Abstract
The present study aims to investigate the effectiveness of bioformulations based on endophytic fungi to control apple scab and Valsa canker disease in two orchards in the Aurès region (Algeria). In both orchards, the results showed that the treatment of senescent apple leaves by invert emulsions containing Trichoderma longibrachiatum and Chaetomium globosum harmed the ascogenesis of winter forms of Venturia inaequalis by reducing the number of ascospore-ejecting asci, the number of morphologically mature asci, and a considerable increase in the immature asci number. This antifungal activity was more essential in soil-incorporated leaves, showing the importance of the combination of treatments with cultural practices to efficiently control the apple scab disease. Furthermore, the disease incidence decreased by 52.63% and 50.68% in R’haouat and Bouhmama orchards, respectively. Moreover, the treatment of Valsa ceratosperma cankers with a biogel containing the endophytic yeast Metschnikowia sp. led to wound healing varying from 43.52% and 87.97% after 120 days but remained more considerable than conventional treatment with Folicur (tebuconazol). The current results open real opportunities concerning the implementation of eco-friendly and potent apple protection systems.
1. Introduction
Apple production is an integral part of the arboriculture sector, constantly improving in terms of production techniques [1,2]. This progress is justified because consumer requirements necessitate a product of high and regular yield, considerable commercial quality, and plasticity regarding cultivation techniques and because the search for new resistant genotypes against notable pests and diseases is required [3].
In 2020, global apple production reached approximately 86.44 million tonnes with a yield of 187.01 cwt/ha [4]. Algeria is a Mediterranean country whose climatic and edaphic conditions in some Northern regions of the country offer a suitable environment for apple cultivation. National apple production reached 566,824 tonnes in 2020 with a yield of 171.63 cwt/ha [4]. This yield is low compared to the global average, attributed to several climatic or agro-technical factors. On the other hand, harmful organisms are frequently encountered on apple trees and are involved in the reduction in production, causing significant damage to production quality [5]. In the Aurès province, apple production was mainly practiced in mountainous areas. The cultivars are mainly Golden Delicious (65%), Starkrimsson (16%), Royal Gala (14%), and Anna (5%), while the area planted by this fruit species increased from 3009.5 ha in 2012 to 3690 ha in 2019 where the average yield was of the order of 243.902 cwt/ha (Batna Agricultural Services Administration).
The main fungal diseases encountered in the Aurès apple orchards are the scab caused by Venturia inaequalis (Cooke G. Winter). The causal agent has a life cycle consisting of two phases; the saprophytic phase that occurs in the litter and the superficial layers of the soil, but this fungus is most often preserved as an overwintering immature pseudothecia [6,7,8], while the parasitic phase occupies a large part of the vegetative and reproductive growing season of the host plant [9]. In spring, from the leaf litter, projections of ascospores, which cause primary infections, occur during each rainy event; they are at the origin of primary infections [7]. After leaf penetration using ascospore arising germ tubes, the growth of V. inaequalis become restricted to the subcuticular level as a multi-layered and compacted mycelial structures known as stromata from which it derives its nutrients until an advanced stage of the disease. The symptoms are visible especially on leaves and fruits by the appearance of initially green, oily spots that turn brown–grey–black. These spots enlarge and may induce cracks that may in turn cause the fruit to dry out. The efficient secondary dissemination of the disease is ensured by conidia resulting from cumulative asexual reproduction during the host’s growing season [10].
Scab infection causes fruit deformation and premature leaf and fruit drop, which increase the sensitivity of the host plant to biotic and abiotic stresses, therefore, crop losses can reach 70% and the fruit can become unmarketed [11]. The mechanisms of scab resistance have been the subject of several studies and reviews [12,13,14,15,16] although most apple cultivars are susceptible to scabs including those known for their high commercial value.
Valsa cankers, also known as Cytospora canker or Leucostomial canker, are induced by the species Valsa mali (Miyabe and G. Yamada) [17]. It is also induced by a second species, Valsa ceratosperma (Tode Maire). The symptoms are characterized by the development of more or less localized cankers on the bark that enlarge and can completely surround the twigs, leading to the death of the latter, the twigs, and the entire tree [18]. Valsa canker is a devastating disease on apples that induces serious economic losses worldwide [19,20].
The occurrence of canker diseases induced by a Valsa complex has recently reached apple orchards in the Aurès that have faced climatic accidents such as hail or alternating cold and hot-dry periods, leading to bursting stems. Apple tree infection is carried out through pruning wounds and fruit scars [21]. Canker develops more rapidly between spring and early summer [21]. While considered a synonym to V. mali [22], V. ceratosperma (Cytospora ceratosperma (Tode G.C. Adams and Rossman)) is currently reclassified as a separate species [23,24,25,26,27,28]. V. ceratosperma is characterized by its spore heterogeneity and polymorphism and its broad host spectrum than V. mali [22,29,30], while rDNA-ITS sequence analysis revealed that this fungus represents heterogeneous species complex [26].
Farmers regularly follow a chemical control approach, repeatedly using a limited number of fungicides, leading to a loss of efficiency in particular given that some producers do not control phytosanitary treatment techniques. This situation has reinforced the arguments for healthy production. However, the technical specificities of the apple production system suggest the development of new approaches, especially the use of protection introns, while preserving the quantitative and qualitative status of the harvest.
