-D-ribofuranosyl group) with antineoplastic and antiviral properties [31]. 2-azabicyclo[2.2.1]heptanes are analogs of aliphatic monoamines (pyrrolidine; piperidine), distributed mainly in the Piperaceae and Rutaceae families, with described antifeedant, analgesic, antipyretic, anti-inflammatory and antioxidant activities [32]. Specifically, 2-azabicyclo[2.2.1]heptane was previously isolated from Delphinium caeruleum Jacquem. ex Cambess. [33]. The flavoring agent vanillic acid (or 4-hydroxy-3-methoxy-benzoic acid) is a phenolic acid associated with lignin following oxidation. It is the intermediate product in the two-step bioconversion of ferulic acid to vanillin [34]. Azelaic acid (or nonanodioic acid) is a naturally occurring acid found in grains such as barley, wheat, and rye, and it serves as a signal that induces the accumulation of salicylic acid, an important component of the defensive response of a plant [35]. Azelaic acid has been reported to manifest its antibacterial effects by inhibiting the synthesis of cellular proteins in both anaerobic bacteria (impeding glycolysis) and aerobic bacteria (inhibiting several oxidoreductive enzymes, including tyrosinase, mitochondrial enzymes of the respiratory chain, thioredoxin reductase, 5-α-reductase, and DNA polymerases) [36]. 1-decene was detected as one of many volatile organic compounds emitted from woodland vegetation, with an emission rate estimated to range from 0.5 to 5 µg·g−1 [37]. The phytochemical α-amino-γ-butyrolactone is a cleavage product of S-adenosylmethionine [38].
3.2. Mode of Action
To provide a tentative explanation of the observed antimicrobial activity, a discussion of the antimicrobial activity of each of the three main extract constituents that were tested in vitro is presented (although concurrent activity from other constituents of the extract and/or synergism among the various phytochemicals cannot be ruled out).
Glycerin, a simple three-carbon tri-alcohol used as a carrier in many medicines and as a plasticizer in gelatin gel capsules, is known to be a bacteriostatic/fungistatic [39], which also features virucidal activity [40]. Concerning its applicability to the preventive and/or curative treatment of plants, glycerin as it is or in a water-based solution has been reported to possess fungicidal and bactericidal properties against some types of phytopathogen fungi and bacteria (Alternaria alternata (Fr.) Keissl., Venturia inaequalis (Cooke) G. Winter, Plasmopara viticola (Berk. & M.A. Curtis) Berl. & de Toni, Cercospora beticola Sacc., Puccinia spp., Fusarium spp., Septoria spp., Botrytis spp., Taphrina spp., Phytophthora infestans (Mont.) de Bary, E. amylovora, and Erysiphe necator Schwein.), although it was noted that it demonstrated greater fungicidal and bactericidal action when used in combination with other substances with a known antimicrobial action, allowing a reduction in the concentration of the latter [41].
The presence of glycerin in plant extracts has been reported, for instance, in Plantago major L. leaf extracts [42]; Allamanda cathartica L. [43]; Cynodon dactylon (L.) Pers. (with an activity comparable to that of streptomycin against S. aureus, E. coli, Salmonella typhi (Schroeter) Warren and Scott, Proteus mirabilis Hauser, and S. pyogens) [44]; Salvadora persica L. (with activity against S. aureus and A. terreus) [45]; and Aphelandra squarrosa Nees (with a glycerin content as high as 46% and strong activity against E. coli) [46].
Regarding vanillic acid, its presence has been reported in extracts with antimicrobial activity from other plants (Table S2), although Angelica sinensis (Oliv.) Diels is currently the largest commercial natural source [47]. The mechanism of action of its antimicrobial activity against clinically important bacteria, by inducing cell lysis, damaging the cell membrane, and causing leakage of intracellular components, has been studied by Li et al. [48]; and it is considered a promising candidate antimicrobial agent not only to treat infections and as a surface disinfectant, but also as a food preservative in the food industry [49].
In relation to azabicyclo[2.2.1]heptane, the presence of azabicyclo derivatives in plant extracts has not yet been thoroughly studied. Nonetheless, 6-azabicyclo[3.2.1]octane was found to be present in an extract of Azadirachta indica A. Juss. and in Ocimum sanctum L. leaves [50]; 1-azabicyclo[3.1.0]hexane was reported in an extract of Melia dubia Cav. leaves [51]; and the highest concentration of an azabicyclo derivative to date was found in an extract of a Dioscorea hispida Dennst. tuber at 23.16% [52], all of which showed antimicrobial activity (Table S2). Nonetheless, as noted above, in this work, the activity of azabicyclo[2.2.1]heptane was low when used alone, which may be tentatively ascribed to solubility problems, given that it improved upon conjugation with COS.
3.3. Antimicrobial Activity Comparison
3.3.1. Comparison with Antimicrobial Activities Reported for Other Q. suber Extracts
The available studies on the antimicrobial activity of Q. suber extracts are summarized in Table S3. Given that no data on activity against the same pathogens have been reported, the comparisons below should be taken with caution. Akroum [53] reported the high in vitro antifungal activity of an acetonic extract of Q. suber acorns against seven pathogenic fungi, with inhibition values in the 20–105 µg·mL−1 range (better than those reported here), which were attributed to the composition of the acorns, rich in phenolic acids and gallic acid derivatives, as well as in proanthocyanidins (present in different species of Quercus spp., mainly Q. ilex L., Q. suber L., and Q. robur L. [54]). However, the aforementioned values were obtained using extracts in acetone (70%), which calls into question their validity (due to the aggressiveness of the extraction medium used). Concerning methanolic or hydromethanolic extracts, Lahlimi-Alami et al. [55] tested the anticandidosic potential of a methanolic extract of cork against five different strains of C. albicans, with minimum inhibitory concentrations in the 12,500–50,000 µg·mL−1 range. These values were similar to those obtained by Hassikou et al. [56] who used methanolic extracts of cork and leaf. This may explain why Touati et al. [6] reported that an hydromethanolic extract of cork failed to inhibit bacterial growth of Listeria innocua (ex Seeliger and Schoofs 1979) Seeliger 1983 and E. coli, given that the maximum concentration assayed was noticeably lower (3000 µg·mL−1). In comparison, the aqueous ammonia extract presented herein would be notably more active. Nonetheless, it should be noted that there would be exceptions, given that Akroum and Rouibah [57] reported an MIC value of 110 µg·mL−1 for a cork methanolic extract against A. alternata. It is also worth noting that, although not comparable (given that inhibition zone values were reported instead of MIC values), other in vitro and in vivo analyses also support the antimicrobial effects of methanolic extracts of oak leaves and stems [13,58].
