Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance
Abstract
1. Introduction
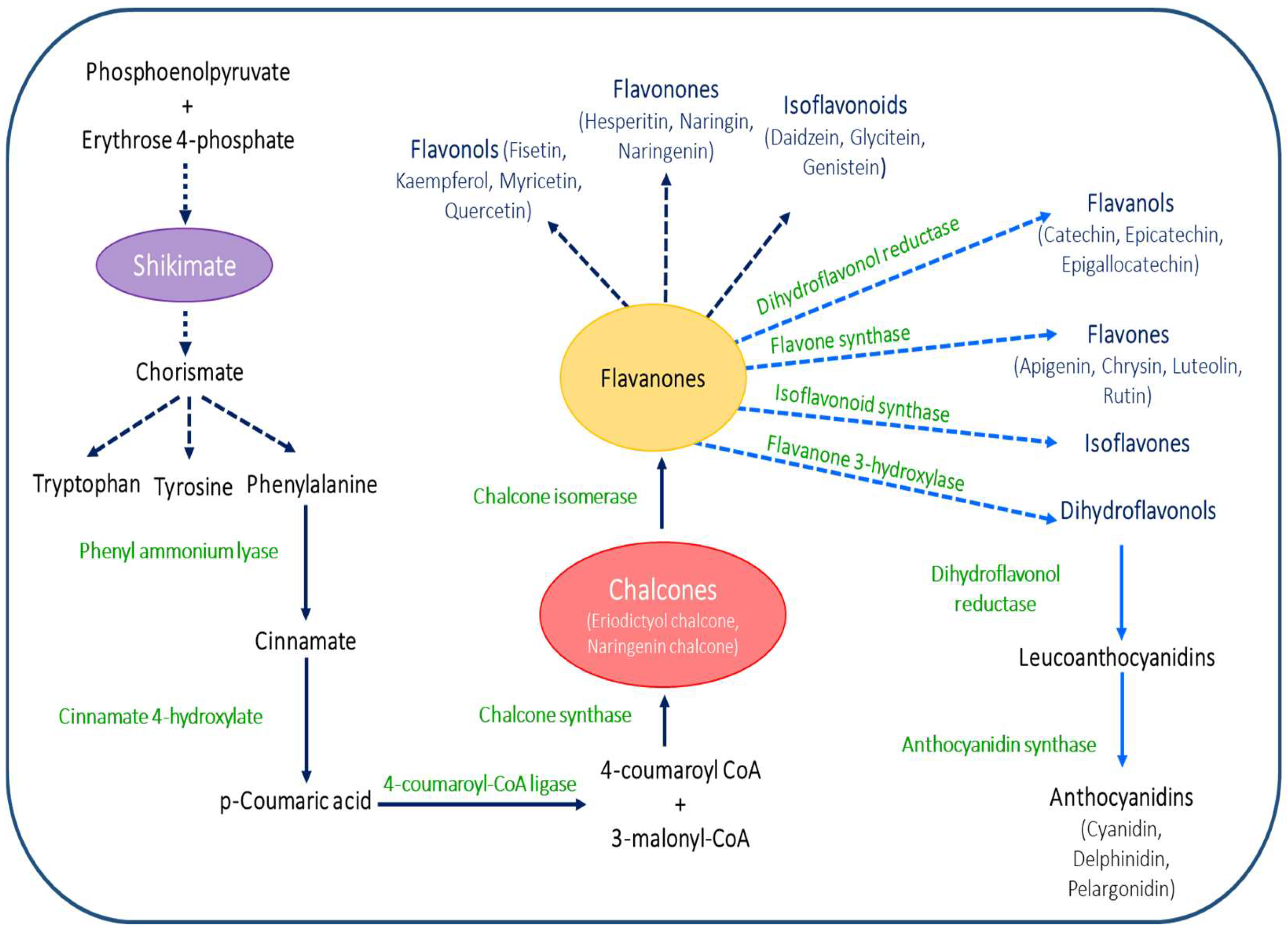
2. Chemistry and Biosynthesis of Flavonoids
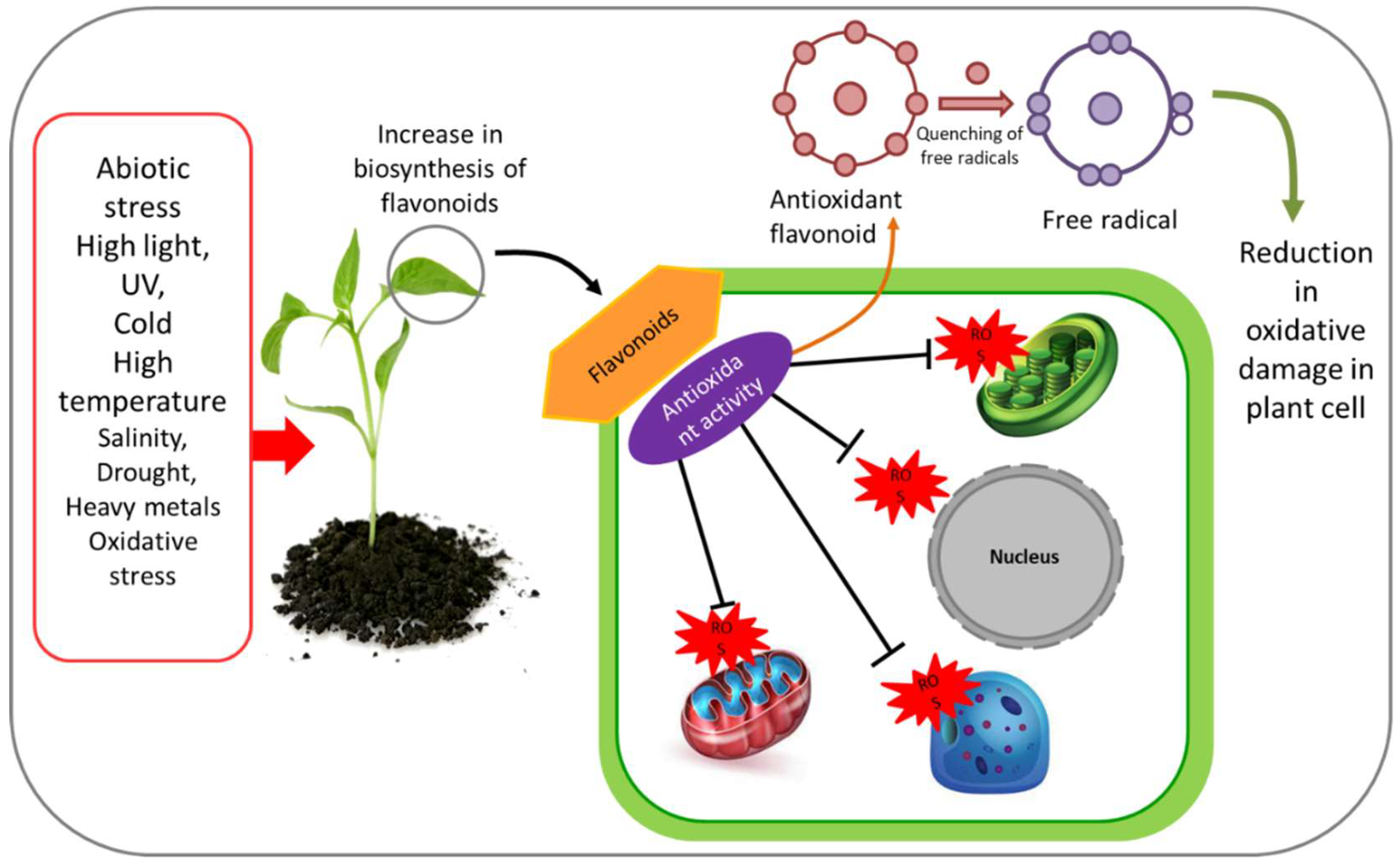
3. Antioxidant Properties of Flavonoids
| Abiotic Stress | Plant Species | Antioxidant Response of Flavonoids | References |
|---|---|---|---|
| UV-B radiation | Medicago sativa | Increased content of flavonoid compound induces enhanced antioxidant capacity of the plant. | [57] |
| UV-B radiation | Kalanchoe pinnata | Increases total flavonoid and quercitrin content, which have antioxidant properties to protect the plant. | [58] |
| UV-B stress and drought | Populus tremula × P. tremuloides | Transgenic line of poplar with high proanthocyanidins content displayed lower hydrogen peroxide content. | [59] |
| Salinity | Zea maize | Improved plant performance under salt stress through antioxidant activities. | [60] |
| Salinity | Arabidopsis thaliana | CrUGT87A1, a UDP-sugar glycosyltransferases (UGTs) gene, improved salt tolerance by increasing antioxidant capacity resulting from the accumulation of flavonoids. | [61] |
| Salinity | Amaranthus tricolor | Increases flavonoid content, which showed the potent antioxidant activity in scavenging ROS. | [62] |
| Salinity | Amaranthus lividus | Increases flavonoid content and the antioxidant capacity of leaves, total flavonoid content scavenged ROS. | [63] |
| Water stress | Chrysanthemum morifoilum | Increases flavonoids (rutin, quercetin, apigenin, and luteolin) and enhanced antioxidant activity. | [64] |
| Drought | Arabidopsis thaliana | Increase in total flavonoid content followed by an increase in antioxidant activity. | [65] |
| Drought | Cistus clusii | prevented oxidative damage. | [66] |
| Drought | Swingle citrumelo | Proline accumulation was concomitant with an increase in antioxidant activity. | [67] |
| Temperature stress | Solanum viarum Dunal | Flavonoids inhibited ROS-mediated oxidative damage. | [68] |
| Heat and salinity | Solanum Lycopersicon | Lower antioxidative damage was observed following a high accumulation of flavonols. | [69] |
| Cadmium stress | Trigonella foenum-graecum | H2S-induced polyamines accumulation was concomitant with an increase in ROS-detoxification capacity. | [70] |
| Cadmium stress | Solanum Lycopersicon | Nitric oxide-induced increase in flavonols resulted in improved antioxidant capacity. | [71] |
| Lead stress | Tritium aestivum | Accumulation of proline was concomitant with a lower level of lipid peroxidation. | [72] |
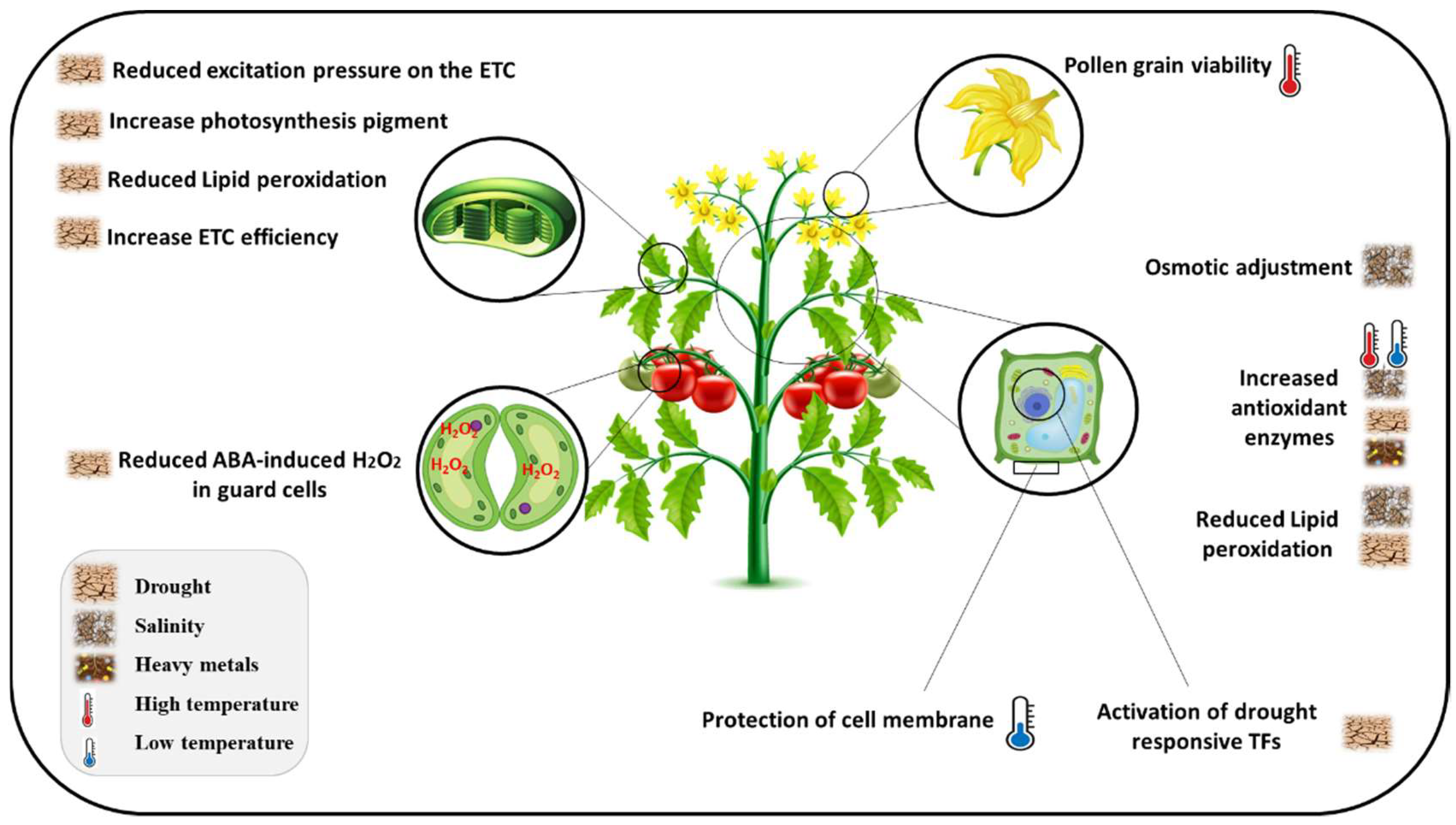
4. Flavonoids-Mediated Defenses against Abiotic Stress
| Abiotic Stress | Concentration/Levels | Duration of Stress | Plant Species | Flavonoids Level under Stress | References |
|---|---|---|---|---|---|
| Salinity | 50 and 100 mM NaCl | 35 days | Amaranthus lividus | An increase was observed in total flavonoid content by 31%. | [77] |
| Salinity | 200 mM NaCl | 3 weeks | Apocynum venetum L. | The total flavonoid content and dihydroquercetin decreased by 20.46% to 23.08%, but an increase in flavonols (quercetin and kaempferol) by 1.6-fold and 2.2-fold was detected in comparison to control. | [78] |