Because of its economic potential and added value, apple production is more predisposed to the establishment of a biological control strategy that can ensure the attaining of a healthy and, therefore, more attractive fruit product within the markets [31,32]. The biological control of apple scab and Valsa canker using endophytic microorganisms has been subject to several studies [33,34,35,36,37,38] revealing several mechanisms of action such as antibiosis, mycoparasitism, and induced resistance [34,38,39]. The phytosanitary products of biological origin must be formulated in order to ensure its effectiveness and stability with regard to the conditions of the natural environment. In this context, we have carried out a series of experiments in two orchards in the Aurès region to test the effectiveness of biological treatments based on mycoendophyte formulations against apple scab and Valsa canker disease.
2. Results
2.1. Apple Scab Incidence after Leaf Treatment
The incidence of scabs decreased over the year of treatment. The disease incidence decreased by 52.63% and 50.68% in R’haouat and Bouhmama orchards, respectively (Figure 1). The determination of the incidence was determined during the same period as the previous year. This indicates that litter treatments with inverted emulsions resulted in a reduction in the infectivity of the inoculum.
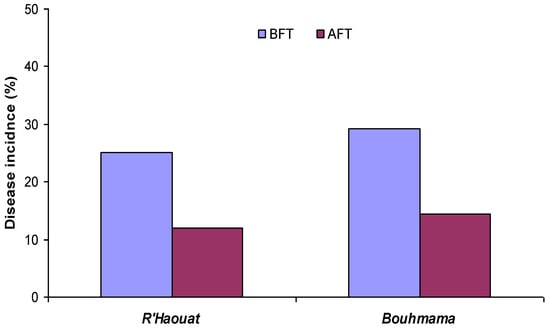
Figure 1.
Apple scab incidence before (BFR) and after (AFT) leaf litter treatment by invert emulsions.
2.2. Effect of Invert Emulsion Application on Wintering Forms of V. inaequalis
After the incubation period, the reproductive structures representing the different stages of maturity of V. inaequalis pseudothecia were inspected in the laboratory.
For unincorporated leaves, the treatments led to a decrease in the average number of asci-ejecting ascospores (AA) compared to untreated leaves. This decrease varies depending on the fungal species used for emulsion-treated leaves containing T. longibrachiatum and C. globosum in the R’Haouat and Bouhmama orchards (p < 0.0001) (Table 1). This decrease is more considerable in incorporated leaves for both orchards (Table 1).

Table 1.
Impact of biological treatments of senescent apple leaves with invert emulsions on numbers of asci-ejecting ascospores (AA), immature asci (IA), and morphologically mature asci (MA) of wintering form of Venturia inaequalis.
In contrast, the average number of immature asci (IA) was increased considerably in unincorporated leaves, especially in those treated with C. globosum emulsion in R’Haouat (Pr < 0.0001). While in the Bouhmama orchard, the reduction was 66.36% and 79.61% after treatment with T. longibrachiatum and C. globosum emulsions, respectively. In addition, the treatment of incorporated leaves led to an increase in IA by 125.85% (T. longibrachiatum emulsion) and by 186.49% (C. globosum emulsion) in the R’Haouat orchard (Pr < 0.0001) and by 164.17% (T. longibrachiatum emulsion) and 232.18% (C. globosum emulsion) in the Bouhmama orchard (Pr < 0.0001) (Table 1).
Finally, the mean number of morphologically mature asci (MA) from unincorporated leaves decreased for those treated with T. longibrachiatum and C. globosum emulsion (Pr < 0.0001) (Table 1). Whereas in the incorporated leaves, this reduction recorded average values of 43.81% (T. longibrachiatum emulsion) and 69.87% (C. globosum emulsion) for the R’Haouat orchard (Pr < 0.0001) and only 9.57% (T. longibrachiatum emulsion) and 69.76% (C. globosum emulsion) for the Bouhmama orchard (Table 1).
2.3. Effect of Biogel Application on Apple Canker
After the treatment of apple branches by the biogel, we noticed a gradual reduction in cankers during the follow-up period (120 days). At the R’Haouat orchard, the mean area of the canker was reduced, resulting in a healing rate of 43.52% (Figure 1), while for the branches treated with tebuconazol, the mean area of the canker was decreased, with a healing rate of 18.18%. Finally, less important healing (11.11%) was also recorded for untreated trees (Figure 2).
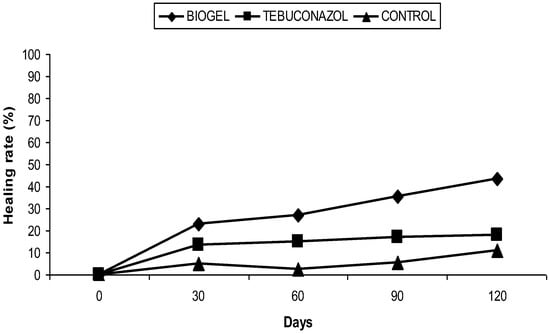
Figure 2.
Effect of biogel application on apple canker in the R’Haouat orchard.
In the second orchard (Bouhmama), the biogel treatment also led to a decrease in the average area of canker, i.e., a healing rate of 87.97% (Figure 3), whereas for the trees treated with tebuconazol, the area of cankers was reduced, resulting in a healing rate of 64.10% (Figure 3). For the untreated trees, the mean area of the cankers was increased from day 0 to day 30, stabilized on days 60 and 90, and reached 188.75 mm2 on the 120th day. Thus, no healing was recorded during this period.
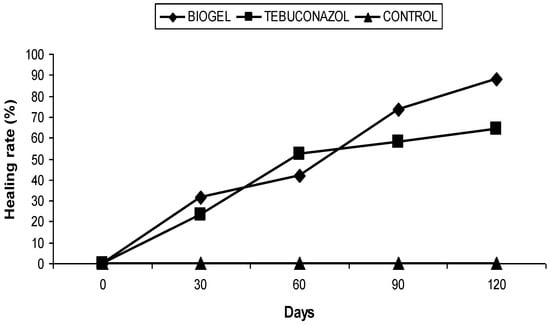
Figure 3.
