Neutrophil Immunomodulatory Activity of Nerolidol, a Major Component of Essential Oils from Populus balsamifera Buds and Propolis
Abstract
1. Introduction
2. Results and Discussion
2.1. Composition of Essential Oil from P. balsamifera Buds and Propolis
| No | RRI | RRI @ | Compound | PBO | PRO | No | RRI | RRI @ | Compound | PBO | PRO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1032 | 1008–1039 a | α-Pinene | 1.8 | 55 | 1703 | 1629–1704 a | Salicylaldehyde | 1.8 | ||
| 2 | 1035 | 1012–1039 a | α-Thujene | 0.3 | 56 | 1704 | 1655–1714 a | γ-Muurolene | T | 0.9 | |
| 3 | 1048 | 1005–1075 b | 2-Methyl-3-buten-2-ol | 3.1 | 57 | 1704 | 1682–1704 a | γ-Curcumene | 0.2 | ||
| 4 | 1057 | 1026–1088 b | Toluene | 0.7 | 58 | 1706 | 1659–1724 a | α-Terpineol | 0.5 | ||
| 5 | 1076 | 1043–1086 a | Camphene | t | 59 | 1709 | 1672–1718 a | α-Terpinyl acetate | 2.7 | ||
| 6 | 1118 | 1085–1130 a | β-Pinene | 0.6 | 60 | 1719 | 1702–1708 b | Zonarene | 0.3 | ||
| 7 | 1132 | 1098–1140 a | Sabinene | 0.1 | 61 | 1726 | 1676–1726 a | Germacrene D | 0.2 | ||
| 8 | 1145 | 1100–1178 b | Ethyl benzene | 0.2 | 62 | 1737 | 1713–1748 a | (Z,E)-α-Farnesene | 0.2 | ||
| 9 | 1174 | 1140–1175 a | Myrcene | 0.3 | 0.2 | 63 | 1740 | 1686–1753 a | α-Muurolene | t | 0.8 |
| 10 | 1176 | 1148–1186 a | α-Phellandrene | t | 64 | 1741 | 1698–1748 a | β-Bisabolene | 0.7 | ||
| 11 | 1188 | 1154–1195 a | α-Terpinene | 0.2 | 65 | 1742 | 1686–1743 a | β-Selinene | 0.4 | ||
| 12 | 1203 | 1178–1219 a | Limonene | 0.5 | 0.6 | 66 | 1744 | 1696–1748 a | α-Selinene | 0.7 | |
| 13 | 1213 | 1186–1231 a | 1,8-Cineole | 10.8 | 0.9 | 67 | 1744 | 1689–1771 a | Benzyl acetate | 0.1 | 0.1 |
| 14 | 1255 | 1222–1266 a | γ-Terpinene | 0.6 | 68 | 1755 | 1711–1756 a | β-Curcumene | 0.4 | ||
| 15 | 1268 | 1249–1266 b | Prenyl acetate | 0.1 | 69 | 1758 | 1714–1763 a | (E,E)-α-Farnesene | 0.7 | 0.2 | |
| 16 | 1272 | 1240–1290 a | Vinyl benzene | 0.8 | 70 | 1771 | 1726–1773 a | γ-Bisabolene | 0.3 | ||
| 17 | 1280 | 1246–1291 a | p-Cymene | 0.7 | 71 | 1773 | 1722–1774 a | δ-Cadinene | 0.1 | 0.2 | |
| 18 | 1290 | 1261–1300 a | Terpinolene | 0.1 | 72 | 1776 | 1735–1782 a | γ-Cadinene | 0.1 | 1.4 | |
| 19 | 1296 | 1267–1312 a | Octanal | 1.1 | 73 | 1784 | 1763–1786 a | (E)-α-Bisabolene | 0.2 | ||
| 20 | 1348 | 1317–1357 a | 6-Methyl-5-hepten-2-one | 0.1 | 74 | 1786 | 1743–1788 a | ar-Curcumene | t | 0.3 | |
| 21 | 1369 | - | 3-Butenyl benzene # | 1.5 | 75 | 1798 | 1727–1809 a | Methyl salicylate | 0.2 | ||
| 22 | 1371 | 1308–1328 b | 2-Methyl-2-butenol | 1.3 | 76 | 1805 | - | Nerolidol oxide der. * | 1.0 | 1.9 | |
| 23 | 1387 | - | MOMP # | 0.3 | 77 | 1819 | 1815 b | α-Cadinene | 0.4 | ||
| 24 | 1388 | 1398 c | DMNT # | 0.1 | 78 | 1825 | 1823 b | Cabreuva oxide VI | 7.9 | ||
| 25 | 1400 | 1390–1432 b | o-Methyl anisole | 1.8 | 79 | 1838 | 1784–1851 a | 2-Phenylethyl acetate | 0.6 | ||
| 26 | 1400 | 1370–1414 a | Nonanal | 0.7 | 80 | 1853 | 1800–1853 a | cis-Calamenene | t | 0.9 | |
| 27 | 1416 | 1397 | 1-Ethenyl-4-methyl benzene d | 0.3 | 81 | 1859 | 1837–1882 b | Benzylacetone | 0.1 | 1.1 | |
| 28 | 1443 | 1425–1459 b | 2,5- Dimethylstyrene | 0.1 | 82 | 1866 | 1842–1866 b | Methyl hydrocinnamate | 0.4 | ||
| 29 | 1450 | 1429–1481 a | trans-Linalool oxide | 0.1 | 0.7 | 83 | 1900 | 1900 e | Nonadecane | 0.3 | |
| 30 | 1478 | 1410–1478 a | cis-Linalool oxide | 0.3 | 84 | 1902 | 1880–1908 b | Benzyl isovalerate | 0.1 | ||
| 31 | 1493 | 1459–1500 a | α-Ylangene | t | 0.1 | 85 | 1932 | 1894–1937 b | 3-Methylbutyl benzoate | 0.1 | |
| 32 | 1497 | 1462–1522 a | α-Copaene | t | 0.3 | 86 | 1941 | 1893–1941 a | α-Calacorene | 0.5 | |
| 33 | 1506 | 1471–1516 a | Decanal | 1.8 | 87 | 1948 | 1988 f | Nerolidol oxide I | tr | ||
| 34 | 1538 | 1551 b | p-Ethyl anisole * | 0.1 | 88 | 1950 | 1950 b | PEMB | 1.4 | ||
| 35 | 1541 | 1481–1555 a | Benzaldehyde | 0.1 | 0.3 | 89 | 2025 | 2016 f | Nerolidol oxide II | 0.9 | |
| 36 | 1553 | 1507–1564 a | Linalool | 1.4 | 90 | 2050 | 1995–2055 a | E-Nerolidol | 64.0 | 14.4 | |
| 37 | 1565 | 1532–1570 a | Linalyl acetate | 0.7 | 91 | 2061 | 1986–2065 a | 4-Ethylguaiacol | 0.4 | ||
| 38 | 1594 | 1559–1609 b | trans-β-Bergamotene | t | 0.2 | 92 | 2080 | 2019–2090 a | Cubenol | 0.5 | |
