Seed Bank Conservation and Incipient Seed Development in Orchids Colonizing Mining Wastes: Results of a Field Pilot Experiment
Abstract
1. Introduction
2. Results
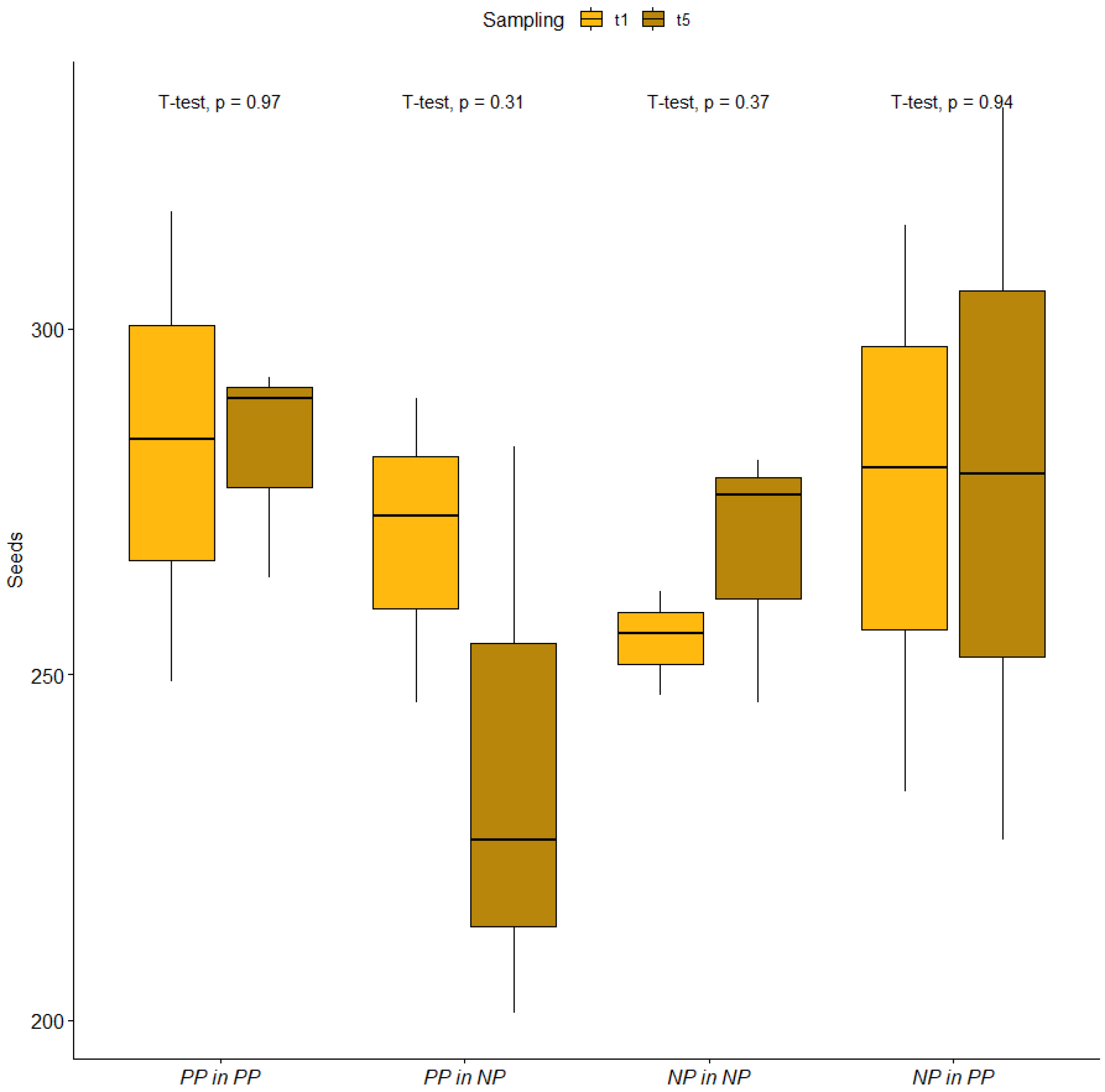
2.1. Seed Conservation during the Experiment and Morphometric Parameters
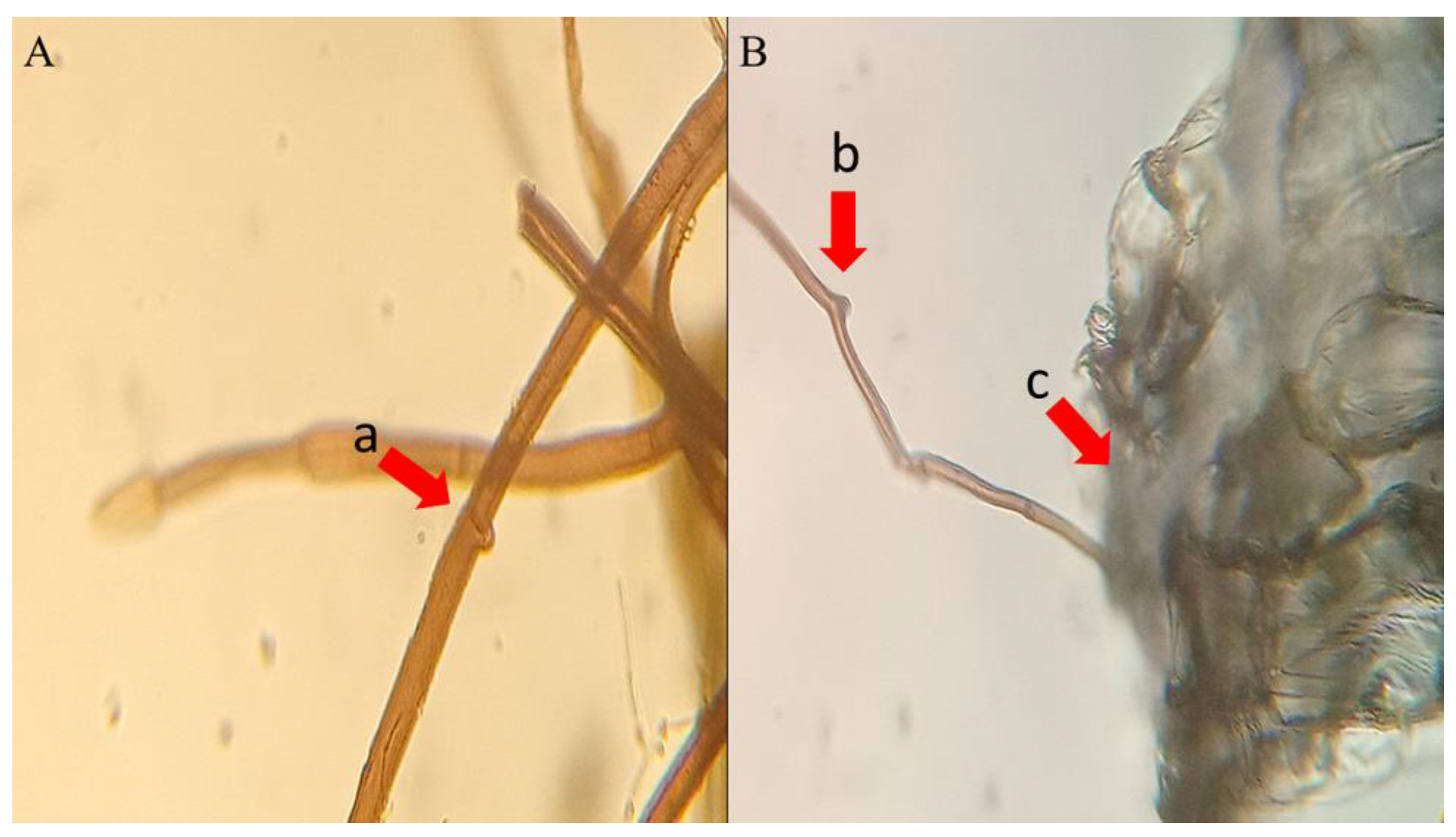
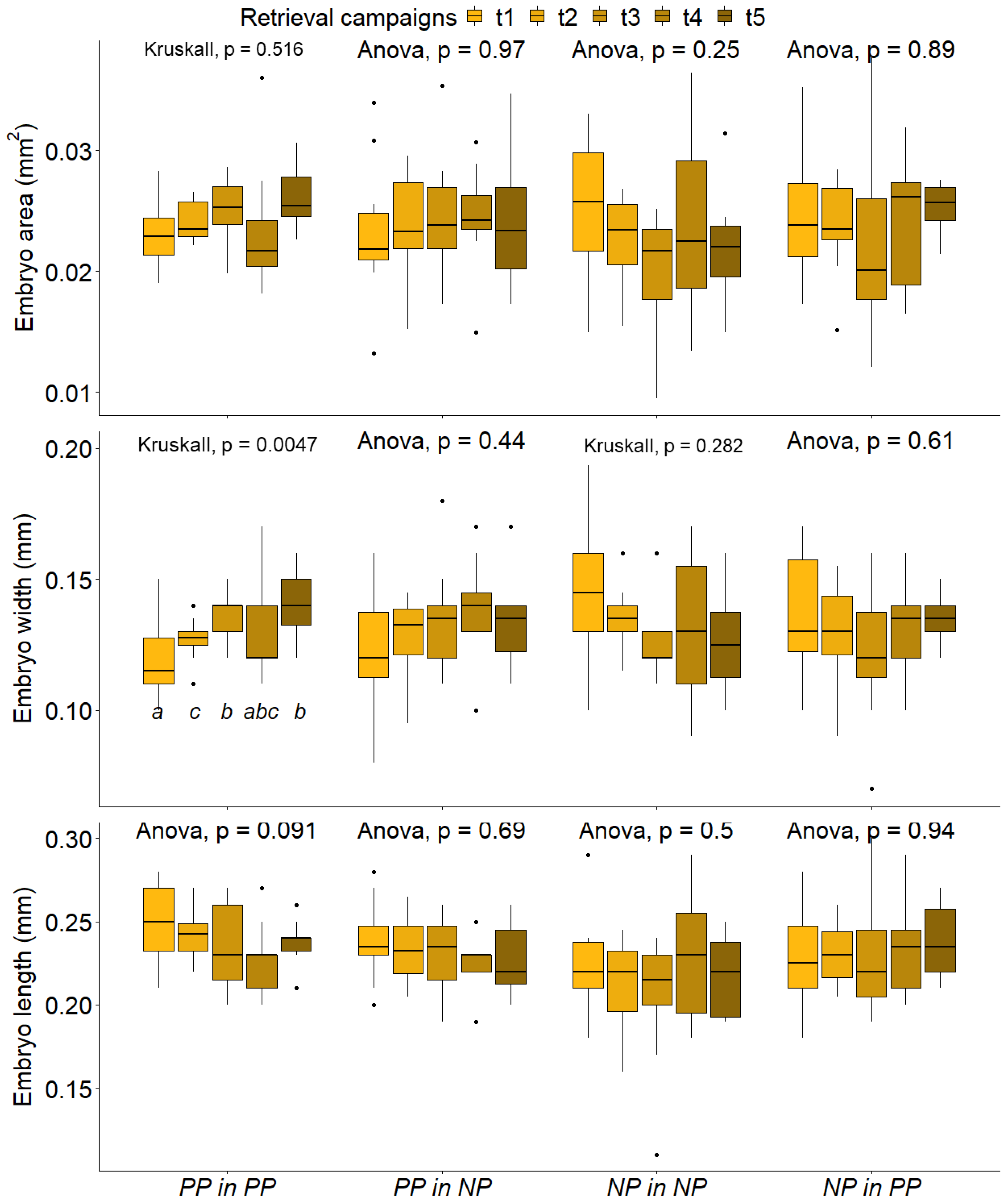
2.2. Developing Embryos
3. Discussion
4. Materials and Methods
4.1. Plant Species
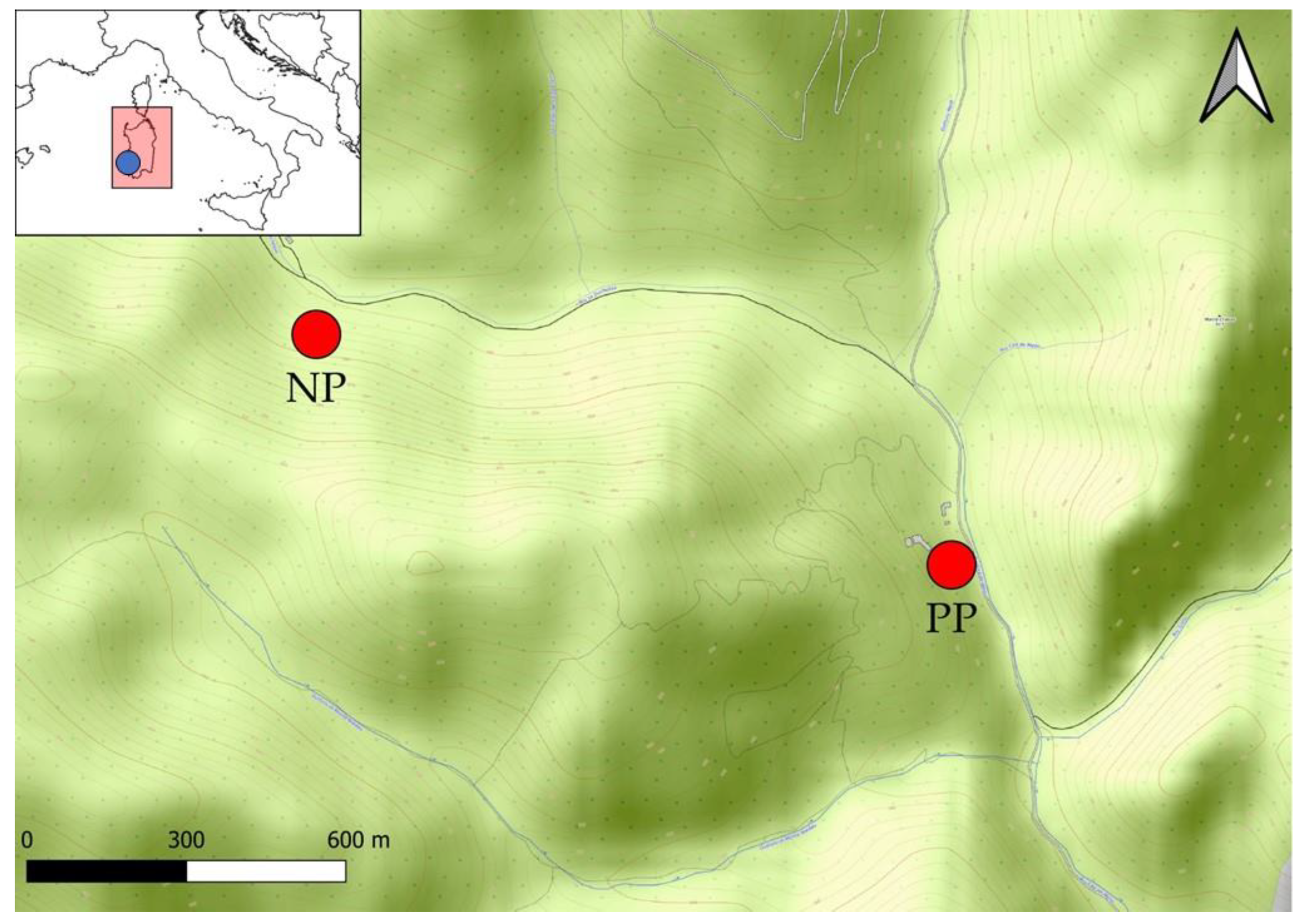
4.2. Contaminated and Control Site
4.3. Seed Collection and Seed Packet Construction
4.4. Experimental Design
4.5. Seeds Observation and Measurements
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thornton, I. Impacts of mining on the environment; some local, regional and global issues. Appl. Geochem. 1996, 11, 355–361. [Google Scholar] [CrossRef]
- Rieuwerts, J.S.; Austin, S.; Harris, E.A. Contamination from historic metal mines and the need for non-invasive remediation techniques: A case study from Southwest England. Environ. Monit. Assess. 2009, 148, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Beane, S.J.; Comber, S.D.W.; Rieuwerts, J.; Long, P. Abandoned metal mines and their impact on receiving waters: A case study from Southwest England. Chemosphere 2016, 153, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Mannu, R.; Pantini, P.; Sassu, A.; Verdinelli, M. A multidiversity approach to investigate the impact of mining exploitation on spider diversity in the abandoned mine district of Montevecchio-Ingurtosu (Sardinia, Italy). Environ. Sci. Pollut. Res. 2020, 27, 32615–32627. [Google Scholar] [CrossRef] [PubMed]