Effect of biogel application on apple canker in Bouhmama orchard.
3. Discussion
The biological control of apple scab disease using fungal antagonists or mycoparasites, including Trichoderma spp. C. globosum, was the object of several works [40,41,42]. In this study, the ascocarp maturity of V. inaequalis is closely related to the physical and chemical conditions that mark the apple litter from the late fall to early spring season [43]. The period during which the pseudothecia undergo a sequence of maturity stages of asci and ascospores were described by Meszka (2015) [44].
We have demonstrated that the treatment of apple senescent leaves with inverted emulsions leads to an alteration in the ascogenic potential and maturity of V. inaequalis ascocarps, considerably reducing the primary inoculum of the pathogen.
The cumulative evolution of the immature asci numbers (IA) and morphologically mature asci (MA) is probably the result of antibiotic activity of the tested endophytes that inhibit or slow down the growth and maturity of these reproductive structures, which multiplies the risks of a fatal exposure of juvenile fruiting bodies to adverse environmental conditions such as the acceleration of leaf biodegradation and, therefore, the suppression of leaf protection that wintering ascocarps benefit from. This maturity slowdown can lead to a delay or even an exclusion from the primary infection, which can be exceeded chronologically when the conditions become unfavorable.
T. longibrachiatum is known for its ability to produce non-volatile and volatile antifungal antibiotics [45,46]. Furthermore, Palani and Lalithakumari (1999) [47] have demonstrated the efficacy of transgenic strains of this fungal species regarding resistant and sensitive forms of V. inaequalis to penconazole, but the role of this fungus may prove to induce tolerance to abiotic stress (salinity) in wheat by improving the antioxidative defense system and gene expression [48].
In addition, certain antagonistic fungi can alter the infectious potential of phytopathogenic organisms by accelerating the decomposition of senescent leaves in combination with antifungal activity; this is the case of Athelia bombacina (Link) Pers. and Coniothyrium sp. [49] and the yeast Saccharomyces cerevisiae (Desm Meyen), which induced the reduction in ascospore production to 95% [50].
Using inverted emulsions containing T. longibrachiatum and C. globosum has proven their effectiveness against wintering forms of V. inaequalis, especially in a formulation containing C. globosum. Indeed, Miedtke and Kennel (1990) [33] showed that C. globosum is an effective antagonist against the perfect stage of V. inaequalis under field conditions. The effectiveness of bio-treatments is much more essential when incorporating senescent leaves to the soil, showing the importance of this cultural practice in optimizing the biological control of apple scab fungus. Carisse and Rolland (2004) [51] have shown that the treatment of apple litter using Microsphaeropsis ochracea (Carisse and Bernier) at the right time has negative consequences on the production and ascospore ejection of V. inaequalis.
Wound dressings have been considered to be a specific treatment method for wounds affecting woody parts of fruit and forest trees [52,53,54]. We have demonstrated that branch treatment with biogel containing the endophytic yeast Metschnikowia sp. led to the healing of the wound caused by the canker more effectively than those treated by tebuconazol. The biological control of tree cankers has been the subject of several studies dealing with the use of microorganisms such as the actinomycete Saccharothrix yanglingensis (Yan et al.), sp. nov. against Valsa mali (Miyabe and G. Yamada) [55], and antagonistic fungi such as Trichoderma viride (Pers.) [56,57] and T. longibrachiatum [58].
Biogels have been designed and used to control several fungal plant diseases [59,60,61], but their application could not be more extended as conventional hydrogels were involved in crop protection against water stress by maintaining a good water status of soil [62] for improving germination potential and seedling performance [63] or to control nutrient releasing from fertilizers [64]. Several mechanisms are involved in preventing plant diseases and conferring biocontrol activity to yeasts’ competition for nutrients and space, the secretion of toxins, enzymes, and volatile organic compounds, direct parasitisation, and induction of resistance [65]. More recently, an assessment of the antimicrobial activity of 10 M. pulcherrima isolates against 10 key potato phytopathogens by the agar-well diffusion method proved the efficiency of this yeast as a biocontrol agent, revealing biological activity attributed to the enzymes Leucine arylamidase, valine arylamidase, α- and β-glucosidase, and esterases [66]. The in situ experiment demonstrated a reduction by 30%–100% in nine phytopathogens on potato seeds [66]. Some yeasts belonging to the genus Metschnikowia were used as biological control agents, mainly against post-harvest diseases of stone and pome fruit, as for M. pulcherrima (Pitt and M.W. Mill) [67,68,69] and M. andauensis (O. Molnár and Prillinger) [70]. Using a metabolomic analysis of yeast-fungus co-culture, Millan et al. (2022) [71] detected 35 extracellular differential metabolites produced by Metschnikowia pulcherrima involved in the biocontrol of gray mold. In this, 3-amino-5-methylhexanoic acid, biphenyl-2,3-diol, and sinapaldehyde were the most active in reducing the infection of tomato and apple fruits up to 90–100%. The same authors showed that these metabolites negatively affected the cell membrane integrity and mycelial morphology of the causal agent (Botrytis cinerea) and increased the intracellular level of reactive oxygen species (ROS).