| 39 | 1602 | 1582–1604 b | MHDO | 0.3 | 93 | 2088 | 2049–2088 b | Methyl-o-anisate | 0.2 | ||
| 40 | 1605 | 1600–1642 b | epi-Bicyclosesquiphellandrene | t | 94 | 2156 | 2156 g | α-Bisabolol oxide B | 0.9 | ||
| 41 | 1608 | 1542–1628 b | β-Copaene | t | 95 | 2164 | 2170–2187 b | Fokienol | 0.4 | 2.2 | |
| 42 | 1611 | 1564–1630 a | Terpinen-4-ol | 0.7 | 96 | 2179 | 2164–2210 b | 4-Ethylphenol | 0.4 | ||
| 43 | 1616 | 1580–1616 a | Hotrienol | 0.6 | 97 | 2187 | 2151–2198 b | T-Cadinol | 3.1 | ||
| 44 | 1630 | 1609–1687 a | Terpinen-4-yl acetate | 0.6 | 98 | 2205 | 2204–2205 b | Eremoligenol | 1.1 | ||
| 45 | 1638 | 1548–1638 a | β-Cyclocitral | 0.3 | 99 | 2209 | 2151–2209 b | T-Muurolol | 0.7 | ||
| 46 | 1641 | 1583–1656 a | Methyl benzoate | 0.5 | 100 | 2214 | - | α-Guaiol # | 0.8 | ||
| 47 | 1641 | - | 1,4-Dihydronaphthalene # | 1.5 | 101 | 2219 | 2150–2233 b | δ-Cadinol | 0.4 | ||
| 48 | 1661 | 1624–1668 a | Alloaromadendrene | 0.4 | 102 | 2232 | 2178–2234 a | α-Bisabolol | 7.1 | ||
| 49 | 1668 | 1627–1668 a | (Z)-β-Farnesene | 0.1 | 0.3 | 103 | 2250 | 2186–2250 a | α-Eudesmol | 3.0 | |
| 50 | 1671 | 1607–1699 a | Acetophenone | 0.6 | 104 | 2255 | 2180–2255 a | α-Cadinol | 1.5 | ||
| 51 | 1677 | 1672–1692 b | epi-Zonarene | 0.1 | 105 | 2257 | 2196–2272 a | β-Eudesmol | 3.6 | ||
| 52 | 1685 | 1640–1706 a | Ethyl benzoate | 0.2 | 106 | 2655 | 2565–2655 a | Benzyl benzoate | 3.7 | 6.1 | |
| 53 | 1688 | 1664–1688 b | Selina-4,11-diene | 0.3 | 1.5 | 107 | 2785 | 2760–2810 a | Benzyl salicylate | 1.6 | |
| 54 | 1694 | 1670–1694 b | p-Vinylanisole | 0.7 | |||||||
2.2. Effect of PBO, PRO, and Nerolidol on Neutrophil Ca2+ Influx
2.3. Effect of PBO, PRO, and Nerolidol on Neutrophil Chemotaxis
2.4. Identification of Potential Protein Targets for Nerolidol
3. Materials and Methods
3.1. Material
3.2. Materials
3.3. Essential Oil Extraction
3.4. Gas Chromatography–Flame Ionization Detector (GC-FID) and Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.5. Isolation of Human Neutrophils
3.6. Ca2+ Mobilization Assay
3.7. Chemotaxis Assay
3.8. Cytotoxicity Assay
3.9. Molecular Modeling
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Saelao, P.; Borba, R.S.; Ricigliano, V.; Spivak, M.; Simone-Finstrom, M. Honeybee microbiome is stabilized in the presence of propolis. Biol. Lett. 2020, 16, 20200003. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Perera, C.O.; Tandean, S. Can propolis be a useful adjuvant in brain and neurological disorders and ınjuries? A Systematic Scoping Review of the Latest Experimental Evidence. Biomedicines 2021, 9, 1227. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, antibacterial, antifungal, and antiparasitic properties of propolis: A review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Ravalia, M.; Kripal, K.; Chandrasekaran, K.; Fearnley, J.; Perera, C.O. Propolis in metabolic syndrome and ıts associated chronic diseases: A narrative review. Antioxidants 2021, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Felitti, R.; Fearnley, J.; Ravalia, M. The use of propolis in dentistry, oral health, and medicine: A review. J. Oral. Biosci. 2021, 63, 23–34. [Google Scholar] [CrossRef]
- Hermansyah, D.; Zulhendri, F.; Perera, C.O.; Firsty, N.N.; Chandrasekaran, K.; Abdulah, R.; Herman, H.; Lesmana, R. The potential use of propolis as an adjunctive therapy in breast cancers. Integr. Cancer 2022, 21, 15347354221096868. [Google Scholar] [CrossRef]
- Zulhendri, F.; Perera, C.O.; Tandean, S.; Abdulah, R.; Herman, H.; Christoper, A.; Chandrasekaran, K.; Putra, A.; Lesmana, R. The potential use of propolis as a primary or an adjunctive therapy in respiratory tract-related diseases and disorders: A systematic scoping review. Biomed. Pharm. 2022, 146, 112595. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent progress in pharmacological research of propolis. Phytother. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Yang, J.; Pi, A.; Yan, L.; Li, J.; Nan, S.; Zhang, J.; Hao, Y. Research progress on therapeutic effect and mechanism of propolis on wound healing. Evid. Based Complement Altern. Med. 2022, 2022, 5798941. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A. Perspectives for uses of propolis in therapy against ınfectious diseases. Molecules 2022, 27, 4594. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, P.; Muninathan, N.; Megalatha, S.T.; Suresh, A.; Kumar, K.S.; Jhansi, N.; Kalaivani, K.; Krishnamoorthy, G. An ınsight into anticancer effect of propolis and ıts constituents: A review of molecular mechanisms. Evid. Based Complement Altern. Med. 2022, 2022, 5901191. [Google Scholar] [CrossRef] [PubMed]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Magnavacca, A.; Sangiovanni, E.; Racagni, G.; Dell’Agli, M. The antiviral and immunomodulatory activities of propolis: An update and future perspectives for respiratory diseases. Med. Res. Rev. 2022, 42, 897–945. [Google Scholar] [CrossRef]
- Dos Santos, F.F.; Morais-Urano, R.P.; Cunha, W.R.; de Almeida, S.G.; Cavallari, P.; Manuquian, H.A.; Pereira, H.A.; Furtado, R.; Santos, M.F.C.; Amdrade, E.S.M.L. A review on the anti-inflammatory activities of Brazilian green, brown and red propolis. J. Food Biochem. 2022, 46, e14350. [Google Scholar] [CrossRef]
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical diversity and challenges in quality control. Phytochem. Rev. 2022, 21, 1887–1911. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. How diverse is the chemistry and plant origin of Brazilian propolis? Apidologie 2021, 52, 1075–1097. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Babickaite, L.; Majiene, D.; Ramanauskiene, K. Comparison of ethanolic and aqueous Populus balsamifera L. bud extracts by different extraction methods: Chemical composition, antioxidant and antibacterial activities. Pharmaceuticals 2021, 14, 1018. [Google Scholar] [CrossRef]
- Moerman, D.E. Native American Medicinal Plants: An Ethnobotanical Dictionary; Timber Press: Portland, OR, USA, 2009; p. 799. [Google Scholar]
- Wang, K.; Zhang, J.L.; Ping, S.; Ma, Q.X.; Chen, X.; Xuan, H.Z.; Shi, J.H.; Zhang, C.P.; Hu, F.L. Anti-inflammatory effects of ethanol extracts of Chinese propolis and buds from poplar (Populus x canadensis). J. Ethnopharmacol. 2014, 155, 300–311. [Google Scholar] [CrossRef]
- Kis, B.; Pavel, I.Z.; Avram, S.; Moaca, E.A.; San Juan, M.H.; Schwiebs, A.; Radeke, H.H.; Muntean, D.; Diaconeasa, Z.; Minda, D.; et al. Antimicrobial activity, in vitro anticancer effect (MCF-7 breast cancer cell line), antiangiogenic and immunomodulatory potentials of Populus nigra L. buds extract. BMC Complement Med. 2022, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Pannucci, E.; D’Eliseo, D.; Ieri, F.; Romani, A.; Santi, L.; Bernini, R.; Sabatti, M.; Velotti, F. Perspectives on Populus spp. (Salicaceae) bud extracts as antioxidant and anti-inflammatory agents. Nat. Prod. Res. 2022, 36, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Kis, B.; Avram, S.; Pavel, I.Z.; Lombrea, A.; Buda, V.; Dehelean, C.A.; Soica, C.; Yerer, M.B.; Bojin, F.; Folescu, R.; et al. Recent advances regarding the phytochemical and therapeutic uses of Populus nigra L. Buds. Plants 2020, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- Okinczyc, P.; Szumny, A.; Szperlik, J.; Kulma, A.; Franiczek, R.; Zbikowska, B.; Krzyzanowska, B.; Sroka, Z. Profile of polyphenolic and essential oil composition of polish propolis, black poplar and aspens buds. Molecules 2018, 23, 1262. [Google Scholar] [CrossRef] [PubMed]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory Activities of Selected Essential Oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Haran, G.H.; Calisto, V.; Jothi, G.; Quintans, J.S.S.; Cuevas, L.E.; Narain, N.; Junior, L.J.Q.; Cipolotti, R.; et al. Essential oils and its bioactive compounds modulating cytokines: A systematic review on anti-asthmatic and immunomodulatory properties. Phytomedicine 2020, 73, 152854. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. Antimicrobial, antioxidant, and ımmunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Ozek, G.; Schepetkin, I.A.; Yermagambetova, M.; Ozek, T.; Kirpotina, L.N.; Almerekova, S.S.; Abugalieva, S.I.; Khlebnikov, A.I.; Quinn, M.T. Innate ımmunomodulatory activity of cedrol, a component of essential oils ısolated from juniperus species. Molecules 2021, 26, 7644. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical composition and ımmunomodulatory activity of essential oils from Rhododendron albiflorum. Molecules 2021, 26, 3652. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical composition and ımmunomodulatory activity of Hypericum perforatum essential oils. Biomolecules 2020, 10, 916. [Google Scholar] [CrossRef]