| Drought | Stop watering | 5 days | Arabidopsis thaliana L. | Quercetin 3-O-glucoside and cyanidin 3-O-glucoside exhibited approximately10-fold higher activity than kaempferol 3-O-glucoside, whereas a slight reduction in total flavonoid content was observed. | [79] |
| Drought | Osmotic potential of 0.49 MPa | 48 h | Triticum aestivum L. | Significant increase in total flavonoid content was detected by 143% in cultivars aikang 58 compared with Chinese spring (115%). | [80] |
| Drought | Soil water content 25% (±2.5%) | At three-leaf seedling stage | Zea mays L. | Flavonol in guard cells was observed 1.7-fold higher compared to control. | [81] |
| Copper | 200 mg L−1 | 35 days | Belamcanda chinensis | Increased generation of 11 kinds of flavonoids. | [82] |
| Copper and Zinc | 200–500 ppm | 28 days | (Lycopersicon esculentum Mill | Accumulation of flavonoids increased (1.44, 0.93 mg QE/g DW) compared to the control (0.18, 0.13 mg QE/g DW) in roots and leaves, respectively. | [83] |
| UV-B and drought | 40% drought-stressed | 8 weeks | Ligustrum vulgare L. | Increases in the biosynthesis of quercetin-3-O-rutinoside, luteolin 7-O-glucoside, and echinacosid were observed. | [53] |
| Extreme temperature and high CO2 levels | Light intensity 700 PAR and ambient CO2 (400 µmol mol−1) | 35–39 days | Lactuca sativa L. | Increased accumulation of quercetin-3-O-glucoside, quercetin-3-O-glucuronide, luteolin7-O-glucoside, cyanidin derivatives (61%), and cyanidin-3-O-glucoside (28%), while lower accumulations of kaempferol, myricetin, quercitrin (99–94%), and rutin were found under high light condition. Total flavonoid content increased by 7.5-fold in comparison to control. | [84] |
| Stress | Stress Level | Duration of Stress | Plant Species | Flavonoids Modulation | Function of Flavonoids | Reference |
|---|---|---|---|---|---|---|
| Drought | Drought (mild drought stress) | 24 h | Tea (Camellia sinensis) | Accumulation phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H), 4-coumarateCoA ligase (4CL), chalcone synthase (CHS), and dihydrofavonol 4-reductase (DFR). | Increase in flavonoid content was concomitant with stress tolerance in plant. | [85] |
| Drought | 15–25% of soil water-holding capacity | 8 days | Tea (C. sinensis) | Accumulation of endogenous flavonoids, including: C4H, CHS, F3′5′H, F3H, kaempferol, quercetin, and myricetin triggered by fulvic acid. | Increase in flavonoid content took part in improved tolerance of plants against drought. | [86] |
| Drought | 8% PEG 6000 | 7 days | Maize (Zea mays) Pigeon pea (Cajanus cajan) | Accumulation of endogenous flavonoids, including: genistein, genistin, and pterostilbene. | ABA and CcMYB114 improve drought tolerance by regulating the accumulation of flavonoids. | [81,87] |
| Drought | Stopped watering | 3 weeks | Arabidopsis (A. thaliana) | Accumulation of endogenous flavonoids triggered by ectopic expression of Arabidopsis glycosyltransferase gene (UGT76E11). | Activation of stress-related transcription factors. | [88] |