In the present study, we showed that this yeast is useful for protecting apple trees against Valsa ceratosperma canker if we include it in a gelled formulation. The progressive evolution of wound healing on trees treated with biogel showed a prolonged efficacy of the bioformulation and probably a covering or colonization of plant parts infected by the pathogen that suggests, in addition to the antibiotic effect, a dressing activity that lasts from 3 to 4 months. In the present work, endophyte yeast Metschnikowia sp. has been incorporated into a formulation containing compounds that are not harmful to the environment such as sodium alginate produced by brown algae Macrocystis pyrifera (L.) (C. Agardh) and Laminaria digitata (Hudson) [72]. This product is characterized by a process known as ionic gelation resulting from the cross-linking network formed from the bonding of Ca+2 ions with polyguluronic portions of the polymer units of β(1,4)-D-mannuronic acid and α(1,4)-L-guluronic acid [72]. The alginates not only contribute to the mechanical appearance of the gel but are also considered as a carbohydrate source for the yeast. The gel extracted from the Linum usitatissimum seeds was added to improve the mechanical stability of the gel; in fact, the mucilaginous gel of this plant consists essentially of arabinoxylan-type oligosaccharides and pectin [73]. On an experimental scale, the arabinoxylan polymer was used to control the powdery mildew, Rhynchosporiose, and Ramularia leaf spots on barley [74].
4. Materials and Methods
4.1. The Studied Orchards
Two apple orchards were considered for this study that was carried out during the period from December 2020 to May 2021. The first is located in R’Haouat (35°30′09.91″ N, 5°54′21.89″ E, 1400 m) with 226 apple trees (cv. Golden Delicious) spaced 4 m × 4 m, irrigated by the drip system, and fertilized only with organic manure. The chemical treatments were applied to control codling moth (Cydia pomonella L.) and mainly scabs and powdery mildew. The second is located in the Bouhmama region, at the southeastern edge of Chelia Mountain (35°18′55.95″ N, 6°42′11.54″ E, 1320 m) with 250 Golden Delicious trees among a total of 460 trees. The irrigation is provided by the drip system and the chemical treatments are mainly applied to control codling moths, mites, and apple scabs. All the trees in both orchards were grafted on MM106 rootstock and planted in 2014.
During the study year, producers have not used pesticides to allow the normal course of the experiment; only the application of bioformulated products that we have designed have been carried out.
4.2. Fungal Endophytes
Three endophytic mycotaxa were used as biological substrates for bioformulations: Trichoderma longibrachiatum (Rifai) (accession MF144551) and Chaetomium globosum (Kunze), both isolated from healthy leaves of native Hyoscyamus albus L. (Solanaceae) as well as Metschnikowia sp. isolated from the green fruits of Parthenocissus quinquefolia (L.) (Planch). Ampelidaceae Fungi are part of a fungal collection established at the plant protection laboratory. The cultural preservation was established according to the standards of the former IMI (International Mycological Institute) [75].
4.3. Pathogenic Fungi
The targeted fungi were: Venturia inaequalis, the causal agent of apple scab, and Valsa ceratosperma inducing branch canker of the apple. The incidence of apple scab and Valsa canker was determined in the year preceding the current study (25.11–14.10% and 29.6–10.4% in R’Haouat and Bouhmama orchards, respectively).
4.4. Preparation of the Bioformulations
4.4.1. The Invert Emulsions
They were designed using T. longibrachiatum and C. globosum as biological fractions. Each bioformulation comprises one aqueous phase and another oily phase. The aqueous phase contains additional conidial suspension of the endophyte (approximately from 106 to 107 spores.mL−1) and salicylic acid (0.5 g/500 mL sterilized distilled water), while the oily phase includes a low viscosity vegetable oil to which we have added Tween 80 (0.1%) (3 mL) and sodium alginate (1.1% w/v) (3 mL) [76]. Both phases were homogenized by mechanical agitation to become yellowish-green (T. longibrachiatum) and light-yellow (C. globosum) emulsions. The emulsions were preserved in the refrigerator (at from 2° to 4 °C).
4.4.2. The Biogel
A Biogel formulation containing Metschnikowia sp. suspension was prepared. The cell suspension of the yeast was prepared (107 cell.mL−1) to which we added Tween 80 (0.1%) and then sodium alginate (2% w/v). The mixture was homogenized by magnetic stirring for 30 min, after which we added oat bran and talc, then the contents we stirred magnetically for one hour. After stirring, the mucilaginous gel extracted from the linseed (Linum usitatissimum L.) was added. The resulting gel was kept in the refrigerator (2 °C) for 6 h before applying to the cankers.
4.5. Invert Emulsion Application on Wintering Forms of V. inaequalis
The inverted emulsions were applied to the apple senescent leaves (at the litter level) to target the wintering forms of V. inaequalis. The choice of this leaf stage is justified by the fact that V. inaequalis is a hemibiotrophic fungus that overwinters in senescent and dead leaves, in which microscopic flasked black fruiting bodies (pseudothecia) are developed. These fruiting bodies mature in early spring, ejecting the ascospores in suitable weather conditions. Sub-plots of 4 m2 (1 m × 4 m) were selected randomly from each orchard. After repeated cleaning, they were covered with polyester plates for 48 h. Subsequently, senescent leaves were collected and placed on each sub-plot. After 12 h, the leaves were sprayed with emulsions. Each sub-plot is treated with a bioformulation containing a fungal bio-substratum alone. Ten sub-plots have been treated. The sub-plots have been divided into two classes according to a cultural modality; four leaves were left on the soil surface (unincorporated litter), whereas the other four were incorporated into the soil manually (incorporated litter). The remaining two sub-plots (unincorporated and incorporated litter) formed the untreated controls. A second treatment was carried out on the same subplots 15 days after the first, following the same procedures mentioned above.
The sub-plots were covered with a light layer of sterile mesh sleeves and replaced each week by a new tissue; the incubation of the leaves lasted 42 days.