- Caboi, R.; Cidu, R.; Fanfani, L.; Zuddas, P.; Zanzari, A.R. Geochemistry of the high-PCO2 waters in Logudoro, Sardinia, Italy. Appl. Geochem. 1993, 8, 153–160. [Google Scholar] [CrossRef]
- Damian, G.E.; Micle, V.; Sur, I.M.; Chirilă Băbău, A.M. From Environmental Ethics to Sustainable Decision-Making: Assessment of Potential Ecological Risk in Soils Around Abandoned Mining Areas-Case Study “Larga de Sus mine” (Romania). J. Agric. Environ. Ethics 2019, 32, 27–49. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Ernst, W.H.O.; van der Ent, A.; Malaisse, F.; Ginocchio, R. Metallophytes: The Unique Biological Resource, Its Ecology and Conservational Status in Europe, Central Africa and Latin America. Ecol. Ind. Pollut. 2010, 18, 7–40. [Google Scholar]
- Whiting, S.N.; Reeves, R.D.; Richards, D.; Johnson, M.S.; Cooke, J.A.; Malaisse, F.; Paton, A.; Smith, J.A.C.; Angle, J.S.; Chaney, R.L.; et al. Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Restor. Ecol. 2004, 12, 106–116. [Google Scholar] [CrossRef]
- van der Ent, A.; van Vugt, R.; Wellinga, S. Ecology of Paphiopedilum rothschildianum at the type locality in Kinabalu Park (Sabah, Malaysia). Biodivers. Conserv. 2015, 24, 1641–1656. [Google Scholar] [CrossRef]
- Mikavica, I.; Ranđelović, D.; Đorđević, V.; Gajić, G.; Mutić, J. Orchid species Anacamptis morio as a potential bioremediator of As, Cd and Pb. J. Appl. Eng. Sci. 2020, 18, 413–421. [Google Scholar] [CrossRef]