- Ozek, G.; Schepetkin, I.A.; Utegenova, G.A.; Kirpotina, L.N.; Andrei, S.R.; Ozek, T.; Baser, K.H.C.; Abidkulova, K.T.; Kushnarenko, S.V.; Khlebnikov, A.I.; et al. Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils. J. Leukoc. Biol. 2017, 101, 1361–1371. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kushnarenko, S.V.; Ozek, G.; Kirpotina, L.N.; Sinharoy, P.; Utegenova, G.A.; Abidkulova, K.T.; Ozek, T.; Baser, K.H.; Kovrizhina, A.R.; et al. Modulation of human neutrophil responses by the essential oils from Ferula akitschkensis and their constituents. J. Agric. Food Chem. 2016, 64, 7156–7170. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kushnarenko, S.V.; Ozek, G.; Kirpotina, L.N.; Utegenova, G.A.; Kotukhov, Y.A.; Danilova, A.N.; Ozek, T.; Baser, K.H.; Quinn, M.T. Inhibition of human neutrophil responses by the essential oil of Artemisia kotuchovii and ıts constituents. J. Agric. Food Chem. 2015, 63, 4999–5007. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Neutrophil ımmunomodulatory activity of (-)-borneol, a major component of essential oils extracted from Grindelia squarrosa. Molecules 2022, 27, 4897. [Google Scholar] [CrossRef]
- Campra, N.A.; Montironi, I.D.; Reinoso, E.B.; Raviolo, J.; Moreno, F.R.; Maletto, B.; Cariddi, L.N. A natural oil increases specific anti-OVA IgG levels and induces a cellular immune response combined with aluminum hydroxide. J. Leukoc. Biol. 2021, 109, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Kuttan, G. Augmentation of humoral and cell mediated immune responses by Thujone. Int. Immunopharmacol. 2011, 11, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Khlebnikov, A.I.; Schepetkin, I.A.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Kirpotina, L.N.; Quinn, M.T. Inhibition of t cell receptor activation by semi-synthetic sesquiterpene lactone derivatives and molecular modeling of their interaction with glutathione and tyrosine kinase ZAP-70. Molecules 2019, 24, 350. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Mitchell, P.T.; Kishkentaeva, A.C.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Quinn, M.T. The natural sesquiterpene lactones arglabin, grosheimin, agracin, parthenolide, and estafiatin inhibit T cell receptor (TCR) activation. Phytochemistry 2018, 146, 36–46. [Google Scholar] [CrossRef]
- Zonfrillo, M.; Andreola, F.; Krasnowska, E.K.; Sferrazza, G.; Pierimarchi, P.; Serafino, A. Essential Oil from Eucalyptus globulus (Labill.) activates complement receptor-mediated phagocytosis and stimulates podosome formation in human monocyte-derived macrophages. Molecules 2022, 27, 3488. [Google Scholar] [CrossRef]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharm. 2022, 146, 112514. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; DeLeo, F.R.; Quinn, M.T. Microbes and the fate of neutrophils. Immunol. Rev. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.X.; Kubes, P. The neutrophil’s role during health and disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef] [PubMed]
- Malech, H.L.; DeLeo, F.R.; Quinn, M.T. The role of neutrophils in the immune system: An overview. Methods Mol. Biol. 2020, 2087, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.P.; Peh, H.Y.; Tan, W.S.D.; Pahima, H.; Maffia, P.; Tiligada, E.; Levi-Schaffer, F. Granulocyte-targeted therapies for airway diseases. Pharm. Res. 2020, 157, 104881. [Google Scholar] [CrossRef]
- Liao, H.R.; Kao, Y.Y.; Leu, Y.L.; Liu, F.C.; Tseng, C.P. Larixol inhibits fMLP-induced superoxide anion production and chemotaxis by targeting the βγ subunit of Gi-protein of fMLP receptor in human neutrophils. Biochem. Pharm. 2022, 201, 115091. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Klein, R.A.; Quinn, M.T. Neutrophil Immunomodulatory activity of farnesene, a component of Artemisia dracunculus essential oils. Pharmaceuticals 2022, 15, 642. [Google Scholar] [CrossRef]