3.3.2. Comparison of Efficacy with Other Natural Compounds
The results of a literature survey on the effectiveness of bioactive substances of natural origin on M. fructigena, M. laxa, and N. parvum fungal pathogens, on the oomycete P. cactorum, as well as on E. amylovora and P. syringae pv. syringae bacteria are compiled in Table S4. Even if the data correspond to the same pathogens, the inhibition values listed below should be used with caution because the susceptibility profile varies depending on the isolates, the procedure for obtaining the extract, the solvent, and the testing techniques used, and also because the units used to express them differ significantly.
In the case of the Monilinia species, the activity of the aqueous ammonia extract of Q. suber cork, and that of its conjugate with COS, would be higher than those of the methanolic or n-hexane extracts of Prunus laurocerasus L., Cornus mas L., Morus nigra L., Morus alba L., and Rosa canina L. described by Onaran and Yanar [59], and also higher than that of the aqueous extract of Punica granatum L. peel [60]. It would be similar to those obtained by Mamoci et al. [61] for the n-hexane extracts of Dittrichia viscosa (L.) Greuter and Ferula communis L. It is worth noting that the lower inhibition values reported by El Khetabi et al. [62] may not be directly compared, as in that study, the essential oils were used as biofumigants. The same applies to the percentage inhibition rate reported by Andreu et al. [63], given that the units differ.
As for N. parvum, our research group conducted previous trials with other natural extracts against this plant pathogen using the same isolate. The efficacy of the cork extract can, thus, be directly compared to those obtained with extracts of Equisetum arvense L., Urtica dioica L., and Silybum marianum (L.) Gaertn. [64,65], which were lower. However, substantially higher efficacy was reported for the extract from roots of Rubia tinctorum L., in which inhibition was reached at 250 µg·mL−1 [66].
Concerning P. cactorum, the commercial CUSTOSTM formulated Allium-based extract (MIC = 100 μg∙mL−1) [67], ethanolic extracts of Pinus spp. (MIC = 100 μg∙mL−1) [68], and the aqueous ammonia extract of U. tomentosa bark (MIC = 187.5 μg∙mL−1) [30] would be more effective than the Q. suber bark extract and its conjugate presented herein (with MIC values of 750 and 375 μg∙mL−1, respectively). On the other hand, commercial essential oils were minimally effective in the inhibition of this oomycete [69,70].
Regarding antibacterial activity, the inhibition results against E. amylovora would be comparable to those obtained by Fontana et al. [71] for the hydroethanolic, methanolic, and maltodextrin-conjugated extracts of Moringa oleifera Lam. leaves, with MIC values of 1000 μg∙mL−1; and with those obtained for hydromethanolic extracts of P. granatum fruits [72], of flowers or leaves of Hibiscus syriacus L. [73], and of flowers or leaves of Limonium binervosum (G.E.Sm.) C.E. Salmon [74], which were previously tested in our research group against the same isolate, with inhibition values in the 750–1500 μg∙mL−1 range. Finally, the lowest inhibitory levels for P. syringae pv. syringae, reported for commercial essential oils by Shabani et al. [75], cannot be directly compared, since the essential oils were utilized as biofumigants. The same holds true for the findings of Islam et al. [76], where a different methodology and different units were employed.
3.3.3. Comparison of Efficacy with Conventional Fungicides
Concerning the in vitro inhibitory activity of the COS−Q. suber bark aqueous ammonia extract conjugate complex, it was higher than that of fosetyl-Al and much higher than that of azoxystrobin against the four pathogens, with MIC values of 1000, 750, 750, and 375 µg·mL−1 against M. fructigena, M. laxa, N. parvum, and P. cactorum, respectively (vs. >2000, >2000, >2000, and 2000 µg·mL−1 for fosetyl-Al; and >625,000, >625,000, >625,000, and ca. 6250 µg·mL−1 for azoxystrobin, respectively). However, in the ex situ bioassays, a ten times higher dose of the Q. suber-based conjugate complex (3750 µg·mL−1) was required to achieve full protection against P. cactorum. This dose represents approximately twice that of fosetyl-Al and half that of azoxystrobin. Thus, the activity of the non-optimized formulation based on the proposed natural product with a view to in-field applications may be regarded as comparable to those of these two commercial synthetic fungicides. However, future research aimed at optimizing the formulation (e.g., via combination with coadjuvants specifically designed to facilitate bark penetration) or the use of controlled release strategies (e.g., nanocarrier encapsulation [77]) may result in enhanced performance. Assessment of different treatment exposure times and application methods (e.g., spraying), which are more representative of the reality of field treatments, and inclusion of direct comparisons with conventional fungicides would also be relevant aspects to be addressed in follow-up studies.
4. Materials and Methods
4.1. Vegetal Material
The origin of the bark was the Alcornocal de Valdegalindo in Foncastín, Valladolid, Spain (coordinates: 41.445766, −5.014038; elevation: 707 m) (Figure S2. The bark samples (n = 10) were thoroughly mixed, dried, and reduced to a fine powder. According to Mota et al. [2], to prevent any damage to the trees, the corking procedure used to obtain the samples to be investigated was performed manually and during the transition from spring to summer, when their physiological circumstances were ideal for extraction.
4.2. Reagents
High-molecular weight chitosan (CAS No. 9012-76-4; MW: 310,000–375,000 Da) was supplied by Hangzhou Simit Chem. & Tech. Co. (Hangzhou, China). NeutraseTM 0.8 L enzyme was supplied by Novozymes A/S (Bagsværd, Denmark). Glycerin (CAS No. 56-81-5), vanillic acid 98% (4-hydroxy-3-methoxybenzoic acid, CAS No. 100-76-5), and ammonium hydroxide, 50% v/v aq. soln. (CAS No. 1336-21-6) were acquired from Alfa Aesar. 2-Azabicyclo[2.2.1]heptane (CAS No. 279-24-3), acetic acid (purum, 80% in H2O; CAS No. 64-19-7), tryptic soy agar (TSA, CAS No. 91079-40-2), and tryptic soy broth (TSB, CAS No. 8013-01-2) were supplied by Sigma–Aldrich Química (Madrid, Spain). Potato dextrose agar (PDA) was purchased from Becton Dickinson (Bergen County, NJ, USA). Alkir® fungicide coadjuvant was purchased from De Sangosse Ibérica (Valencia, Spain).