- Jurkiewicz, A.; Turnau, K.; Mesjasz-Przybyowicz, J.; Przybyowicz, W.; Godzik, B. Heavy metal localisation in mycorrhizas of Epipactis atrorubens (Hoffm.) Besser (Orchidaceae) from zinc mine tailings. Protoplasma 2001, 218, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Esfeld, K.; Hensen, I.; Wesche, K.; Jakob, S.S.; Tischew, S.; Blattner, F.R. Molecular data indicate multiple independent colonizations of former lignite mining areas in Eastern Germany by Epipactis palustris (Orchidaceae). Biodivers. Conserv. 2008, 17, 2441–2453. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Kull, T.; Tali, K. Mycorrhizal interactions of orchids colonizing Estonian mine tailings hills. Am. J. Bot. 2008, 95, 156–164. [Google Scholar] [CrossRef] [PubMed]
- De Agostini, A.; Caltagirone, C.; Caredda, A.; Cicatelli, A.; Cogoni, A.; Farci, D.; Guarino, F.; Garau, A.; Labra, M.; Lussu, M.; et al. Heavy metal tolerance of orchid populations growing on abandoned mine tailings: A case study in Sardinia Island (Italy). Ecotoxicol. Environ. Saf. 2020, 189, 110018. [Google Scholar] [CrossRef]
- De Agostini, A.; Cortis, P.; Cogoni, A.; Gargiulo, R.; Fenu, G. Epipactis tremolsii seed diversity in two close but extremely different populations: Just a case of intraspecific variability? Plants 2020, 9, 1625. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.N.; Whigham, D.F. Seed ecology of dust seeds in situ: A new study technique and its application in terrestrial orchids. Am. J. Bot. 1993, 80, 1374–1378. [Google Scholar] [CrossRef]
- Turner, S.R.; Lewandrowski, W.; Elliott, C.P.; Merino-Martín, L.; Miller, B.P.; Stevens, J.C.; Erickson, T.E.; Merritt, D.J. Seed ecology informs restoration approaches for threatened species in water-limited environments: A case study on the short-range Banded Ironstone endemic Ricinocarpos brevis (Euphorbiaceae). Aust. J. Bot. 2017, 65, 661–677. [Google Scholar] [CrossRef]