- Piochon-Gauthier, M.; Legault, J.; Sylvestre, M.; Pichette, A. The essential oil of Populus balsamifera buds: Its chemical composition and cytotoxic activity. Nat. Prod. Comm. 2014, 9, 257–260. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Vinogorova, V.T. GC-MS analysis of compounds extracted from buds of Populus balsamifera and Populus nigra. Z. Nat. C 2003, 58, 355–360. [Google Scholar] [CrossRef]
- Bankova, V.S.; Christov, R.S.; Tejera, A.D. Lignans and other constituents of propolis from the Canary Islands. Phytochemistry 1998, 49, 1411–1415. [Google Scholar] [CrossRef]
- de Albuquerque, I.L.; Alves, L.A.; Lemos, T.L.G.; Dorneles, C.A.; de Morais, M.O. Constituents of the essential oil of Brazilian green propolis from Brazil. J. Essent. Oil Res. 2008, 20, 414–415. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Marostica, M.R.; Daugsch, A.; Moraes, C.S.; Queiroga, C.L.; Pastore, G.M.; Park, Y.K. Comparison of volatile and polyphenolic compounds in Brazilian green propolis and its botanical origin Baccharis dracunculifolia. Cienc. Tecnol. Alime. 2008, 28, 178–181. [Google Scholar] [CrossRef]
- Miguel, M.G.; Nunes, S.; Cruz, C.; Duarte, J.; Antunes, M.D.; Cavaco, A.M.; Mendes, M.D.; Lima, A.S.; Pedro, L.G.; Barroso, J.G.; et al. Propolis volatiles characterisation from acaricide-treated and -untreated beehives maintained at Algarve (Portugal). Nat. Prod. Res. 2013, 27, 743–749. [Google Scholar] [CrossRef]
- Melliou, E.; Stratis, E.; Chinou, I. Volatile constituents of propolis from various regions of Greece—Antimicrobial activity. Food Chem. 2007, 103, 375–380. [Google Scholar] [CrossRef]
- Bankova, V.; Christov, R.; Popov, S.; Pureb, O.; Bocari, G. Volatile Constituents of Propolis. Z. Nat. C 1994, 49, 6–10. [Google Scholar] [CrossRef]
- Bankova, V.; Christov, R.; Kujumgiev, A.; Marcucci, M.C.; Popova, S. Chemical-Composition and Antibacterial Activity of Brazilian Propolis. Z. Nat. C 1995, 50, 167–172. [Google Scholar] [CrossRef]
- Borčić, I.; Radonić, A.; Grzunov, K. Comparison of the volatile constituents of propolis gathered in different regions of Croatia. Flavour Frag. J. 1996, 11, 311–313. [Google Scholar] [CrossRef]
- Klopell, F.C.; Lemos, M.; Sousa, J.P.B.; Comunello, E.; Maistro, E.L.; Bastos, J.K.; de Andrade, S.F. Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae). Z. Nat. C 2007, 62, 537–542. [Google Scholar] [CrossRef]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Chanotiya, C.S. The essential oil composition of Melaleuca leucadendra L. grown in India: A novel source of (E)-nerolidol. Ind. Crop. Prod. 2015, 69, 224–227. [Google Scholar] [CrossRef]
- Marques, A.M.; Barreto, A.L.S.; Curvelo, J.A.D.; Romanos, M.T.V.; Soares, R.M.D.; Kaplan, M.A.C. Antileishmanial activity of nerolidol-rich essential oil from Piper claussenianum. Rev. Bras. Farm. 2011, 21, 908–914. [Google Scholar] [CrossRef]
- De Carvalho, R.B.F.; De Almeida, A.A.C.; Campelo, N.B.; Lellis, D.; Nunes, L.C.C. Nerolidol and its pharmacological application in treating neurodegenerative diseases: A review. Recent Pat. Biotechnol. 2018, 12, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Limberger, R.P.; Sobral, M.; Henriques, A.T. Intraspecific volatile oil variation in Myrceugenia cucullata (Myrtaceae). Biochem. Syst. Ecol. 2005, 33, 287–293. [Google Scholar] [CrossRef]
- Kramer, R.; Abraham, W.R. Volatile sesquiterpenes from fungi: What are they good for? Phytochem. Rev. 2012, 11, 15–37. [Google Scholar] [CrossRef]
- Nguikwie, S.K.; Nyegue, M.A.; Belinga, F.N.F.; Ngane, R.A.N.; Romestand, B.; Kouzayha, A.; Casabianca, H.; Zono, P.H.A.; Menut, C. The chemical composition and antibacterial activities of the essential oils from three aframomum species from Cameroon, and their potential as sources of (E)-(R)-nerolidol. Nat. Prod. Comm. 2013, 8, 829–834. [Google Scholar] [CrossRef]
- Green, S.A.; Chen, X.Y.; Nieuwenhuizen, N.J.; Matich, A.J.; Wang, M.Y.; Bunn, B.J.; Yauk, Y.K.; Atkinson, R.G. Identification, functional characterization, and regulation of the enzyme responsible for floral (E)-nerolidol biosynthesis in kiwifruit (Actinidia chinensis). J. Exp. Bot. 2012, 63, 1951–1967. [Google Scholar] [CrossRef]