| Salt | 300 mM NaCl | 14 days | Arabidopsis (A. thaliana) | Accumulation of endogenous flavonoids including: chalcone, dihydrokaempferole, and quercetin. | Act in MYB111-regulated salt stress response. | [89] |
| Salt | 100, 150, and 200 mM NaCl | 19 days | Maize (Z. mays) | Exogenous application of α-tocopherol in combination with selenium (Na2SeO4 (0.5 mM) + a-tocopherol (200 ppm)). | Improved plant performance under salt stress through antioxidant defense. | [60] |
| Salt | 150 mM NaCl | 5 days | Tomato (Solanum Lycopersicon L.) | Exogenous application of vanillic acid (4- hydroxy-3-methoxy benzoic acid) (50 μM). | Increase in the activity of AsA-GSH cycle and glyoxalase system and a further increase in accumulation of osmolytes. Improved K+ accumulation and restricted Na+ accumulation. Increase in superoxide dismutase (SOD), catalase (CAT), and ascorbic acid (AsA). | [90] |
| Salt | 100 mM NaCl | 8 days | Bean (Phaseolus vulgaris) | Exogenous application of naringenin (0.1–0.4 mM). | Regulation of cellular redox, chloroplast antioxidant system, and photosynthesis. | [91] |
| Heavy metals | 150 mg L−1 of Pb2 þ (which corresponds to 724 μM Pb(NO3)2) | Incubated for 2 h | Lupin | Incubation of seedlings with catechin before exposure to lead stress (5, 10, and 20 μg mL−1 of catechin equivalents). | Increased root growth and reduced accumulation of ROS, lipid peroxidation, and cell death. | [92] |
| Heavy metals | Wastewater | 100 days | Lettuce and turnip | Accumulation of endogenous flavonoids, including putrescine and spermidine. | Counteract the oxidative stress. | [93] |
| High temperature | 37 °C (day), 25 °C (night) | During growth period | Tomato (Solanum Lycopersicon L.) | Accumulation of endogenous flavonoids. | Reducing the abundance of ROS, enhancing fertility. | [94] |
| High temperature | Moderate (36 °C/24 °C day/night) or severe (42 °C/26 °C day/night) | During the growth period since the pod’s color changed to an individual level | Soybean (Glycine max) | Accumulation of endogenous flavonoids, including tocopherols, flavonoids, phenylpropanoids, and ascorbate precursors. | Scavenging of heat-induced ROS damage during seed maturity. | [95] |
| Air pollutant | Sulfur dioxide (SO2), NO2, carbon monoxide (CO), hydrocarbons (HC), and airborne particulate material (APM) | During growth period | Spartium junceum L., Lagerstroemia indica L., Th uja orientalis L., and Petunia hybrida L. w | Accumulation of endogenous flavonoids. | Reduced ROS accumulation in pollen grain and improved development of pollen tube and germination. | [96] |
| Air pollutant | O3 stress (300 nL L−1) | 6 h | Medicago truncatula | Accumulation of endogenous phenolic compounds. | Phenols were oxidized red/purple pigments and resulted in the accumulation of antioxidant compounds. | [97] |
4.1. Drought and Salinity
4.2. Toxic Metal/Metalloids
4.3. Extreme Temperature
4.4. Atmospheric Pollutants
4.5. Light Stress
4.6. Other Stresses
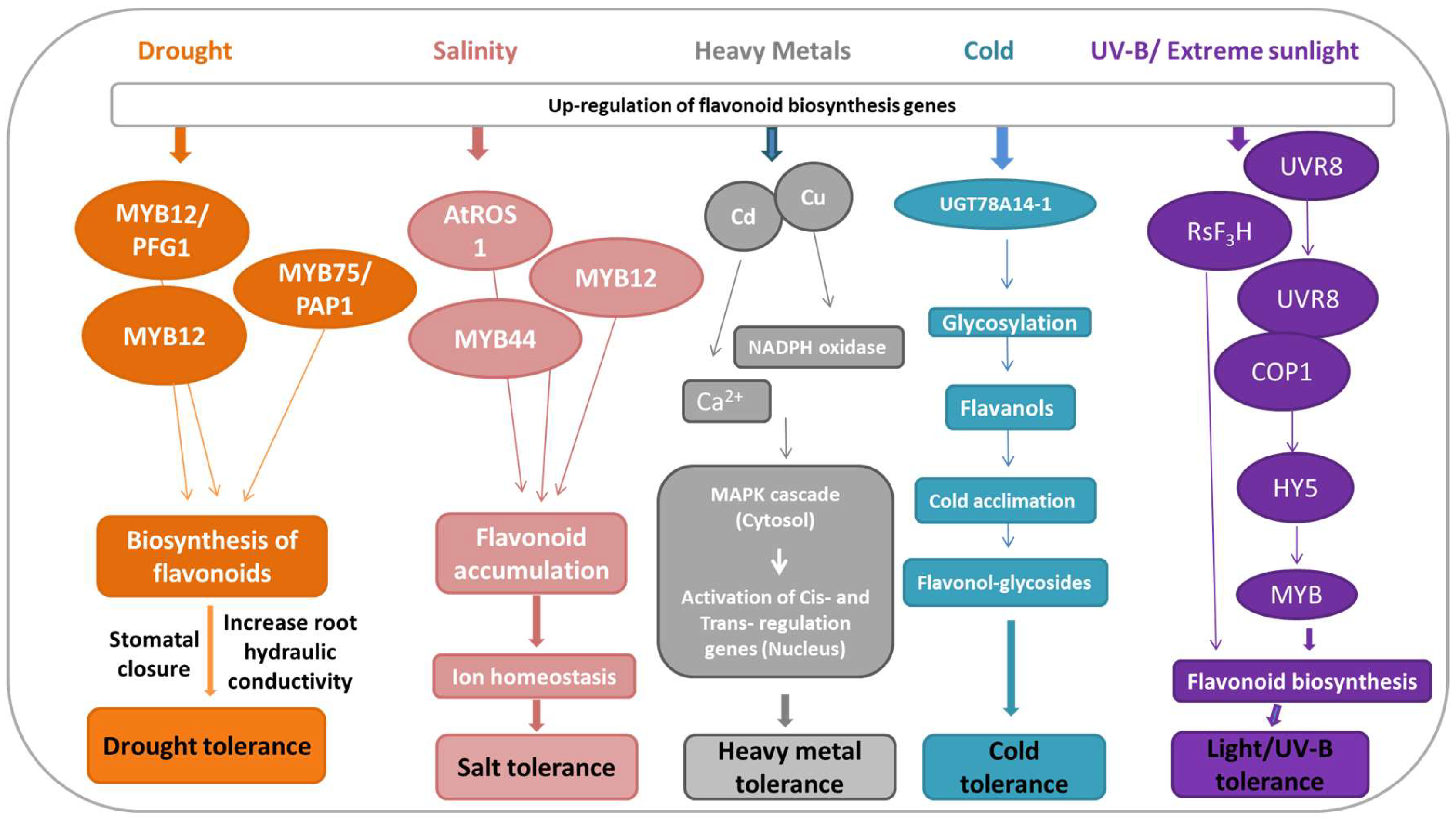