The leaves were sampled and transferred to the laboratory to evaluate the effectiveness of the invert emulsions based on the determination and quantification of ascogenic phases indicating the development and maturity of the wintering pseudothecia of V. inaequalis. Thus, the mean number of asci-ejecting ascospores (AA), the mean number of immature asci (IA), and morphologically mature asci (MA) were quantified.
4.6. Invert Emulsion Application on Wintering Forms of V. inaequalis
The cankers were treated using the elaborated biogel; 24 wounds (cankers) detected in 14 trees that were treated in the first orchard (R’Haouat) and 21 were detected in 16 trees in the second (Bouhmama). It should be noted that an apple tree may contain more than one canker. The bark surrounding the canker was first removed and then the biogel was applied, forming a gelatinous layer from 5 mm to 12 mm thick, covering the entire wound. The conventional treatment with Folicur® WG (Bayer Crop Science, Leverkusen, Germany) (tebuconazol) at a dose of 40 g/100 L was applied on 10 other cankers, while some cankers (8 for the R’Haouat orchard and 10 for the Bouhmama orchard) did not undergo any treatment.
The treatments were performed on 28 January and 28 February. Careful monitoring of cankers’ evolutions was carried out over 120 days to assess the effectiveness of biogel and Folicur by measuring the wound healing rate. Photos were taken for each canker, first just before treatment and then 30, 60, 90, and 120 days after the last treatment. The resulting photos were then converted to Tiff format and processed via the ImageJ software [77]. First calibrated to a 1 mm unit, the area of the canker (mm2) was calculated based on the determination of the depression area that remains in the center of the canker. The canker area was calculated by an algorithm used by the software after spectral scanning.
4.7. Statistical Analysis
An analysis of the variance with the LSD Fisher test (α = 0.05) was adopted to characterize the effect of the biological substrate (fungal species) of emulsion on the mean number of asci-ejecting ascospores and immature asci, as well as the morphologically mature asci.
5. Conclusions
This study showed that the application of bioformulations derived from endophytic fungi can have a positive effect on the phytosanitary status of apple trees and provide natural protection against scab and Valsa canker disease. Inverse emulsion based on Chaetomium globosum and Trichoderma longibrachiatum shows promise as a biopesticide. We have also shown the importance of cultural practices (the incorporation of litter leaves) in increasing the effectiveness of the formulation, indicating the importance of combining different approaches to apple scab control. Furthermore, using new biogel formulated with the endophytic yeast, Metschnikowia sp. offers a new perspective on the natural control of Valsa cankers on apple trees. The use of a biological control involving antagonistic mycoendophytes is much more promising because introduces the conception of suitable formulations without forgetting good cultural practices as part of an integrated disease management system. Based on the results of this study, the bioformulations derived from endophytic fungi based on Chaetomium globosum and Trichoderma longibrachiatum could be recommended in crop protection systems for efficient diseases control, as well as in healthy products.
Author Contributions
Conceptualization, O.A.B. and R.B.; methodology, O.A.B. and R.B.; software, R.B.; validation, N.Y.R.; formal analysis, O.A.B. and T.A.; investigation, O.A.B.; resources, O.A.B., T.A. and N.Y.R.; data curation, R.B.; writing—original draft preparation, O.A.B., N.Y.R. and A.V.P.; writing—review and editing, O.A.B., N.Y.R., R.R.G. and A.V.P.; visualization, D.E.K.; supervision, O.A.B., N.Y.R.; project administration, D.E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This paper has been supported by the RUDN University Strategic Academic Leadership Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- O’Rourke, D. World Production, trade, consumption and economic outlook for apples. In Apples: Botany, Production and Uses; Ferree, D.C., Warrington, I.J., Eds.; CAB International: Wallingford, UK, 2003; pp. 15–29. [Google Scholar]
- Koch, T. Differential Resistance and Virulence in the Interaction of Malus × domestica and Venturia inaequalis. Ph.D. Thesis, Swiss Federal Institute of Technology, Zurich, Switzerland, 1998; 98p. [Google Scholar] [CrossRef]
- Hampson, C.R.; Kemp, H. Characteristics of important commercial apple cultivars. In Apples: Botany, Production and Uses; Ferree, D.C., Warrington, I.J., Eds.; CAB International: Wallingford, UK, 2003; pp. 61–89. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/fr/#data/QCL (accessed on 2 August 2022).