Commercial fungicides used for comparison purposes, viz. Ortiva® (azoxystrobin 25%; reg. no. 22000; Syngenta, Basel, Switzerland), Vondozeb® (mancozeb 75%; reg. no. 18632; UPL Iberia, Barcelona, Spain), and Fosbel® (fosetyl-Al 80%, reg. no. 25502; Probelte, Murcia, Spain) were kindly provided by the Plant Health and Certification Service (CSCV) of Gobierno de Aragón.
4.3. Phytopathogen Isolates
M. laxa (MYC-1580) and M. fructigena (MYC-1579) were supplied by the Mycology lab at the Center for Research and Agrifood Technology of Aragón (CITA, Zaragoza, Spain) as subcultures on PDA. P. cactorum (CRD Prosp/59) and the bacterial isolate P. syringae pv. syringae (CRD 17/105) were supplied by the Regional Diagnostic Center of Aldearrubia (Junta de Castilla y León, Spain) as subcultures in PDA or TSA, respectively. The N. parvum isolate (code ITACYL_F111, isolate Y-091-03-01c) was supplied in a lyophilized vial (later reconstituted and refreshed as a subculture on PDA) by the Instituto Tecnológico Agrario de Castilla y León (ITACYL; Valladolid, Spain). E. amylovora (NCPPB 595) was obtained from the Spanish Type Culture Collection (CECT; Valencia, Spain).
4.4. Preparation of Bark Extracts, Chitosan Oligomers, and Conjugate Complexes
The preparation of the Q. suber bark extract followed the method previously described in [30]; briefly, the bark powder sample was first digested in an aqueous ammonia solution for 2 h, then sonicated in pulsed mode (with a 2 min stop every 2.5 min) for 10 min using a probe-type ultrasonicator (model UIP1000hdT; 1000 W, 20 kHz; Hielscher Ultrasonics, Teltow, Germany), and then allowed to stand for 24 h. Finally, the solution was centrifuged at 9000 rpm for 15 min, and the supernatant was filtered through Whatman No. 1 paper.
Chitosan oligomers (COS) were prepared according to the procedure described in the work by Santos-Moriano et al. [78], with the modifications indicated in [77]. In brief, commercial chitosan (MW = 310–375 kDa) was dissolved in aqueous 1% (w/w) acetic acid, and, after filtration, the filtrate was neutralized with aqueous 4% (w/w) NaOH. The precipitate was collected and washed thoroughly with hot distilled water, ethanol, and acetone. Purified chitosan was obtained by drying. The degree of deacetylation (DD) was determined to be 90% according to Sannan et al. [79]. A total of 20 g of purified chitosan was dissolved in 1000 mL of Milli-Q water by adding 20 g of citric acid under constant stirring at 60 °C. Once dissolved, the commercial proteolytic preparation NeutraseTM 0.8 L (a protease from Bacillus amyloliquefaciens Priest, Goodfellow, Shute & Berkeley) was added to obtain a product enriched in deacetylated chitooligosaccharides and to degrade the polymer chains. The mixture was sonicated for 3 min in 1 min of sonication/1 min without sonication cycles to keep the temperature in the 30–60 °C range. The molar mass of the COS samples was determined by measuring the viscosity, in agreement with the method outlined by Yang et al. [80], in a solvent of 0.20 mol·L−1 NaCl + 0.1 mol·L−1 CH3COOH at 25 °C using an Ubbelohde capillary viscometer. The molar mass was determined using the Mark–Houwink equation [η] = 1.81 × 10−3 M0.93 [81]. At the end of the process, a solution with a pH in the four to six interval with oligomers of molecular weight <2 kDa was obtained, with a polydispersity index of 1.6, within the usual range reported in the literature [82].
The COS–bark extract and COS−main bioactive compounds conjugate complexes were obtained by mixing the respective solutions in a 1:1 (v/v) ratio, followed by sonication for 15 min in 5 3-min pulses (so that the temperature did not exceed 60 °C). Attenuated total-reflectance Fourier transform infrared (ATR-FTIR) spectroscopy was used to confirm the formation of the conjugate complexes.
4.5. Extract and Conjugate Complexe Characterization
The aqueous ammonia Q. suber bark extract was studied by gas chromatography–mass spectrometry (GC-MS) at the Research Support Services (STI) at Universidad de Alicante (Alicante, Spain), using a gas chromatograph model 7890A coupled to a quadrupole mass spectrometer model 5975C (both from Agilent Technologies, Santa Clara, CA, USA). Chromatographic conditions were as follows: 3 injections/vial, injection volume = 1 µL; injector temperature = 280 °C, in splitless mode; initial oven temperature = 60 °C, after 2 min, followed by an increase of 10 °C/min up to a final temperature of 300 °C, after 15 min. The chromatographic column used for the separation of the compounds was an Agilent Technologies HP-5MS UI of 30 m in length, 0.250 mm in diameter, and with 0.25 µm film. The conditions of the mass spectrometer were as follows: temperature of the electron impact source of the mass spectrometer = 230 °C and of the quadrupole = 150 °C; ionization energy = 70 eV. Test mixture 2 for apolar capillary columns according to Grob (Supelco 86501) and PFTBA tuning standards were used for equipment calibration. The identification of the components was based on a comparison of their mass spectra and retention time with those of the authentic compounds and by computer matching with the database of the National Institute of Standards and Technology (NIST11) and Adams [83].
4.6. In Vitro Antimicrobial Activity Assessment
The antifungal/anti-oomycete activity of the different treatments (including Q. suber bark extract, some of its main constituents, the conjugates of all of them, and certain commercial synthetic fungicides) was determined by the agar dilution method according to the EUCAST antifungal susceptibility testing standard procedures [84], incorporating stock solution aliquots into the pouring PDA medium to provide final concentrations in the 62.5–1500 μg·mL−1 range. Mycelial plugs (⌀ = 5 mm), from the margin of 1-week-old PDA cultures of M. laxa, M. fructigena, and N. parvum, and 2-week-old cultures of P. cactorum, were transferred to plates, incorporating the above treatment concentrations (3 plates/concentration, with 2 replicates). Neofusicoccum parvum and P. cactorum plates were incubated at 25 °C in the dark for 1 and 2 weeks, respectively. In the case of M. laxa and M. fructigena, incubation was carried out at 22 °C in the dark for 1 week. PDA medium without any modification was used as the control. Mycelial growth inhibition was estimated according to the formula ((dc − dt)/dc) × 100, where dc and dt represent the mean diameters of the control and treated fungal colonies, respectively. The effective concentrations (EC50 and EC90) were estimated using PROBIT analysis in IBM SPSS Statistics v.25 software (IBM; Armonk, NY, USA). The level of interaction, i.e., the synergy factor, was estimated according to Wadley’s method [85].