5. Flavonoids-Mediated Abiotic Stress Signaling
6. Molecular and Genetic Approaches in Tailoring Flavonoids Biosynthesis and Regulation under Abiotic Stress
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Austen, N.; Walker, H.J.; Lake, J.A.; Phoenix, G.K.; Cameron, D.D. The regulation of plant secondary metabolism in response to abiotic stress: Interactions between heat shock and elevated CO2. Front. Plant Sci. 2019, 10, 1463. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Saeed-ur-Rahman; Bilal, M.; Huang, D.-F. Role of flavonoids in plant interactions with the environment and against human pathogens—A review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, polyphenols and tannins: An overview. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, A., Clifford, M.N., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 1–24. [Google Scholar] [CrossRef]
- Griesbach, R.J. Biochemistry and genetics of flower color. In Plant Breeding Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 89–114. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef]
- Kolb, C.A.; Käser, M.A.; Kopecky, J.; Zotz, G.; Riederer, M.; Pfündel, E.E. Effect of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol. 2001, 127, 863–875. [Google Scholar] [CrossRef]
- Agati, G.; Stefano, G.; Biricolti, S.; Tattini, M. Mesophyll distribution of antioxidant flavonoids in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann. Bot. 2009, 104, 853–863. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Int. J. Pharm. Sci. Technol. 2011, 6, 12–35. [Google Scholar]
- Baskar, V.; Venkatesh, R.; Ramalingam, S. Flavonoids (antioxidant systems) in higher plants and their response to stresses. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: Cham, Switzerland, 2018; pp. 253–268. [Google Scholar] [CrossRef]
- Mathesius, U. Flavonoid Functions in Plants and Their Interactions with Other Organisms. Plants 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Preuß, A.; Matern, U. Multifunctional flavonoid dioxygenases: Flavonol and anthocyanin biosynthesis in Arabidopsis thaliana L. Phytochemistry 2010, 71, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Yu, O. Metabolic engineering of flavonoids in plants and microorganisms. Appl. Microbiol. Biotechnol. 2011, 91, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef]
- Winkel, B.S. Metabolic channeling in plants. Annu. Rev. Plant Biol. 2004, 55, 85–107. [Google Scholar] [CrossRef]
- Stracke, R.; Favory, J.J.; Gruber, H.; Bartelniewoehner, L.; Bartels, S.; Binkert, M.; Funk, M.; Weisshaar, B.; Ulm, R. The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Environ. 2010, 33, 88–103. [Google Scholar] [CrossRef]
- Stracke, R.; Ishihara, H.; Huep, G.; Barsch, A.; Mehrtens, F.; Niehaus, K.; Weisshaar, B. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007, 50, 660–677. [Google Scholar] [CrossRef]
- Shirley, B.W.; Kubasek, W.L.; Storz, G.; Bruggemann, E.; Koornneef, M.; Ausubel, F.M.; Goodman, H.M. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995, 8, 659–671. [Google Scholar] [CrossRef]