- Phillips, R.D.; Reiter, N.; Peakall, R. Orchid conservation: From theory to practice. Ann. Bot. 2020, 126, 345–362. [Google Scholar] [CrossRef]
- Reiter, N.; Menz, M.H.M. Optimising conservation translocations of threatened Caladenia (Orchidaceae) by identifying adult microsite and germination niche. Aust. J. Bot. 2022, 70, 231–247. [Google Scholar] [CrossRef]
- Richards, A.J.; Swan, G.A. Epipactis leptochila (Godfery) Godfery and E. phyllanthes G.E.Sm. occurring in South Northumberland on lead and zinc soils. Watsonia 1976, 11, 1–5. [Google Scholar]
- Szarek-Łukaszewska, G. Vegetation of reclaimed and spontaneously vegetated Zn-Pb mine wastes in Southern Poland. Polish J. Environ. Stud. 2009, 18, 717–733. [Google Scholar]
- Bell, T.J.; Bowles, M.L.; Zettler, L.W.; Pollack, C.A.; Ibberson, J.E. Environmental and management effects on demographic processes in the U.S. threatened Platanthera leucophaea (Nutt.) Lindl. (Orchidaceae). Plants 2021, 10, 1308. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.A.J. Successful recruitment following translocation of a threatened terrestrial orchid (Diuris tricolor) into mining rehabilitation in the Hunter Valley of NSW. Ecol. Manag. Restor. 2021, 22, 204–207. [Google Scholar] [CrossRef]
- Volis, S.; Blecher, M. Quasi in situ: A bridge between ex situ and in situ conservation of plants. Biodivers. Conserv. 2010, 19, 2441–2454. [Google Scholar] [CrossRef]