The antibacterial activity was assessed according to the CLSI standard M07–11 [86], using the agar dilution method to determine the minimum inhibitory concentrations (MIC). In short, an isolated colony of E. amylovora was incubated at 30 °C for 18 h in a TSB liquid medium. Serial dilutions were then performed, starting from a concentration of 108 CFU·mL−1, to obtain a final inoculum of ~104 CFU·mL−1. The bacterial suspensions were then delivered to the surface of the TSA plates, to which the treatments had previously been added at concentrations ranging from 62.5 to 1500 μg·mL−1. Plates were incubated at 30 °C for 24 h, and readings were taken after 24 h. In the case of P. syringae pv. syringae, the same procedure was followed, although it was grown at 25 °C for 48 h. MICs were determined in the agar dilutions as the lowest concentrations of the bioactive products at which no bacterial growth was visible. All experiments were run in triplicate, with 3 plates per treatment/concentration.
4.7. Protection Tests on Artificially Inoculated Excised Stems
The efficacy of the treatment was tested by artificial inoculation of excised stems in controlled laboratory conditions. Inoculation was performed according to the procedure proposed by Matheron [87], with the modifications described by Álvarez Bernaola [88]. Briefly, using a grafting knife, young stems of healthy ‘Garnem’ (Prunus amygdalus × P. persica) rootstock with a 1.5 cm diameter were cut into 10 cm long sections. The excised stem pieces were immediately wrapped in moistened sterile absorbent paper, while the produced wounds were painted with Mastix®.
In the laboratory, the freshly excised stem segments were first immersed in a NaClO 3% solution for 10 min, immersed in ethanol 70% for 10 min, and then thoroughly rinsed four times with bidistilled sterile water, to avoid superficial contaminants in the tissue. Some of the stem segments (n = 10) were soaked for 1 h in distilled water as a control, while the remaining stem segments were soaked for 1 h in aqueous solutions to which an appropriate amount of the conjugate complex of COS–Q. suber bark extract had been added to obtain MIC, MIC × 5, and MIC × 10 concentrations (n = 10 segments/concentration). Alkir® coadjuvant (1% v/v) was added to all the solutions (including the control) to facilitate the moistening and penetration of the treatment into the bark.
The stem pieces were allowed to dry, and the bark was carefully removed with a scalpel to reveal the cambium. The bark was then placed on an agar Petri dish, and subsequently inoculated by placing a plug (⌀ = 5 mm), from the margin of 2-week-old PDA cultures of P. cactorum, on the center of the inner surface of the bark. After inoculation, stem segments were incubated in a humid chamber for 3–4 days at 24 °C, 95–98% RH.
The efficacy of the treatments was evaluated by measuring the lengths of the cankers that developed at the inoculation sites. Finally, the P. cactorum strain was re-isolated and morphologically identified from the lesions to fulfill Koch’s postulates.
4.8. Statistical Analyses
The results of the in vitro mycelium growth inhibition experiments were statistically analyzed using one-way analysis of variance (ANOVA), followed by a post hoc comparison of means using the Tukey test at p < 0.05, given that the homogeneity and homoscedasticity requirements were met, according to the Shapiro–Wilk and Levene tests. For the ex situ assays on excised ‘Garnem’ stems, in which normality and homoscedasticity requirements were not met, the Kruskal–Wallis nonparametric test was used instead, with the Conover–Iman test for post hoc multiple pairwise comparisons. R v.4.2.2 statistical software was used for all the statistical analyses [89].
5. Conclusions
In vitro antifungal/anti-oomycete and antibacterial tests showed the moderate–high activity of the aqueous ammonia extract of Q. suber cork, with EC90/MIC values ranging from 675 to 3450 μg·mL−1, depending on the pathogen tested. Upon conjugation with chitosan oligomers, substantial antimicrobial activity enhancement was observed, resulting in inhibitory values in the 340–801 μg·mL−1 range. The observed in vitro activity was lower than that of mancozeb, but remarkably higher than those of azoxystrobin and fosetyl-Al commercial fungicides, which were taken as a reference, and is among the highest reported for other natural compounds. In a first approximation, glycerin and vanillic acid, identified by gas chromatography–mass spectroscopy and tested as pure compounds, would be responsible for the observed activity. To assess the applicability of the treatment in more realistic conditions, the COS–Q. suber bark extract conjugate complex was further tested ex situ for the protection of excised stems artificially inoculated with P. cactorum, observing that a 10 times higher dose than the MIC determined in vitro (i.e., 3750 μg·mL−1) was required to achieve high protection, which would be twice the dose recommended for fosetyl-Al (2000 μg·mL−1) and approximately half that of azoxystrobin (ca. 6250 μg·mL−1). In view of these promising results, a possible valorization pathway for leftovers from the bottle stopper industry (cork fragments, granulates, and dust) as a source of phytochemicals for crop protection can be put forward.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11243415/s1, Figure S1. GC–MS chromatogram of Q. suber bark aqueous ammonia extract; Figure S2. Quercus suber tree in the Alcornocal de Valdegalindo, Foncastín, Valladolid, Spain; Table S1. Chemical species identified in Q. suber bark aqueous ammonia extract by GC–MS; Table S2. Examples of antimicrobial activity reported in the literature for other natural products rich in glycerin, vanillic acid, and azabicyclo derivatives; Table S3. Inhibition values reported in the literature for Q. suber extracts against pathogenic microorganisms; Table S4. Inhibitory values reported in the literature for bioactive natural substances against the pathogens under study. References [90,91,92,93,94,95] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, J.M.-G. and J.J.B.-V.; methodology, V.G.-G. and B.L.-V.; validation, J.C.-G. and B.L.-V.; formal analysis, E.S.-H., V.G.-G., J.C.-G. and P.M.-R.; investigation, E.S.-H., V.G.-G., J.C.-G., J.J.B.-V., J.B.-G., B.L.-V., J.M.-G. and P.M.-R.; resources, V.G.-G. and J.M.-G.; writing—original draft preparation, E.S.-H., V.G.-G., J.C.-G., J.J.B.-V., J.B.-G., B.L.-V., J.M.-G. and P.M.-R.; writing—review and editing, E.S.-H., V.G.-G. and P.M.-R.; visualization, E.S.-H.; supervision, P.M.-R.; project administration, J.M.-G. and P.M.-R.; funding acquisition, J.M.-G. and P.M.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Junta de Castilla y León under project VA258P18, with FEDER co-funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to their relevance to an ongoing Ph.D. thesis.