- Borevitz, J.O.; Xia, Y.J.; Blount, J.; Dixon, R.A.; Lamb, C. Activation tagging identifies a conserved MYB regulator of Phenylpropanoid Biosynthesis. Plant Cell 2000, 12, 2383–2393. [Google Scholar] [CrossRef]
- Butelli, E.; Licciardello, C.; Zhang, Y.; Liu, J.; Mackay, S.; Bailey, P.; Reforgia-to-Recupero, G.; Martin, C. Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. Plant Cell 2012, 24, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant flavonoids—Biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Misra, P.; Chandrashekar, K.; Trivedi, P.K. Development of AtMYB12-expressing transgenic tobacco callus culture for production of rutin with biopesticidal potential. Plant Cell Rep. 2012, 31, 1867–1876. [Google Scholar] [CrossRef]
- Burbulis, I.E.; Winkel-Shirley, B. Interactions among enzymes of the Arabidopsis flavonoid biosynthetic pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 12929–12934. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Saslowsky, D.; Winkel-Shirley, B. Localization of flavonoid enzymes in Arabidopsis roots. Plant J. 2001, 27, 37–48. [Google Scholar] [CrossRef]
- Saslowsky, D.E.; Warek, U.; Winkel, B.S. Nuclear localization of flavonoid enzymes in Arabidopsis. J. Biol. Chem. 2005, 280, 23735–23740. [Google Scholar] [CrossRef]
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef]
- Pourcel, L.; Irani, N.G.; Lu, Y.; Riedl, K.; Schwartz, S.; Grotewold, E. The formation of anthocyanic vacuolar inclusions in Arabidopsis thaliana and implications for the sequestration of anthocyanin pigments. Mol. Plant 2010, 3, 78–90. [Google Scholar] [CrossRef]
- Gomez, C.; Conejero, G.; Torregrosa, L.; Cheynier, V.; Terrier, N.; Ageorges, A. In vivo grapevine anthocyanin transport involves vesicle-mediated trafficking and the contribution of anthoMATE transporters and GST. Plant J. 2011, 67, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Grace, S.C.; Logan, B.A. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos. Trans. R. Soc. B 2000, 355, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Antioxidants and Antioxidant Enzymes in Higher Plants, 1st ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Hatier, J.H.B.; Gould, K.S. Foliar anthocyanins as modulators of stress signals. J. Theor. Biol. 2008, 253, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Di Ferdinando, M.; Brunetti, C.; Fini, A.; Tattini, M. Flavonoids as antioxidants in plants under abiotic stresses. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012; pp. 159–179. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]
- Schroeter, H.; Boyd, C.; Spencer, J.P.; Williams, R.J.; Cadenas, E.; Rice-Evans, C. MAPK signaling in neurodegeneration: Influences of flavonoids and of nitric oxide. Neurobiol. Aging 2002, 23, 861–880. [Google Scholar] [CrossRef]