- Bell, S.A.J. Translocation of threatened terrestrial orchids into non-mined and post-mined lands in the Hunter Valley of New South Wales, Australia. Restor. Ecol. 2020, 28, 1396–1407. [Google Scholar] [CrossRef]
- Fenu, G.; Bacchetta, G.; Charalambos, S.C.; Fournaraki, C.; del Galdo, G.P.G.; Gotsiou, P.; Kyratzis, A.; Piazza, C.; Vicens, M.; Pinna, M.S.; et al. An early evaluation of translocation actions for endangered plant species on Mediterranean islands. Plant Divers. 2019, 41, 94–104. [Google Scholar] [CrossRef]
- Fenu, G.; Bacchetta, G.; Christodoulou, C.S.; Cogoni, D.; Fournaraki, C.; del Galdo Gian Pietro, G.; Gotsiou, P.; Kyratzis, A.; Piazza, C.; Vicens, M.; et al. A common approach to the conservation of threatened island vascular plants: First results in the mediterranean basin. Diversity 2020, 12, 157. [Google Scholar] [CrossRef]
- Abeli, T.; D’Agostino, M.; Orsenigo, S.; Bartolucci, F.; Accogli, R.; Albani Rocchetti, G.; Alessandrelli, C.; Amadori, A.; Amato, F.; Angiolini, C.; et al. IDPlanT: The Italian database of plant translocation. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2021, 155, 1174–1177. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Dixon, K.W.; Jersáková, J.; Těšitelová, T. Germination and seedling establishment in orchids: A complex of requirements. Ann. Bot. 2015, 116, 391–402. [Google Scholar] [CrossRef]
- Whigham, D.; Mccormick, M.; Brooks, H.; Josey, B.; Floyd, R.; Applegate, J. Isotria medeoloides, a North American threatened orchid: Fungal abundance may be as important as light in species management. Plants 2021, 10, 1924. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Pedersen, H.Æ. Cypripedium calceolus germination in situ: Seed longevity, and dormancy breakage by long incubation and cold winters. Eur. J. Environ. Sci. 2012, 1, 69–70. [Google Scholar] [CrossRef]