- Turechek, W.W. Apple Diseases and their Management. In Diseases of Fruits and Vegetables; Naqvi, S.A.M.H., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; Volume 1, pp. 1–108. [Google Scholar]
- Bernier, J.; Carisse, O.; Paulitz, T.C. Fungal communities isolated from dead apple leaves from orchards in Québec. Phytoprotection 1996, 77, 129–134. [Google Scholar] [CrossRef][Green Version]
- Trapman, M. Copper free production of organic apples: Three years experience in the Netherlands. In Journées Techniques Fruits et Légumes Biologiques; ITAB: Paris, France, 2009; pp. 61–89. [Google Scholar]
- Bowen, J.K.; Mesarich, C.H.; Bus, V.G.M.; Beresford, R.M.; Plummer, K.M.; Templeton, M.D. Venturia inaequalis: The causal agent of apple scab. Mol. Plant Pathol. 2011, 12, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Hebrard, M.A. La Tavelure en Vergers de Pommiers: Suivi de la Contamination Primaire et Evaluation de Nouvelles Substances Actives SDHI Contre Venturia inaequalis. Master’s Thesis, Université de Rennes I, Rennes, France, 2013; 79p. [Google Scholar]
- Smereka, K.J.; MacHardy, W.E.; Kausch, A.P. Cellular differentiation in Venturia inaequalis ascospores during germination and penetration of apple leaves. Can. J. Bot. 1987, 65, 2549–2561. [Google Scholar] [CrossRef]
- Jha, G.; Thakur, K.; Thakur, P. The Venturia Apple Pathosystem: Pathogenicity Mechanisms and Plant Defense Responses. J. Biomed. Biotechnol. 2009, 2009, 680160. [Google Scholar] [CrossRef]
- Szkolnik, M. Relative susceptibility to scab and production of conidia among 30 apple varieties. In Proceedings of the Apple and Pear Scab Workshop, Kansas City, Missouri, 11 July 1976; New York Agricultural Experimental Station Special Report; Jones, A.L., Gilpatrick, J.D., Eds.; New York State Agricultural Experiment Station Cornell University: Geneva, NY, USA, 1978; Volume 28, pp. 11–14. [Google Scholar]
- Cusin, R.; Revers, L.F.; Maraschin, F.S. New biotechnological tools to accelerate scab-resistance trait transfer to apple. Genet. Mol. Biol. 2017, 40, 305–311. [Google Scholar] [CrossRef]
- Gessler, C.; Petrot, I. Vf scab resistance of Malus. Trees 2012, 26, 95–108. [Google Scholar] [CrossRef]
- Höfer, M.; Flachowsky, H.; Schröpfer, S.; Peil, A. Evaluation of Scab and Mildew Resistance in the Gene Bank Collection of Apples in Dresden-Pillnitz. Plants 2021, 10, 1227. [Google Scholar] [CrossRef]
- Zelmene, K.; Kārkliņa, K.; Ikase, L.; Lācis, G. Inheritance of Apple (Malus X domestica (L.) Borkh) Resistance against Apple Scab (Venturia inaequalis (Cooke)Wint.) in Hybrid Breeding Material Obtained by Gene Pyramiding. Horticulturae 2022, 8, 772. [Google Scholar] [CrossRef]
- Wang, S.; Hu, T.; Wang, Y.; Luo, Y.; Michailides, T.J.; Cao, K. New understanding on infection processes of Valsa canker of apple in China. Eur. J. Plant Pathol. 2016, 146, 531–540. [Google Scholar] [CrossRef]
- Tamura, O.; Saito, I. Histopathological changes of apple bark infected by Valsa ceratosperma (Tode ex Fr.) Maire during dormant and growing periods. Ann. Phytopathol. Soc. Jpn. 1982, 48, 490–498. [Google Scholar] [CrossRef]
- Saville, R.; Olivieri, L. Fungal diseases of fruit: Apple cankers in Europe. In Integrated Management of Diseases and Insect Pests of Tree Fruit; Xu, X., Fountain, M., Eds.; Burleigh Dodds: Cambridge, HJ, USA, 2019; pp. 59–83. [Google Scholar]
- Wang, X.L.; Wei, J.L.; Huang, L.L.; Kang, Z.S. Re-evaluation of pathogens causing Valsa canker on apple in China. Mycologia 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Kotoda, N.; Kato, H.; Soejima, J. Resistance sources to Valsa canker (Valsa ceratosperma) in a germplasm collection of diverse Malus species. Plant Breed 2007, 126, 449–453. [Google Scholar] [CrossRef]
- Kobayashi, T. Taxonomic studies of Japanese Diaporthaceae with special reference to their life-histories. Bull. Gov. For. Exp. Stn. Tokyo 1970, 226, 1–242. [Google Scholar]
- Lu, Y.J. Studies on the pathogenic fungus of pear canker disease. Acta Phytopathol. Sin. 1992, 22, 197–203. [Google Scholar]
- Vasilyeva, L.; Kim, W.G. Valsa mali Miyabe et Yamada, the causal fungus of apple tree canker in east Asia. Mycobiology 2000, 28, 153–157. [Google Scholar] [CrossRef]
- Montuschi, C.; Collina, M. First record of Valsa ceratosperma on pear in Italy. Inf. Agrar. 2003, 59, 55–57. [Google Scholar]
- Adams, G.C.; Wingfield, M.J.; Common, R.; Roux, J. Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Stud. Mycol. 2005, 52, 1–142. [Google Scholar]
- Wang, X.L.; Zang, R.; Yin, Z.Y.; Kang, Z.S.; Huang, L.L. Delimiting cryptic pathogen species causing apple Valsa canker with multilocus data. Ecol. Evol. 2014, 4, 1369–1380. [Google Scholar] [CrossRef]
- Rossman, A.Y.; Adams, G.C.; Cannon, P.F.; Castlebury, L.A.; Crous, P.W.; Gryzenhout, M.; Jaklitsch, W.M.; Mejia, L.C.; Stoykov, D.; Udayanga, D.; et al. Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus 2015, 6, 145–154. [Google Scholar] [CrossRef]
- Spielman, L.J. A monograph of Valsa on hardwood in North America. Can. J. Bot. 1985, 63, 1355–1378. [Google Scholar] [CrossRef]