- Juchelka, D.; Steil, A.; Witt, K.; Mosandl’, A. Chiral compounds of essential oils. XX. Chirality evaluation and authenticity profiles of neroli and petitgrain oils. J. Essent. Oil Res. 1996, 8, 487–497. [Google Scholar] [CrossRef]
- Naves, Y.R. Presenee de nltrolidol dans les huiles essentielles de papilionae. Helv. Chim. Acta 1947, 30, 278–286. [Google Scholar] [CrossRef]
- Naves, Y.R. Etudes sur les matières végétales volatiles LXI. Présence de nérolidol dans les huiles essentielles de papilionacées. Helv. Chim. Acta 1948, 31, 408–417. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 43101. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook NIST Standard Reference Database Number 69. Last Update to Data: 2022. Available online: Https://webbook.nist.gov/chemistry/ (accessed on 25 August 2022).
- Viljoen, A.M.; Moolla, A.; van Vuuren, S.F.; Baser, K.H.C.; Demirci, B.; Ozek, T. A seasonal variation study of the chemical composition and antimicrobial activity of the essential oil of Agathosma ovata (Thunb.) Pillans (Rutaceae). J. Essent. Oil Res. 2006, 18, 30–36. [Google Scholar] [CrossRef]
- Boneva, S.; Vassilev, K. Gas chromatographic separation of epoxystyrenes on Carbowax 20 M capillary column. Chromatographia 1996, 43, 208–210. [Google Scholar] [CrossRef]
- Weyerstahl, P.; Marschall, H.; Son, P.T.; Giang, P.M. Constituents of the flower essential oil of Aglaia odorata Lour. from Vietnam. Flavour Frag. J. 1999, 14, 219–224. [Google Scholar] [CrossRef]
- Şen, A.; Kürkçüoğlu, M.; Bitiş, L.; Doğan, A.; Başer, K.H. Essential oil composition of different parts of Tanacetum cilicicum (Boiss.) Grierson. Nat. Volatiles Essent. Oils 2020, 7, 18–28. [Google Scholar] [CrossRef]
- Can, O.D.; Ozkay, U.D.; Kiyan, H.T.; Demirci, B. Psychopharmacological profile of Chamomile (Matricaria recutita L.) essential oil in mice. Phytomedicine 2012, 19, 306–310. [Google Scholar] [CrossRef]
- Dixit, N.; Kim, M.H.; Rossaint, J.; Yamayoshi, I.; Zarbock, A.; Simon, S.I. Leukocyte function antigen-1, kindlin-3, and calcium flux orchestrate neutrophil recruitment during inflammation. J. Immunol. 2012, 189, 5954–5964. [Google Scholar] [CrossRef]
- Ali, H.; Richardson, R.M.; Haribabu, B.; Snyderman, R. Chemoattractant receptor cross-desensitization. J. Biol. Chem. 1999, 274, 6027–6030. [Google Scholar] [CrossRef]
- Chan, W.K.; Tan, L.T.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Lapczynski, A.; Letizia, C.S.; Api, A.M. Fragrance material review on cis-nerolidol. Food Chem. Toxicol. 2008, 46, S245–S246. [Google Scholar] [CrossRef]
- McGinty, D.; Letizia, C.S.; Api, A.M. Addendum to fragrance material review on nerolidol (isomer unspecified). Food Chem. Toxicol. 2010, 48, S43–S45. [Google Scholar] [CrossRef]
- Krist, S.; Banovac, D.; Tabanca, N.; Wedge, D.E.; Gochev, V.K.; Wanner, J.; Schmidt, E.; Jirovetz, L. Antimicrobial activity of nerolidol and its derivatives against airborne microbes and further biological activities. Nat. Prod. Comm. 2015, 10, 143–148. [Google Scholar] [CrossRef]
- Silva, M.P.N.; Oliveira, G.L.S.; de Carvalho, R.B.F.; de Sousa, D.P.; Freitas, R.M.; Pinto, P.L.S.; de Moraes, J. Antischistosomal Activity of the Terpene Nerolidol. Molecules 2014, 19, 3793–3803. [Google Scholar] [CrossRef] [PubMed]
- AbouLaila, M.; Sivakumar, T.; Yokoyama, N.; Igarashi, I. Inhibitory effect of terpene nerolidol on the growth of Babesia parasites. Parasitol. Int. 2010, 59, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Azimullah, S.; Khair, S.B.A.; Ojha, S.; Haque, M.E. Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci. 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Jaiswal, G.; Brar, J.; Kumar, P. Neuroprotective effect of nerolidol in traumatic brain injury associated behavioural comorbidities in rats. Toxicol. Res. 2021, 10, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Irfan, H.M.; Alamgeer; Jahan, S.; Shahzad, M.; Latif, M.B. Nerolidol: A potential approach in rheumatoid arthritis through reduction of TNF-alpha, IL-1beta, IL-6, NF-kB, COX-2 and antioxidant effect in CFA-induced arthritic model. Inflammopharmacol 2022, 30, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Badrealam, K.F.; Kuo, C.H.; Daddam, J.; Asokan Shibu, M.; Lin, K.H.; Ho, T.J.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Small molecule compound nerolidol attenuates hypertension induced hypertrophy in spontaneously hypertensive rats through modulation of Mel-18-IGF-IIR signalling. Phytomedicine 2021, 84, 153450. [Google Scholar] [CrossRef]