- Tešitelová, T.; Tešitel, J.; Jersáková, J.; Říhová, G.; Selosse, M.A. Symbiotic germination capability of four Epipactis species (Orchidaceae) is broader than expected from adult ecology. Am. J. Bot. 2012, 99, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, H.; Waud, M.; Merckx, V.S.F.T.; Lievens, B.; Brys, R. Mycorrhizal diversity, seed germination and long-term changes in population size across nine populations of the terrestrial orchid Neottia ovata. Mol. Ecol. 2015, 24, 3269–3280. [Google Scholar] [CrossRef] [PubMed]
- Buyun, L.I.; Cherevchenko, T.M.; Kovalska, L.A.; Ivannikov, R.V. Reproductive biology of Angraecum eburneum subsp. superbum (Orchidaceae) under glasshouse conditions. Environ. Exp. Biol. 2015, 13, 33–39. [Google Scholar]
- Medrano, M.; Guitian, P.; Guitian, J. Patterns of fruit and seed set within inflorescences of Pancratium maritimum (Amaryllidaceae): Nonuniform pollination, resource limitation, or architectural effects? Am. J. Bot. 2000, 87, 493–501. [Google Scholar] [CrossRef]
- Delforge, P. Orchids of Europe, North Africa and the Middle East; Timber Press: Portland, OR, USA, 2006. [Google Scholar]
- Agenzia Regionale per la Protezione dell’Ambiente della Sardegna (Arpas)—Dipartimento Specialistico Regionale Meteo Climatico. Available online: http://www.sar.sardegna.it/pubblicazioni/riepiloghimensili/mensili.asp (accessed on 3 March 2022).
- Bacchetta, G.; Fenu, G.; Mattana, E.; Piotto, B.; Virevaire, M. Manuale per la Raccolta, Studio, Conservazione e Gestione Ex Situ del Germoplasma; APAT: Rome, Italy, 2006; Volume 37. [Google Scholar]