Acknowledgments
The authors would like to acknowledge Pilar Blasco and Pablo Candela from the Technical Research Services of the University of Alicante for conducting the GC–MS analysis. The authors would also like to acknowledge José Luis Palomo Gómez from the Aldearrubia Regional Diagnostic Center (Junta de Castilla y León) for providing the P. cactorum and P. syringae pv. syringae isolates used in the study.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bugalho, M.N.; Caldeira, M.C.; Pereira, J.S.; Aronson, J.; Pausas, J.G. Mediterranean cork oak savannas require human use to sustain biodiversity and ecosystem services. Front. Ecol. Environ. 2011, 9, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Mota, S.; Pinto, C.; Cravo, S.; Rocha e Silva, J.; Afonso, C.; Sousa Lobo, J.M.; Tiritan, M.E.; Cidade, H.; Almeida, I.F. Quercus suber: A promising sustainable raw material for cosmetic application. Appl. Sci. 2022, 12, 4604. [Google Scholar] [CrossRef]
- Gonçalves, F.; Correia, P.; Silva, S.P.; Almeida-Aguiar, C.; Bowater, L. Evaluation of antimicrobial properties of cork. FEMS Microbiol. Lett. 2016, 363, fnv231. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2013, 50, 345–365. [Google Scholar] [CrossRef]
- Carriço, C.; Ribeiro, H.M.; Marto, J. Converting cork by-products to ecofriendly cork bioactive ingredients: Novel pharmaceutical and cosmetics applications. Ind. Crops Prod. 2018, 125, 72–84. [Google Scholar] [CrossRef]
- Touati, R.; Santos, S.A.O.; Rocha, S.M.; Belhamel, K.; Silvestre, A.J.D. The potential of cork from Quercus suber L. grown in Algeria as a source of bioactive lipophilic and phenolic compounds. Ind. Crops Prod. 2015, 76, 936–945. [Google Scholar] [CrossRef]
- Coquet, C.; Bauza, E.; Oberto, G.; Berghi, A.; Farnet, A.; Ferré, E.; Peyronel, D.; Dal Farra, C.; Domloge, N. Quercus suber cork extract displays a tensor and smoothing effect on human skin: An in vivo study. Drugs Under Exp. Clin. Res. 2005, 31, 89–99. [Google Scholar]
- Coquet, C.; Ferré, E.; Peyronel, D.; Dal Farra, C.; Farnet, A.M. Identification of new molecules extracted from Quercus suber L. cork. Comptes Rendus Biol. 2008, 331, 853–858. [Google Scholar] [CrossRef]
- Fernandes, A.; Sousa, A.; Mateus, N.; Cabral, M.; de Freitas, V. Analysis of phenolic compounds in cork from Quercus suber L. by HPLC–DAD/ESI–MS. Food Chem. 2011, 125, 1398–1405. [Google Scholar] [CrossRef]
- Pinto, J.; Oliveira, A.S.; Lopes, P.; Roseira, I.; Cabral, M.; Bastos, M.d.L.; Guedes de Pinho, P. Characterization of chemical compounds susceptible to be extracted from cork by the wine using GC-MS and 1H NMR metabolomic approaches. Food Chem. 2019, 271, 639–649. [Google Scholar] [CrossRef]
- Borrero, C.; Castillo, S.; Casanova, E.; Segarra, G.; Trillas, M.I.; Castaño, R.; Avilés, M. Capacity of composts made from agriculture industry residues to suppress different plant diseases. Acta Hortic. 2013, 1013, 459–463. [Google Scholar] [CrossRef]
- Garcia, H.; Ferreira, R.; Martins, C.; Sousa, A.F.; Freire, C.S.R.; Silvestre, A.J.D.; Kunz, W.; Rebelo, L.P.N.; Silva Pereira, C. Ex situ reconstitution of the plant biopolyester suberin as a film. Biomacromolecules 2014, 15, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Subhashini, S.; Begum, S.M.; Rajesh, G. Antimicrobial characterisation combining spectrophotometric analysis of different oak species. Int. J. Herb. Med. 2016, 4, 32–35. [Google Scholar]
- Rungjindamai, N.; Jeffries, P.; Xu, X.-M. Epidemiology and management of brown rot on stone fruit caused by Monilinia laxa. Eur. J. Plant Pathol. 2014, 140, 1–17. [Google Scholar] [CrossRef]
- Balsells-Llauradó, M.; Silva, C.J.; Usall, J.; Vall-llaura, N.; Serrano-Prieto, S.; Teixidó, N.; Mesquida-Pesci, S.D.; de Cal, A.; Blanco-Ulate, B.; Torres, R. Depicting the battle between nectarine and Monilinia laxa: The fruit developmental stage dictates the effectiveness of the host defenses and the pathogen’s infection strategies. Hortic. Res. 2020, 7, 167. [Google Scholar] [CrossRef]
- Delgado-Cerrone, L.; Mondino-Hintz, P.; Alaniz-Ferro, S. Botryosphariaceae species associated with stem canker, die-back and fruit rot on apple in Uruguay. Eur. J. Plant Pathol. 2016, 146, 637–655. [Google Scholar] [CrossRef]
- Di Francesco, A.; Rusin, C.; Di Foggia, M.; Marceddu, S.; Rombolà, A.; Botelho, R.V.; Baraldi, E. Characterization of apple cultivar susceptibility to Neofusicoccum parvum Brazilian strains. Eur. J. Plant Pathol. 2020, 156, 939–951. [Google Scholar] [CrossRef]
- Donat, V.; Biosca, E.G.; Peñalver, J.; López, M.M. Exploring diversity among Spanish strains of Erwinia amylovora and possible infection sources. J. Appl. Microbiol. 2007, 103, 1639–1649. [Google Scholar] [CrossRef]
- Peng, L.; Yang, S.; Zhang, Y.; Haseeb, H.; Song, S.; Xu, X.; Yang, M.; Zhang, J. Characterization and genetic diversity of Pseudomonas syringae pv. syringae isolates associated with rice bacterial leaf spot in heilongjiang, china. Biology 2022, 11, 720. [Google Scholar] [CrossRef]
- Usall, J.; Casals, C.; Sisquella, M.; Palou, L.; De Cal, A. Alternative technologies to control postharvest diseases of stone fruits. Stewart Postharvest Rev. 2015, 11, 1–6. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; FHoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Lawal, O.; Bello, A.; Idem, R. The role of methyl diethanolamine (MDEA) in preventing the oxidative degradation of CO2 loaded and concentrated aqueous monoethanolamine (MEA)−MDEA blends during CO2 absorption from flue gases. Ind. Eng. Chem. Res. 2005, 44, 1874–1896. [Google Scholar] [CrossRef]