- Yu, Y.; Velu, P.; Ma, Y.; Vijayalakshmi, A. Nerolidol induced apoptosis via PI3K/JNK regulation through cell cycle arrest in MG-63 osteosarcoma cells. Env. Toxicol. 2022, 37, 1750–1758. [Google Scholar] [CrossRef]
- Raj, V.; Venkataraman, B.; Almarzooqi, S.; Chandran, S.; Ojha, S.K.; Attoub, S.; Adrian, T.E.; Subramanya, S.B. Nerolidol Mitigates Colonic Inflammation: An Experimental Study Using both In Vivo and In Vitro Models. Nutrients 2020, 12, 2032. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Boligon, A.A.; Grando, T.H.; MF, D.E.S.; AS, D.A.S.; Stefani, L.M.; Baldisserotto, B.; Monteiro, S.G. Solving the challenge of the blood-brain barrier to treat infections caused by Trypanosoma evansi: Evaluation of nerolidol-loaded nanospheres in mice. Parasitology 2017, 144, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.; Macchiarulo, A.; Costantino, G.; Pellicciari, R. Pharmacophore model for bile acids recognition by the FPR receptor. J. Comput. Aided Mol. Des. 2006, 20, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, D.; Shen, W.; Dong, H.F.; Wang, J.M.; Oppenheim, J.J.; Howard, M.Z. Characterization of chenodeoxycholic acid as an endogenous antagonist of the G-coupled formyl peptide receptors. Inflamm. Res. 2000, 49, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mellon, R.D.; Yang, L.; Dong, H.; Oppenheim, J.J.; Howard, O.M. Regulatory effects of deoxycholic acid, a component of the anti-inflammatory traditional Chinese medicine Niuhuang, on human leukocyte response to chemoattractants. Biochem. Pharm. 2002, 63, 533–541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pamplona, F.A.; Ferreira, J.; Menezes de Lima, O., Jr.; Duarte, F.S.; Bento, A.F.; Forner, S.; Villarinho, J.G.; Bellocchio, L.; Wotjak, C.T.; Lerner, R.; et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 21134–21139. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, S.; Wang, J.; Xia, F.; Wan, J.B.; Lu, J.; Ye, R.D. Dual modulation of formyl peptide receptor 2 by aspirin-triggered lipoxin contributes to its anti-inflammatory activity. FASEB J. 2020, 34, 6920–6933. [Google Scholar] [CrossRef]
- Das, U.N. Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution. Biomolecules 2021, 11, 1873. [Google Scholar] [CrossRef]
- Park, J.; Langmead, C.J.; Riddy, D.M. New advances in targeting the resolution of ınflammation: Implications for specialized pro-resolving mediator GPCR drug discovery. ACS Pharm. Transl. 2020, 3, 88–106. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, G.; Zhou, Y.; Lin, C.; Chen, S.; Lin, Y.; Mai, S.; Huang, Z. Reverse Screening Methods to Search for the Protein Targets of Chemopreventive Compounds. Front. Chem. 2018, 6, 138. [Google Scholar] [CrossRef]
- Lu, D.J.; Furuya, W.; Grinstein, S. Involvement of multiple kinases in neutrophil activation. Blood Cells 1993, 19, 343–349. [Google Scholar]
- Bokoch, G.M. Chemoattractant signaling and leukocyte activation. Blood 1995, 86, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Khlebnikov, A.I.; Giovannoni, M.P.; Kirpotina, L.N.; Cilibrizzi, A.; Quinn, M.T. Development of small molecule non-peptide formyl peptide receptor (FPR) ligands and molecular modeling of their recognition. Curr. Med. Chem. 2014, 21, 1478–1504. [Google Scholar] [CrossRef] [PubMed]
- He, H.Q.; Ye, R.D. The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition. Molecules 2017, 22, 455. [Google Scholar] [CrossRef]
- Knall, C.; Young, S.; Nick, J.A.; Buhl, A.M.; Worthen, G.S.; Johnson, G.L. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J. Biol. Chem. 1996, 271, 2832–2838. [Google Scholar] [CrossRef]