- Old, K.M.; Kobayashi, T.A.Y. Valsa teleomorph for Cytospora eucalypticola. Mycol. Res. 1991, 95, 1253–1256. [Google Scholar] [CrossRef]
- Gao, L.Q.; Berrie, A.; Yang, J.R.; Xu, X.M. Within- and between-orchard variability in the sensitivity of Venturia inaequalis to myclobutanil, a DMI fungicide, in the UK. Pest Manag. Sci. 2009, 65, 1241–1249. [Google Scholar] [CrossRef]
- Zhang, F.; Xue, H.; Lu, X.; Zhang, B.; Wang, F.; MaY, Z.Z. Autotetraploidization enhances drought stress tolerance in two apple cultivars. Trees 2015, 29, 1773–1780. [Google Scholar] [CrossRef]
- Miedtke, U.; Kennel, W. Athelia bombacina and Chaetomium globosum as antagonists of the perfect stage of the apple scab pathogen (Venturia inaequalis) under field conditions. J. Plant Dis. Prot. 1990, 97, 24–32. [Google Scholar]
- Xin, Y.F.; Shang, J.J. Bio-control trials of Chaetomium spirale ND35 against apple canker. J. For. Res. 2005, 16, 121–124. [Google Scholar]
- Carisse, O.; Meloche, C.; Boivin, G.; Jobin, T. Action thresholds for summer fungicide sprays and sequential classification of apple scab incidence. Plant Dis. 2009, 93, 490–498. [Google Scholar] [CrossRef]
- Li, Z.; Gao, X.; Kang, K.; Huang, L.; Fan, D.; Yan, X.; Kang, Z. Saccharothrix yanglingensis Strain Hhs.015 Is a Promising Biocontrol Agent on Apple Valsa Canker. Plant Dis. 2016, 100, 510–514. [Google Scholar] [CrossRef]
- Shuttleworth, L.A. Alternative disease management strategies for organic apple production in the United Kingdom. CABI Agric. Biosci. 2021, 2, 34. [Google Scholar] [CrossRef]
- Valetti, L.; Lima, N.B.; Cazón, L.I.; Crociara, C.; Ortega, L.; Pastor, S. Mycoparasitic Trichoderma isolates as a biocontrol agent against Valsa ceratosperma, the causal agent of apple valsa canker. Eur. J. Plant Pathol. 2022, 163, 923–935. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Fernández, I.; Sánchez-Guzmán, M.J.; Jung, S.C.; Pascual, J.A.; Pozo, M.J. Deciphering the hormonal signalling network behind the systemic resistance induced by Trichoderma harzianum in tomato. Front. Plant Sci. 2013, 4, 206. [Google Scholar] [CrossRef] [PubMed]
- Marois, J.J.; Mitchell, D.J.; Sonada, R.M. Biological control of Fusarium crown rot of tomato under field conditions. Phytopathology 1981, 71, 1257–1260. [Google Scholar] [CrossRef]
- Carisse, O.; Philion, V.; Rolland, D.; Bernier, J. Effect of fall application of fungal antagonists on spring ascospore production of apple scab pathogen, Venturia inaequalis. Phytopathology 2000, 90, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Narayanasami, P. Mechanisms of action of fungal biological control agents. In Biological Management of Diseases of Crops; Characteristics of Biological Control Agents; Nayaranassami, P., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 1, pp. 99–200. [Google Scholar]
- Gadoury, D.M.; Seem, R.C.; Stenvand, A. Ascospore discharge in Venturia inaequalis. Nor. J. Agric. Sci. Suppl. 1994, 17, 205–219. [Google Scholar]
- Meszka, B. Study of Venturia inaequalis pseudothecia development and apple scab severity under Polish conditions. Folia Hortic. 2015, 27, 107–114. [Google Scholar] [CrossRef]
- Dennis, C.; Webster, J. Antagonistic properties of species-groups of Trichoderma. I. Production of non-volatile antibiotics. Trans. Br. Mycol. Soc. 1971, 57, 25–39. [Google Scholar] [CrossRef]
- Dennis, C.; Webster, J. Antagonistic properties of species-groups of Trichoderma. II. Production of volatile antibiotics. Trans. Br. Mycol. Soc. 1971, 57, 41–48. [Google Scholar] [CrossRef]
- Palani, P.V.; Lalithakumari, D. Inhibition of Venturia inaequal is by genetically improved Trichoderma longibrachiatum strains. J. Plant Dis. Prot. 1999, 106, 460–465. [Google Scholar]
- Zhang, S.; Gan, Y.; Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef]
- Philion, V.; Carisse, O.; Paulitz, T. In vitro evaluation of fungal isolates for their ability to influence leaf rheology, production of pseudothecia, and ascospores of Venturia inaequalis. Eur. J. Plant Pathol. 1997, 103, 441–452. [Google Scholar] [CrossRef]
- Porsche, F.M.; Pfeiffer, B.; Kollar, A. A new phytosanitary method to reduce the ascospore potential of Venturia inaequalis. Plant Dis. 2017, 101, 414–420. [Google Scholar] [CrossRef][Green Version]
- Carisse, O.; Rolland, D. Effect of timing of application of the biological control agent Microsphaeropsis ochracea on the production and ejection pattern of ascospores by Venturia inaequalis. Phytopathology 2004, 94, 1305–1314. [Google Scholar] [CrossRef]
- Seaby, D.A.; Swinburne, T.R. Protection of pruning wounds on apple trees from Nectria galligena Bres. Using modified pruning shears. Plant Pathol. 1976, 25, 50–54. [Google Scholar] [CrossRef]
- Shigo, A.L.; Wilson, C.L. Wound dressings on red maple and American elm: Effectiveness after five years. Arboric. J. 1977, 3, 81–87. [Google Scholar] [CrossRef]
- Shigo, A.L.; Shortle, W.C. Wound dressings: Results of studies over thirteen years. Arboric. J. 1984, 8, 193–210. [Google Scholar] [CrossRef]