- Xie, H.-B.; Ma, F.; Wang, Y.; He, N.; Yu, Q.; Chen, J. Quantum chemical study on ·Cl-initiated atmospheric degradation of monoethanolamine. Environ. Sci. Technol. 2015, 49, 13246–13255. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Hoffmann, S.; Blomberg, L.G. Gas chromatographic—mass spectrometric analysis of compounds generated upon thermal degradation of some stationary phases in gas chromatography—Part II. J. High. Resolut. Chromatogr. 1985, 8, 734–740. [Google Scholar] [CrossRef]
- Michal, H.; Blanka, K.; Miloš, K. Chiral and nonchiral GC×GC/TOFMS analysis of natural compounds: The case of possible aggregation pheromones of chinese bark beetles Ips shangrila and Ips nitidus. In Gas Chromatography in Plant Science, Wine Technology, Toxicology and Some Specific Applications; Bekir, S., Çelikbıçak, O., Eds.; IntechOpen: Rijeka, Croatia, 2012; pp. 325–346. [Google Scholar] [CrossRef]
- Cunha, M.; Lourenço, A.; Barreiros, S.; Paiva, A.; Simões, P. Valorization of cork using subcritical water. Molecules 2020, 25, 4695. [Google Scholar] [CrossRef]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crops Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Batista, M.; Rosete, M.; Ferreira, I.; Ferreira, J.; Duarte, C.; Matias, A.; Poejo, J.; Crespo, J.; Valério, R.; Fraga, M. Extracto Hidro-Glicólico de Cortiça, Processo para a Sua Preparação, Formulações Compreendendo o Referido Extracto e Sua Utilização. WO2015152746A1, 1 April 2014. [Google Scholar]
- Sánchez-Hernández, E.; Balduque-Gil, J.; Barriuso-Vargas, J.J.; Casanova-Gascón, J.; González-García, V.; Cuchí-Oterino, J.A.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Holm oak (Quercus ilex subsp. ballota (Desf.) Samp.) bark aqueous ammonia extract for the control of invasive forest pathogens. Int. J. Mol. Sci. 2022, 23, 11882. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Martín-Ramos, P.; Martín-Gil, J.; Santiago-Aliste, A.; Hernández-Navarro, S.; Oliveira, R.; González-García, V. Bark extract of Uncaria tomentosa L. for the control of strawberry phytopathogens. Horticulturae 2022, 8, 672. [Google Scholar] [CrossRef]
- Canonico, P.G.; Jahrling, P.B.; Pannier, W.L. Antiviral efficacy of pyrazofurin against selected rna viruses. Antivir. Res. 1982, 2, 331–337. [Google Scholar] [CrossRef]
- Kumar, V.; Bhatt, V.; Kumar, N. Amides from Plants: Structures and Biological Importance; Atta-ur-Rahman, F.R.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 56, pp. 287–333. [Google Scholar]
- Pan, Y.-j. A novel lactam from Delphinium caeruleum. J. Zhejiang Univ. Sci. A 2000, 1, 186–187. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Delattre, M.; Haon, M.; Thibault, J.-F.; Ceccaldi, B.C.; Brunerie, P.; Asther, M. A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and Pycnoporus cinnabarinus. J. Biotechnol. 1996, 50, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Tschaplinski, T.J.; Wang, L.; Glazebrook, J.; Greenberg, J.T. Priming in systemic plant immunity. Science 2009, 324, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Hojo, Y.; Saito, Y.; Tanimoto, T.; Hoefen, R.J.; Baines, C.P.; Yamamoto, K.; Haendeler, J.; Asmis, R.; Berk, B.C. Fluid shear stress attenuates hydrogen peroxide-induced c-Jun NH2-terminal kinase activation via a glutathione reductase-mediated mechanism. Circ. Res. 2002, 91, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.; Zimmerman, P.; Wildermuth, M. Natural volatile organic compound emission rate estimates for U.S. woodland landscapes. Atmos. Environ. 1994, 28, 1197–1210. [Google Scholar] [CrossRef]
- Mudd, S.H. The mechanism of the enzymatic cleavage of S-adenosylmethionine to α-amino-γ-butyrolactone. J. Biol. Chem. 1959, 234, 1784–1786. [Google Scholar] [CrossRef]
- Stout, E.I.; McKessor, A. Glycerin-based hydrogel for infection control. Adv. Wound Care 2012, 1, 48–51. [Google Scholar] [CrossRef]
- Mackie, D.P. The Euro Skin Bank: Development and application of glycerol-preserved allografts. J. Burn Care Rehabil. 1997, 18, s7–s9. [Google Scholar] [CrossRef]
- Linser, A. Glycerine as Fungicide or Bactericide Active. Substance. Patent WO/2002/069708, 12 September 2002. [Google Scholar]
- Jamilah, J.; Sharifa, A.; Sharifah, N. GC-MS analysis of various extracts from leaf of Plantago major used as traditional medicine. World Appl. Sci. J. 2012, 17, 67–70. [Google Scholar]
- Prabhadevi, V.; Sahaya, S.S.; Johnson, M.; Venkatramani, B.; Janakiraman, N. Phytochemical studies on Allamanda cathartica L. using GC–MS. Asian Pac. J. Trop. Biomed. 2012, 2, S550–S554. [Google Scholar] [CrossRef]
- Jatin, R.R.; Priya, R.S. Determination of bioactive components of Cynodon dactylon by GC-MS analysis & its in vitro antimicrobial activity. Int. J. Pharm. Life Sci. 2016, 7, 4880–4885. [Google Scholar]
- Hameed, R.H.; Mohammed, G.J.; Hameed, I.H. Characterization of antimicrobial metabolites produced by Salvadora persica and analysis of its chemical compounds using GC-MS and FTIR. Indian J. Public Health Res. Dev. 2018, 9, 241. [Google Scholar] [CrossRef]
- Maria, K.K.; Joanna, G.K.; Katerina, A.D.; Leland, G.K. Chemical composition and antibacterial activity against Escherichia coli of extracts of a common household plant. J. Med. Plants Res. 2021, 15, 56–63. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K.; et al. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Li, Y.; Cai, C.; Zeng, Q.; Zhang, J.; Liu, M.; Sun, Z.; Wang, T.; Yang, M.I.N.; Qian, W. Antibacterial mechanism of vanillic acid on physiological, morphological, and biofilm properties of carbapenem-resistant Enterobacter hormaechei. J. Food Prot. 2020, 83, 576–583. [Google Scholar] [CrossRef]