- Ozek, G.; Ishmuratova, M.; Tabanca, N.; Radwan, M.M.; Goger, F.; Ozek, T.; Wedge, D.E.; Becnel, J.J.; Cutler, S.J.; Can Baser, K.H. One-step multiple component isolation from the oil of Crinitaria tatarica (Less.) Sojak by preparative capillary gas chromatography with characterization by spectroscopic and spectrometric techniques and evaluation of biological activity. J. Sep. Sci. 2012, 35, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. High-throughput screening for small-molecule activators of neutrophils: Identification of novel N-formyl peptide receptor agonists. Mol. Pharm. 2007, 71, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010, 38, W609–W614. [Google Scholar] [CrossRef] [PubMed]
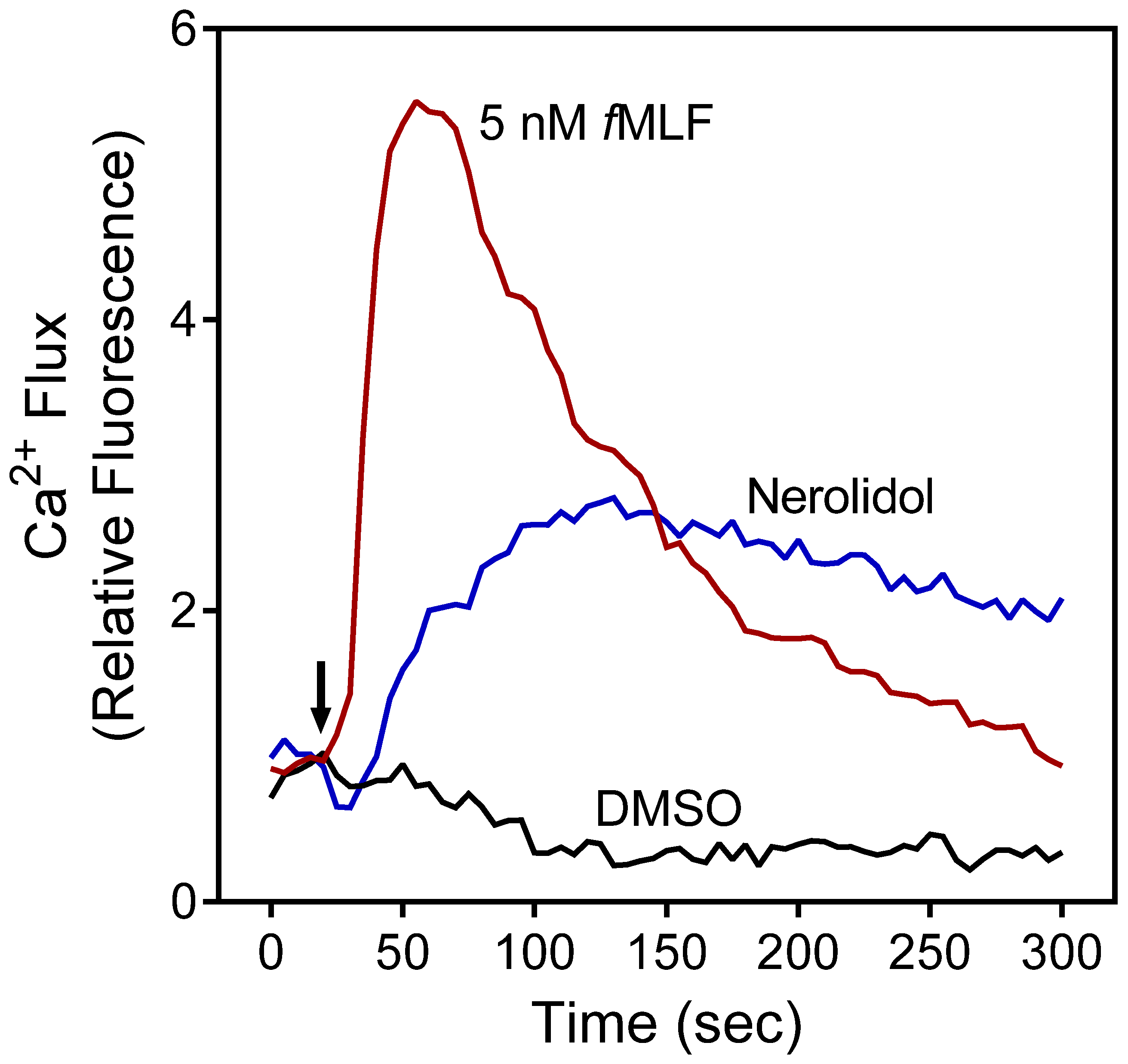
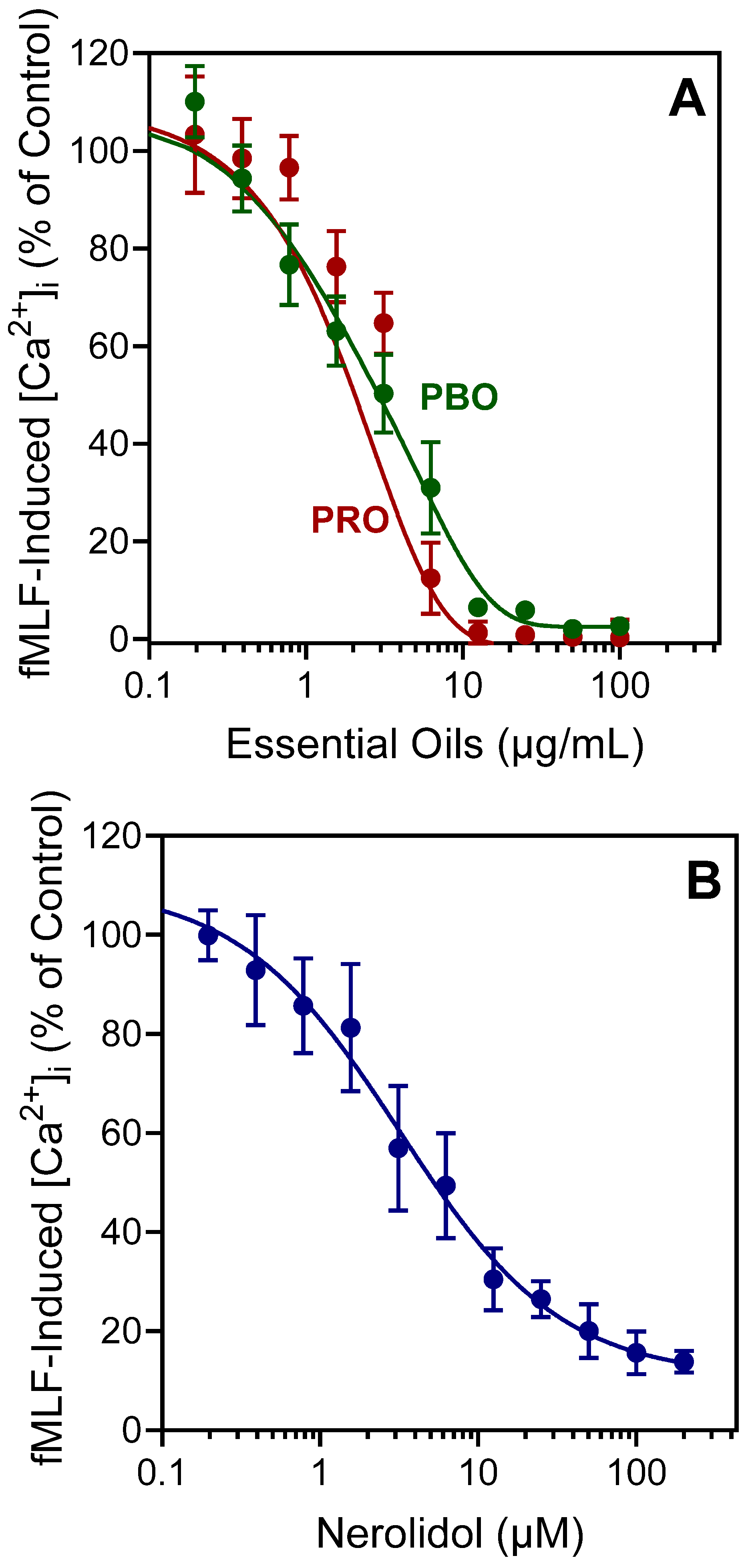
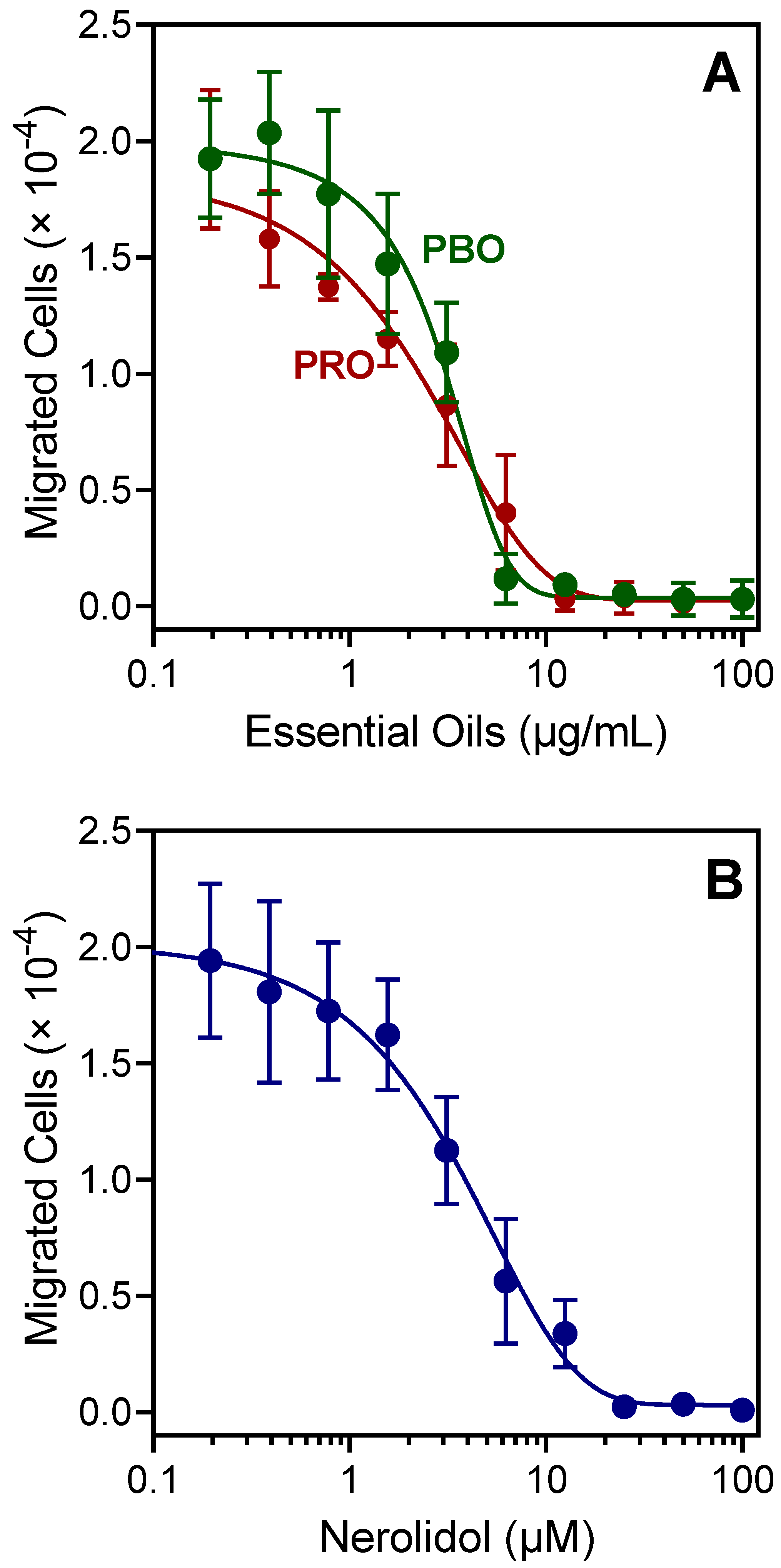
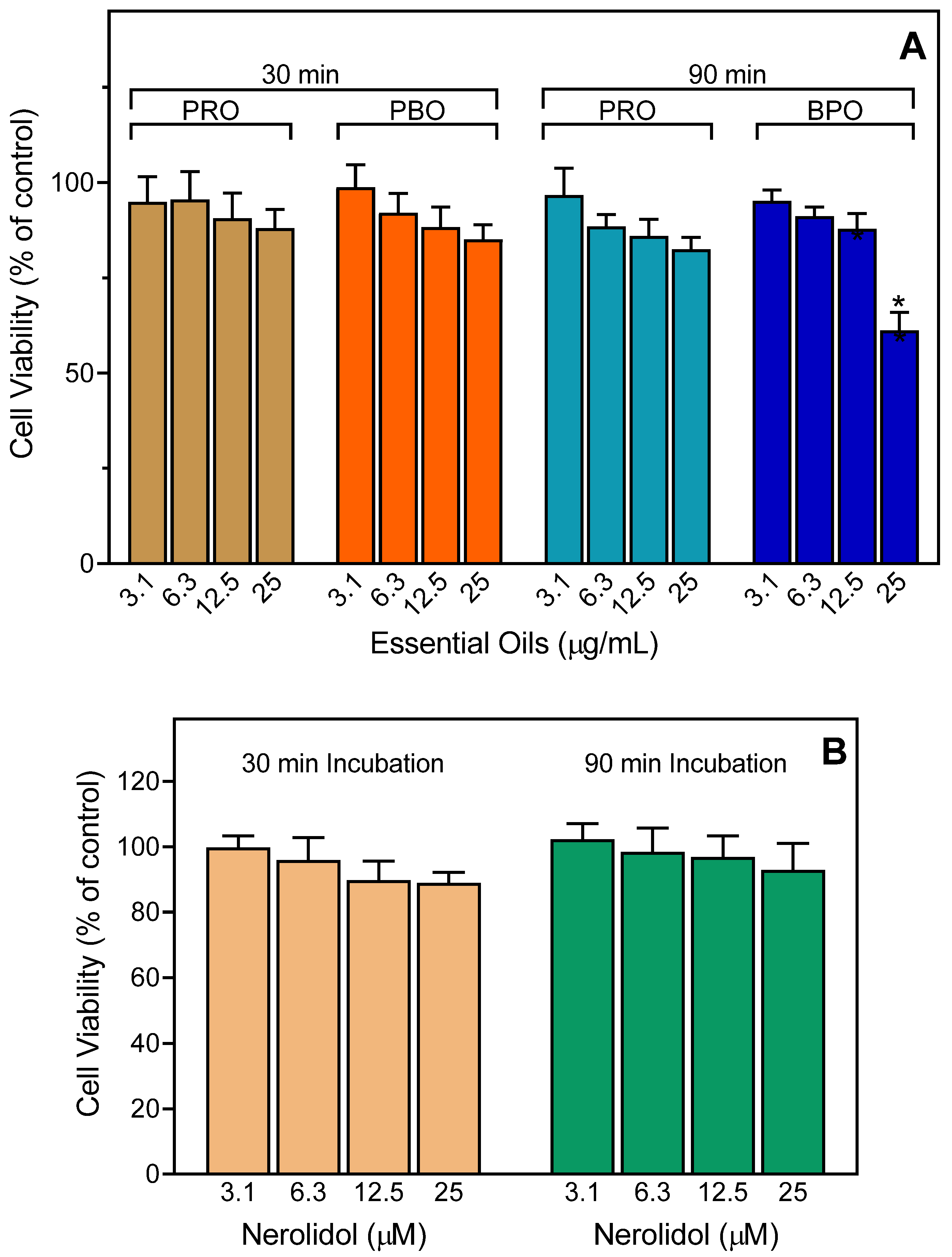
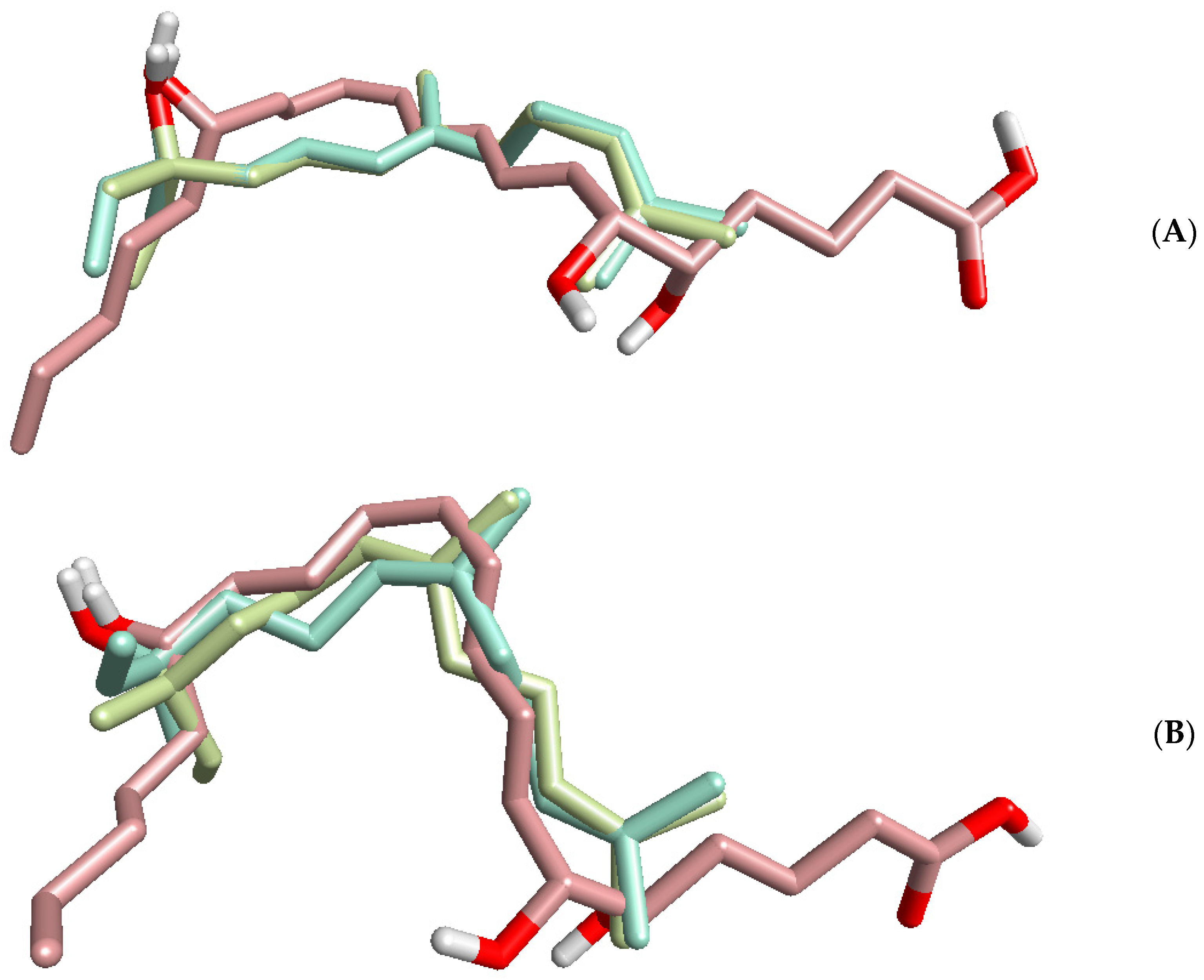
| Source of Essential Oil or Pure Compound | Activation of [Ca2+]i | Inhibition of [Ca2+]i | ||
|---|---|---|---|---|
| fMLF- Induced | WKYMVM- Induced | fMLF-Induced Chemotaxis | ||
| EC50 (μg/mL) | IC50 (μg/mL) | |||
| P. balsamifera | 10.5 ± 1.1 | 1.8 ± 0.6 | 9.4 ± 1.9 | 1.5 ± 0.5 |
| Propolis | 18.3 ± 3.7 | 3.4 ± 0.1 | 0.9 ± 0.3 | 2.9 ± 1.3 |
| EC50 (μM) | IC50 (μM) | |||
| Nerolidol | 0.8 ± 0.1 | 4.0 ± 1.7 | 3.7 ± 0.4 | 3.9 ± 1.3 |
| Property | E-Nerolidol | Z-Nerolidol |
|---|---|---|
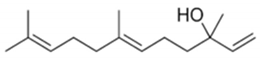 |  | |
| Formula | C15H26O | C15H26O |
| M.W. | 222.37 | 222.37 |
| Heavy atoms | 16 | 16 |
| Fraction Csp3 | 0.6 | 0.6 |
| Rotatable bonds | 7 | 7 |
| H-bond acceptors | 1 | 1 |
| H-bond donors | 1 | 1 |
| MR | 74.0 | 74.0 |
| tPSA | 20.23 | 20.23 |
| iLogP | 3.64 | 3.60 |
| BBB permeation | Yes | Yes |
| Rank | PDB ID | Target Name | Fit Score | Rank | PDB ID | Target Name | Fit Score |
|---|---|---|---|---|---|---|---|
| R-(E)-Nerolidol | S-(E)-Nerolidol | ||||||
| 1 | 1J96 | AKR1C2 | 2.999 | 1 | 1P49 | Steryl-sulfatase | 2.987 |
| 2 | 1E7E | Serum albumin | 2.997 | 2 | 3BMP | BMP2 | 2.983 |
| 3 | 1L6L | Apo A-II | 2.991 | 3 | 2JBP | MAPKAPK2 | 2.981 |
| 4 | 1P49 | Steryl-sulfatase | 2.989 | 4 | 3DEJ | Caspase-3 | 2.979 |
| 5 | 3BMP | BMP2 | 2.982 | 5 | 2Q11 | β-Secretase 1 | 2.977 |
| 6 | 2PIN | NR1A2 | 2.978 | 6 | 2PIN | NR1A2 | 2.977 |
| 7 | 3BGP | Pim-1 | 2.970 | 7 | 1BM6 | Stromelysin-1 | 2.970 |
| 8 | 1PME | ERK2 | 2.966 | 8 | 1L6L | Apo A-II | 2.969 |
| 9 | 1III | Transthyretin | 2.962 | 9 | 1PME | ERK2 | 2.965 |
| 10 | 1TG6 | CLPP | 2.960 | 10 | 1QKU | Estrogen receptor | 2.961 |
| R-(Z)-Nerolidol | S-(Z)-Nerolidol | ||||||
| 1 | 1P49 | Steryl-sulfatase | 2.998 | 1 | 1P49 | Steryl-sulfatase | 3.000 |
| 2 | 2JBP | MAPKAPK2 | 2.991 | 2 | 3BGP | Pim-1 | 2.993 |
| 3 | 3CJF | VEGFR2 | 2.989 | 3 | 1F86 | Transthyretin | 2.992 |
| 4 | 3CGF | JNK3 | 2.989 | 4 | 1L6L | Apo A-II | 2.991 |
| 5 | 3BMP | BMP2 | 2.983 | 5 | 2PG2 | KIF11 | 2.987 |
| 6 | 2PIN | NR1A2 | 2.980 | 6 | 3BMP | BMP2 | 2.982 |
| 7 | 1SHJ | Caspase-7 | 2.970 | 7 | 1IF4 | CA2 | 2.975 |
| 8 | 1YA8 | LCE1 | 2.966 | 8 | 1J96 | AKR1C2 | 2.974 |
| 9 | 1PME | ERK2 | 2.965 | 9 | 1OJ9 | MAO-B | 2.969 |
| 10 | 2O65 | Pim-1 | 2.964 | 10 | 2JBP | MAPKAPK2 | 2.964 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Kokorina, P.I.; Khlebnikov, A.I.; Quinn, M.T. Neutrophil Immunomodulatory Activity of Nerolidol, a Major Component of Essential Oils from Populus balsamifera Buds and Propolis. Plants 2022, 11, 3399. https://doi.org/10.3390/plants11233399
Schepetkin IA, Özek G, Özek T, Kirpotina LN, Kokorina PI, Khlebnikov AI, Quinn MT. Neutrophil Immunomodulatory Activity of Nerolidol, a Major Component of Essential Oils from Populus balsamifera Buds and Propolis. Plants. 2022; 11(23):3399. https://doi.org/10.3390/plants11233399
Chicago/Turabian StyleSchepetkin, Igor A., Gulmira Özek, Temel Özek, Liliya N. Kirpotina, Polina I. Kokorina, Andrei I. Khlebnikov, and Mark T. Quinn. 2022. "Neutrophil Immunomodulatory Activity of Nerolidol, a Major Component of Essential Oils from Populus balsamifera Buds and Propolis" Plants 11, no. 23: 3399. https://doi.org/10.3390/plants11233399
APA StyleSchepetkin, I. A., Özek, G., Özek, T., Kirpotina, L. N., Kokorina, P. I., Khlebnikov, A. I., & Quinn, M. T. (2022). Neutrophil Immunomodulatory Activity of Nerolidol, a Major Component of Essential Oils from Populus balsamifera Buds and Propolis. Plants, 11(23), 3399. https://doi.org/10.3390/plants11233399