- Melidou, M.; Riganakos, K.; Galaris, D. Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: The role of iron chelation. Free Radic. Biol. Med. 2005, 39, 1591–1600. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Guidi, L.; Morassi-Bonzi, L.; Pinelli, P.; Remorini, D.; Degl’Innocenti, E.; Giordano, C.; Massai, R.; Agati, G. On the role of flavonoids in the integrated mechanisms of response of Ligustrum vulgare and Phillyrea latifolia to high solar radiation. New Phytol. 2005, 167, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Matteini, P.; Goti, A.; Tattini, M. Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 2007, 174, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta Biomembr. 2006, 1758, 994–1003. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Malešev, D.; Kuntić, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serb. Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Banjarnahor, S.D.; Artanti, N. Antioxidant properties of flavonoids. Med. J. Indones. 2014, 23, 239–244. [Google Scholar] [CrossRef]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, Q. Antioxidant capacity of flavonoid in soybean seedlings under the joint actions of rare earth element La (III) and ultraviolet-B stress. Biol. Trace Elem. Res. 2009, 127, 69–80. [Google Scholar] [CrossRef]
- Saunders, J.A.; McClure, J.W. The distribution of flavonoids in chloroplasts of twenty-five species of vascular plants. Phytochemistry 1976, 15, 809–810. [Google Scholar] [CrossRef]
- Brown, E.J.; Khodr, H.; Hider, C.R.; Rice-Evans, C.A. Structural dependence of flavonoid interactions with Cu2+ ions: Implications for their antioxidant properties. Biochem. J. 1998, 330, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Y.; Wang, X.; Li, Y.; Han, R. Lower levels of UV-B light trigger the adaptive responses by inducing plant antioxidant metabolism and flavonoid biosynthesis in Medicago sativa seedlings. Funct. Plant Biol. 2019, 46, 896–906. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Nascimento, L.B.; Leal-Costa, M.V.; Menezes, E.A.; Lopes, V.R.; Muzitano, M.F.; Costa, S.S.; Tavares, E.S. Ultraviolet-B radiation effects on phenolic profile and flavonoid content of Kalanchoe pinnata. J. Photochem. Photobiol. B Biol. 2015, 148, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Gourlay, G.; Hawkins, B.J.; Albert, A.; Schnitzler, J.P.; Constabel, C.P. Condensed tannins as antioxidants that protect poplar against oxidative stress from drought and UV-B. Plant Cell Environ. 2022, 45, 362–377. [Google Scholar] [CrossRef]
- Khalil, R.; Yusuf, M.; Bassuony, F.; Haroun, S.; Gamal, A. Alpha-tocopherol reinforce selenium efficiency to ameliorates salt stress in maize plants through carbon metabolism, enhanced photosynthetic pigments and ion uptake. S. Afr. J. Bot. 2022, 144, 1–9. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, Y.; Li, M.; Long, R. CrUGT87A1, a UDP-sugar glycosyltransferases (UGTs) gene from Carex rigescens, increases salt tolerance by accumulating flavonoids for antioxidation in Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 159, 28–36. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef]