- Mattana, E.; Daws, M.I.; Fenu, G.; Bacchetta, G. Adaptation to habitat in Aquilegia species endemic to Sardinia (Italy): Seed dispersal, germination and persistence in the soil. Plant Biosyst. 2012, 146, 374–383. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 10 September 2022).
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 10 September 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Agostini, A.; Cogoni, D.; Cogoni, A.; Vacca, A.; Fenu, G.; Cortis, P. Seed Bank Conservation and Incipient Seed Development in Orchids Colonizing Mining Wastes: Results of a Field Pilot Experiment. Plants 2022, 11, 3315. https://doi.org/10.3390/plants11233315
De Agostini A, Cogoni D, Cogoni A, Vacca A, Fenu G, Cortis P. Seed Bank Conservation and Incipient Seed Development in Orchids Colonizing Mining Wastes: Results of a Field Pilot Experiment. Plants. 2022; 11(23):3315. https://doi.org/10.3390/plants11233315
Chicago/Turabian StyleDe Agostini, Antonio, Donatella Cogoni, Annalena Cogoni, Andrea Vacca, Giuseppe Fenu, and Pierluigi Cortis. 2022. "Seed Bank Conservation and Incipient Seed Development in Orchids Colonizing Mining Wastes: Results of a Field Pilot Experiment" Plants 11, no. 23: 3315. https://doi.org/10.3390/plants11233315
APA StyleDe Agostini, A., Cogoni, D., Cogoni, A., Vacca, A., Fenu, G., & Cortis, P. (2022). Seed Bank Conservation and Incipient Seed Development in Orchids Colonizing Mining Wastes: Results of a Field Pilot Experiment. Plants, 11(23), 3315. https://doi.org/10.3390/plants11233315