- Fan, D.; Li, Y.; Zhao, L.; Li, Z.; Huang, L.; Yan, X. Study on interactions between the major apple Valsa canker pathogen Valsa mali and its biocontrol agent Saccharothrix yanglingensis Hhs.015 using RT-qPCR. PLoS ONE 2016, 11, e0162174. [Google Scholar] [CrossRef]
- Mercer, P.C.; Kirk, S.A. Biological treatments for the control of decay in tree wounds. II. Field tests. Ann. Appl. Biol. 1984, 104, 221–229. [Google Scholar] [CrossRef]
- Spiers, A.G.; Brewster, D.T. Evaluation of chemical and biological treatments for control of Chondrostereum purpureum infection of pruning wounds in willows, apples, and peaches. N. Zeal. J. Crop Hortic. Sci. 1997, 25, 19–31. [Google Scholar] [CrossRef]
- Yi, H.W.; Chi, Y.J. Biocontrol of Cytospora canker of poplar in north-east China with Trichoderma longibrachiatum. For. Pathol. 2011, 41, 299–307. [Google Scholar] [CrossRef]
- Saks, Y.; Barkai-Golan, R. Aloe vera gel activity against plant pathogenic fungi. Postharvest Biol. Technol. 1995, 6, 159–165. [Google Scholar] [CrossRef]
- Amer, G.A. Effectiveness of bio-gel based powder formulations of bacterial biocontrol agents in controlling root rot disease of bean caused by Sclerotium rolfsii. J. Plant Prot. Pathol. 2010, 1, 21–33. [Google Scholar]
- Ramjegathesh, R.; Jayaraman, J. Chitosan for plant disease management–prospects and problems. In Sustainable Crop Disease Management using Natural Products; Ganesan, S., Vadivel, K., Jayaraman, J., Eds.; CAB International: Wallingford, UK, 2015; pp. 198–218. [Google Scholar]
- Ekebafe, L.O.; Ogbeifun, D.E.; Okieimen, F.E. Polymer applications in agriculture. Biokemistri 2011, 23, 81–89. [Google Scholar]
- Huttermann, A.; Zommorodi, M.; Reise, K. Addition of hydrogels to soil for prolonging the survival of Pinus halepensis seedlings subjected to drought. Soil Tillage Res. 1999, 50, 295–304. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Jarosiewicz, A. Use of polysulfone in controlled-release NPK fertilizer formulations. J. Agric. Food Chem. 2002, 50, 4634–4639. [Google Scholar] [CrossRef] [PubMed]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef] [PubMed]
- Steglińska, A.; Kołtuniak, A.; Berłowska, J.; Czyżowska, A.; Szulc, J.; Cieciura-Włoch, W.; Okrasa, M.; Kręgiel, D.; Gutarowska, B. Metschnikowia pulcherrima as a Biocontrol Agent against Potato (Solanum tuberosum) Pathogens. Agronomy 2022, 12, 2546. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Tworkoski, T.J.; Kurtzman, C.P. Biocontrol potential of Metchnikowia pulcherrima strains against blue mold of apple. Phytopathology 2001, 91, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Grebenisan, I.; Cornea, P.; Mateescu, R.; Cimpeanu, C.; Olteanu, V.; Campenu, G.; Stefan, L.A.; Oancea, F.; Lupu, C. Metschnikowia pulcherrima, a new yeast with potential for biocontrol of postharvest fruit rots. XXVII International Horticultural Congress-IHC2006: International Symposium on Sustainability through Integrated and Organic Horticulture. Acta Hortic. 2008, 767, 355–360. [Google Scholar] [CrossRef]
- Türkel, S.; KorukluoLlu, M.; Yavuz, M. Biocontrol activity of the local strain of Metschnikowia pulcherrima on different postharvest pathogens. Biotechnol. Res. Int. 2014, 397167. [Google Scholar] [CrossRef]
- Manso, T.; Nunes, C. Metschnikowia andauensis as a new biocontrol agent of fruit postharvest diseases. Postharvest Biol. Technol. 2011, 61, 64–71. [Google Scholar] [CrossRef]
- Millan, A.F.-S.; Gamir, J.; Farran, I.; Larraya, L. Identification of new antifungal metabolites produced by the yeast Metschnikowia pulcherrima involved in the biocontrol of postharvest plant pathogenic fungi. Postharvest Biol. Technol. 2022, 192, 111995. [Google Scholar] [CrossRef]
- Otari, S.V.; Patil, R.M.; Waghmare, S.R.; Ghosh, S.J.; Pawar, S.H. A novel microbial synthesis of catalytically active Ag–alginate biohydrogel and its antimicrobial activity. Dalton Trans. 2013, 42, 9966–9975. [Google Scholar] [CrossRef] [PubMed]
- Guilloux, K.; Gaillard, I.; Courtois, J.; Courtois, B.; Petit, E. Production of Arabinoxylan-oligosaccharides from Flaxseed (Linum usitatissimum). J. Agric. Food Chem. 2009, 57, 11308–11313. [Google Scholar] [CrossRef]
- Rätsep, J.; Havis, N.D.; Loake, G.J.; Walters, D.R.; McGrann, G.R.D. In-field assessment of an arabinoxylan polymer on disease control in spring barley. Crop Prot. 2018, 109, 102–109. [Google Scholar] [CrossRef]
- Smith, D.; Onions, A.H.S. The preservation and maintenance of living fungi, 2nd ed.; In IMI Technical Handbook, No. 2; CABI Publishing: Wallingford, UK, 1994; 122p. [Google Scholar]
- Bensaci, O.A.; Daoud, H.; Lombarkia, N.; Rouabah, K. Formulation of the endophytic fungus Cladosporium oxysporum Berk. & M. A. Curtis, isolated from Euphorbia bupleuroides subsp. luteola, as a new biocontrol tool against the black bean aphid (Aphis fabae Scop.). J. Plant Prot. Res. 2015, 55, 80–87. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with Image. J. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).