- Delaquis, P.; Stanich, K.; Toivonen, P. Effect of pH on the inhibition of Listeria spp. by vanillin and vanillic acid. J. Food Prot. 2005, 68, 1472–1476. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, M.-C.; Kim, J.-S.; Balasundaram, C.; Jawahar, S.; Heo, M.-S. Identification and antimicrobial activity of combined extract from Azadirachta indica and Ocimum sanctum. Isr. J. Aquac.-Bamidgeh 2010, 62, 85–95. [Google Scholar] [CrossRef]
- Mudhafar, M.; Zainol, I.; Jaafar, C.; Alsailawi, H.; Majhool, A.A.; Alsaady, M. Phytochemical screening and characterization of Melia dubia leaves extract for antimicrobial activity against Escherichia coli and Staphylococcus aureus. Indian J. Ecol. 2020, 47, 493–496. [Google Scholar]
- Suryowati, T.; Sirait, R.H.; Siagian, F.E.; Nursyam, M. Bioactive compound impacting the metabolism and antibacterial activity of gadung tuber (Dioscorea hispida Dennst). J. Phys. Conf. Ser. 2020, 1665, 012030. [Google Scholar] [CrossRef]
- Akroum, S. Antifungal activity of acetone extracts from Punica granatum L., Quercus suber L. and Vicia faba L. J. Mycol. Médicale 2017, 27, 83–89. [Google Scholar] [CrossRef]
- Popović, B.M.; Štajner, D.; Ždero, R.; Orlović, S.; Galić, Z. Antioxidant characterization of oak extracts combining spectrophotometric assays and chemometrics. Sci. World J. 2013, 2013, 134656. [Google Scholar] [CrossRef]
- Lahlimi-Alami, Q.; Layachi, R.; Hassikou, R.; Benjelloun, J.; Amallah, L.; Guennoun, N.; Zaid, Y.; Bouzroud, S. Anticandidosic activity and acute toxicity of Quercus suber L. bark extracts. J. Med. Chem. Sci. 2022, 5, 769–778. [Google Scholar] [CrossRef]
- Hassikou, R.; Oulladi, H.; Arahou, M. Activité antimycosique des extraits du chêne-liège Quercus suber sur Trichophyton rubrum et Candida albicans. Phytothérapie 2014, 12, 206–212. [Google Scholar] [CrossRef]
- Akroum, S.; Rouibah, M. Utilisation d’extraits méthanoliques de plantes pour la protection des cultures de tomates-cerises (Solanum lycopersicum var. cerasiforme) contre l’infection fongique par Alternaria alternata. Biol. Aujourd’hui 2020, 214, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Morales, D. Oak trees (Quercus spp.) as a source of extracts with biological activities: A narrative review. Trends Food Sci. Technol. 2021, 109, 116–125. [Google Scholar] [CrossRef]
- Onaran, A.; Yanar, Y. In vivo and in vitro antifungal activities of five plant extracts against various plant pathogens. Egypt. J. Biol. Pest Control 2016, 26, 405–411. [Google Scholar]
- El Khetabi, A.; Lahlali, R.; Askarne, L.; Ezrari, S.; El Ghadaroui, L.; Tahiri, A.; Hrustić, J.; Amiri, S. Efficacy assessment of pomegranate peel aqueous extract for brown rot (Monilinia spp.) disease control. Physiol. Mol. Plant Pathol. 2020, 110, 101482. [Google Scholar] [CrossRef]
- Mamoci, E.; Cavoski, I.; Simeone, V.; Mondelli, D.; Al-Bitar, L.; Caboni, P. Chemical composition and in vitro activity of plant extracts from Ferula communis and Dittrichia viscosa against postharvest fungi. Molecules 2011, 16, 2609–2625. [Google Scholar] [CrossRef]
- El Khetabi, A.; Ezrari, S.; El Ghadraoui, L.; Tahiri, A.; Ait Haddou, L.; Belabess, Z.; Merah, O.; Lahlali, R. In vitro and in vivo antifungal activities of nine commercial essential oils against brown rot in apples. Horticulturae 2021, 7, 545. [Google Scholar] [CrossRef]
- Andreu, V.; Levert, A.; Amiot, A.; Cousin, A.; Aveline, N.; Bertrand, C. Chemical composition and antifungal activity of plant extracts traditionally used in organic and biodynamic farming. Environ. Sci. Pollut. Res. 2018, 25, 29971–29982. [Google Scholar] [CrossRef]
- Langa-Lomba, N.; Buzón-Durán, L.; Martín-Ramos, P.; Casanova-Gascón, J.; Martín-Gil, J.; Sánchez-Hernández, E.; González-García, V. Assessment of conjugate complexes of chitosan and Urtica dioica or Equisetum arvense extracts for the control of grapevine trunk pathogens. Agronomy 2021, 11, 976. [Google Scholar] [CrossRef]
- Langa-Lomba, N.; Buzón-Durán, L.; Sánchez-Hernández, E.; Martín-Ramos, P.; Casanova-Gascón, J.; Martín-Gil, J.; González-García, V. Antifungal activity against Botryosphaeriaceae fungi of the hydro-methanolic extract of Silybum marianum capitula conjugated with stevioside. Plants 2021, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Langa-Lomba, N.; Sánchez-Hernández, E.; Buzón-Durán, L.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Activity of anthracenediones and flavoring phenols in hydromethanolic extracts of Rubia tinctorum against grapevine phytopathogenic fungi. Plants 2021, 10, 1527. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, O.; Imani, J. Inhibitory effect of CUSTOS, a formulated Allium-based extract, on the growth of some selected plant pathogens. Int. J. Plant Biol. 2022, 13, 44–54. [Google Scholar] [CrossRef]