- Hossain, M.N.; Sarker, U.; Raihan, M.S.; Al-Huqail, A.A.; Siddiqui, M.H.; Oba, S. Influence of Salinity Stress on Color Parameters, Leaf Pigmentation, Polyphenol and Flavonoid Contents, and Antioxidant Activity of Amaranthus lividus Leafy Vegetables. Molecules 2022, 27, 1821. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Rao, M.J.; Xu, Y.; Tang, X.; Huang, Y.; Liu, J.; Deng, X.; Xu, Q. CsCYT75B1, a Citrus CYTOCHROME P450 gene, is involved in accumulation of antioxidant flavonoids and induces drought tolerance in transgenic Arabidopsis. Antioxidants 2020, 9, 161. [Google Scholar] [CrossRef]
- Hernández, I.; Alegre, L.; Munné-Bosch, S. Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under Mediterranean field conditions. Tree Physiol. 2004, 24, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, K.; de Campos, M.K.F.; Domingues, D.S.; Pereira, L.F.P.; Vieira, L.G.E. The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol. Biol. Rep. 2013, 40, 3269–3279. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Prasad, A.; Srivastava, D.; Niranjan, A.; Saxena, G.; Singh, S.S.; Misra, P.; Chakrabarty, D. Genotype-dependent and temperature-induced modulation of secondary metabolites, antioxidative defense and gene expression profile in Solanum viarum Dunal. Environ. Exp. Bot. 2022, 194, 104686. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef] [PubMed]
- Javad, S.; Shah, A.A.; Ramzan, M.; Sardar, R.; Javed, T.; Al-Huqail, A.A.; Ali, H.M.; Chaudhry, O.; Yasin, N.A.; Ahmed, S.; et al. Hydrogen sulphide alleviates cadmium stress in Trigonella foenum-graecum by modulating antioxidant enzymes and polyamine content. Plant Biol. 2022, 24, 618–626. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Wei, X.; You, J.; Wang, W.; Lu, J.; Shi, R. Comparative antioxidative responses and proline metabolism in two wheat cultivars under short term lead stress. Ecotoxicol. Environ. Saf. 2011, 74, 733–740. [Google Scholar] [CrossRef]
- Lasky, J.R.; Des Marais, D.L.; Lowry, D.B.; Povolotskaya, I.; McKay, J.K.; Richards, J.H.; Keitt, T.H.; Juenger, T.E. Natural variation in abiotic stress responsive gene expression and local adaptation to climate in Arabidopsis thaliana. Mol. Biol. Evol. 2014, 31, 2283–2296. [Google Scholar] [CrossRef]
- Llanes, A.; Andrade, A.; Alemano, S.; Luna, V. Metabolomic approach to understand plant adaptations to water and salt stress. In Plant Metabolites and Regulation under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 133–144. [Google Scholar] [CrossRef]
- Taulavuori, K.; Hyöky, V.; Oksanen, J.; Taulavuori, E.; Julkunen-Tiitto, R. Species-specific differences in synthesis of flavonoids and phenolic acids under increasing periods of enhanced blue light. Environ. Exp. Bot. 2016, 121, 145–150. [Google Scholar] [CrossRef]
- Cetinkaya, H.; Kulak, M.; Karaman, M.; Karaman, H.S.; Kocer, F. Flavonoid accumulation behavior in response to the abiotic stress: Can a uniform mechanism be illustrated for all plants. In Flavonoids—From Biosynthesis to Human Health; Intechopen: London, UK, 2017. [Google Scholar]