- Minova, S.; Sešķēna, R.; Voitkāne, S.; Metla, Z.; Daugavietis, M.; Jankevica, L. Impact of pine (Pinus sylvestris L.) and spruce (Picea abies (L.) Karst.) bark extracts on important strawberry pathogens. Proc. Latv. Acad. Sci. Sect. B. Nat. Exact Appl. Sci. 2015, 69, 62–67. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, J.; Shin, S.-C.; Lee, S.-G.; Park, I.-K. Antifungal activity of Myrtaceae essential oils and their components against three phytopathogenic fungi. Flavour Fragr. J. 2008, 23, 23–28. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.S.; Lee, S.G.; Shin, S.C.; Park, I.K. Fumigant antifungal activity of plant essential oils and components from West Indian bay (Pimenta racemosa) and thyme (Thymus vulgaris) oils against two phytopathogenic fungi. Flavour Fragr. J. 2008, 23, 272–277. [Google Scholar] [CrossRef]
- Fontana, R.; Macchi, G.; Caproni, A.; Sicurella, M.; Buratto, M.; Salvatori, F.; Pappadà, M.; Manfredini, S.; Baldisserotto, A.; Marconi, P. Control of Erwinia amylovora growth by Moringa oleifera leaf extracts: In vitro and in planta effects. Plants 2022, 11, 957. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Cuchí-Oterino, J.A.; Martín-Gil, J.; Lorenzo-Vidal, B.; Martín-Ramos, P. Dwarf pomegranate (Punica granatum L. var. nana): Source of 5-HMF and bioactive compounds with applications in the protection of woody crops. Plants 2022, 11, 550. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Physicochemical characterization and antimicrobial activity against Erwinia amylovora, Erwinia vitivora, and Diplodia seriata of a light purple Hibiscus syriacus L. cultivar. Plants 2021, 10, 1876. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Langa-Lomba, N.; Casanova-Gascón, J.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Characterization and antimicrobial activity of a halophyte from the Asturian coast (Spain): Limonium binervosum (G.E.Sm.) C.E.Salmon. Plants 2021, 10, 1852. [Google Scholar] [CrossRef]
- Shabani, B.; Rezaei, R.; Charehgani, H.; Salehi, A. Study on antibacterial effect of essential oils of six plant species against Pseudomonas syringae pv. syringae Van Hall 1902 and Pseudomonas fluorescens Migula 1894. J. Plant Pathol. 2019, 101, 671–675. [Google Scholar] [CrossRef]
- Islam, M.S.; Sultana, R.; Hasan, M.A.; Alam, M.S.; Sikdar, B.; Kamaruzzaman, M.; Islam, M.A. Characterization and biocontrol measures of Pseudomonas syringae pv. syringae associated with citrus blast disease. Vegetos 2020, 33, 555–569. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Santiago-Aliste, A.; Torres-Sánchez, S.; Martín-Ramos, P. Lignin–chitosan nanocarriers for the delivery of bioactive natural products against wood-decay phytopathogens. Agronomy 2022, 12, 461. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2017, 36, 57–67. [Google Scholar] [CrossRef]
- Sannan, T.; Kurita, K.; Iwakura, Y. Studies on chitin, 2. Effect of deacetylation on solubility. Die Makromol. Chem. 1976, 177, 3589–3600. [Google Scholar] [CrossRef]
- Yang, Y.; Shu, R.; Shao, J.; Xu, G.; Gu, X. Radical scavenging activity of chitooligosaccharide with different molecular weights. Eur. Food Res. Technol. 2005, 222, 36–40. [Google Scholar] [CrossRef]
- Maghami, G.G.; Roberts, G.A.F. Evaluation of the viscometric constants for chitosan. Die Makromol. Chem. 1988, 189, 195–200. [Google Scholar] [CrossRef]
- Tian, M.; Tan, H.; Li, H.; You, C. Molecular weight dependence of structure and properties of chitosan oligomers. RSC Adv. 2015, 5, 69445–69452. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- Levy, Y.; Benderly, M.; Cohen, Y.; Gisi, U.; Bassand, D. The joint action of fungicides in mixtures: Comparison of two methods for synergy calculation. EPPO Bull. 1986, 16, 651–657. [Google Scholar] [CrossRef]
- CLSI. CLSI standard M07—Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Matheron, M.E. Seasonal variation in susceptibility of Juglans hindsii and Paradox rootstocks of English walnut trees to Phytophthora citricola. Phytopathology 1985, 75, 970. [Google Scholar] [CrossRef]
- Álvarez Bernaola, L.A. Estudios de Etiología, Epidemiología y Control de un Nuevo Síndrome de Lesiones en Tronco y Ramas Principales de Cítricos Asociado a Phytophthora; Universitat Politècnica de València: Valencia, Spain, 2008. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Nehad, M.G.; Abdulrahaman, S.H. Antimicrobial efficacy of Casuarina equisetifolia extracts against some pathogenic microorganisms. J. Med. Plants Res. 2012, 6, 5819–5825. [Google Scholar]
- Naz, S.; Ahmad, S.; Ajaz Rasool, S.; Asad Sayeed, S.; Siddiqi, R. Antibacterial activity directed isolation of compounds from Onosma hispidum. Microbiol. Res. 2006, 161, 43–48. [Google Scholar] [CrossRef]
- Ouerghemmi, I.; Bettaieb Rebey, I.; Rahali, F.Z.; Bourgou, S.; Pistelli, L.; Ksouri, R.; Marzouk, B.; Saidani Tounsi, M. Antioxidant and antimicrobial phenolic compounds from extracts of cultivated and wild-grown Tunisian Ruta chalepensis. J. Food Drug Anal. 2017, 25, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Ćurković-Perica, M.; Hrenović, J.; Kugler, N.; Goić-Barišić, I.; Tkalec, M. Antibacterial activity of Pinus pinaster bark extract and its components against multidrug-resistant clinical isolates of Acinetobacter baumannii. Croat. Chem. Acta 2015, 88, 133–137. [Google Scholar] [CrossRef]
- Salamone, A.; Zizzo, G.V.; Scarito, G. The antimicrobial activity of water extracts from Labiatae. Acta Hortic. 2006, 465–470. [Google Scholar] [CrossRef]
- Carezzano, M.E.; Sotelo, J.P.; Primo, E.; Reinoso, E.B.; Paletti Rovey, M.F.; Demo, M.S.; Giordano, W.F.; Oliva, M.D.L.M.; Flemetakis, E. Inhibitory effect of Thymus vulgaris and Origanum vulgare essential oils on virulence factors of phytopathogenic Pseudomonas syringae strains. Plant Biol. 2017, 19, 599–607. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).