- Sarker, U.; Oba, S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, J.; Ren, T.; Du, H.; Liu, H.; Li, Y.; Zhang, C. Salt stress decreases seedling growth and development but increases quercetin and kaempferol content in Apocynum venetum. Plant Biol. 2020, 22, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Mori, T.; Saito, K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e29518. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef]
- Li, B.; Fan, R.; Sun, G.; Sun, T.; Fan, Y.; Bai, S.; Guo, S.; Huang, S.; Liu, J.; Zhang, H.; et al. Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species. Plant Soil 2021, 461, 389–405. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Zhang, X.; Xie, G.; Qin, M. Copper stress-induced changes in biomass accumulation, antioxidant activity and flavonoid contents in Belamcanda chinensis calli. Plant Cell Tissue Organ Cult. 2020, 142, 299–311. [Google Scholar] [CrossRef]
- Badiaa, O.; Yssaad, H.A.R.; Topcuoglu, B. Effect of heavy metals (Copper and Zinc) on proline, polyphenols and flavonoids content of tomato (Lycopersicon esculentum Mill.). Plant Arch. 2020, 20, 2125–2137. [Google Scholar]
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.F.; Muñoz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, Y.U.; Ding, Y.; Qian, W.; Qiu, C.; Xie, H.; Sun, L.; Jiang, Z.; Ma, Q.; Wang, L.; et al. Exogenous abscisic acid induces the lipid and flavonoid metabolism of tea plants under drought stress. Sci. Rep. 2020, 10, 12275. [Google Scholar] [CrossRef]
- Sun, J.; Qiu, C.; Ding, Y.; Wang, Y.; Sun, L.; Fan, K.; Gai, Z.; Dong, G.; Wang, J.; Li, X.; et al. Fulvic acid ameliorates drought stress-induced damage in tea plants by regulating the ascorbate metabolism and flavonoids biosynthesis. BMC Genom. 2020, 21, 411. [Google Scholar] [CrossRef]
- Yang, W.; Li, N.; Fan, Y.; Dong, B.; Song, Z.; Cao, H.; Du, T.; Liu, T.; Qi, M.; Niu, L.; et al. Transcriptome analysis reveals abscisic acid enhancing drought resistance by regulating genes related to flavonoid metabolism in pigeon pea. Environ. Exp. Bot. 2021, 191, 104627. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.M.; Meng, X.F.; Lin, J.S.; Li, Y.J.; Hou, B.K. Ectopic expression of glycosyltransferase UGT 76E11 increases flavonoid accumulation and enhances abiotic stress tolerance in Arabidopsis. Plant Biol. 2018, 20, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fan, R.; Guo, S.; Wang, P.; Zhu, X.; Fan, Y.; Chen, Y.; He, K.; Kumar, A.; Shi, J.; et al. The Arabidopsis MYB transcription factor, MYB111 modulates salt responses by regulating flavonoid biosynthesis. Environ. Exp. Bot. 2019, 166, 103807. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Alp, F.N.; Kucukoduk, M.; Turkan, I. Naringenin induces tolerance to salt/osmotic stress through the regulation of nitrogen metabolism, cellular redox and ROS scavenging capacity in bean plants. Plant Physiol. Biochem. 2020, 157, 264–275. [Google Scholar] [CrossRef]
- Izbiańska, K.; Arasimowicz-Jelonek, M.; Deckert, J. Phenylpropanoid pathway metabolites promote tolerance response of lupine roots to lead stress. Ecotoxicol. Environ. Saf. 2014, 110, 61–67. [Google Scholar] [CrossRef]
- Ahmed, H.R.; Ahmed, H.H.; Hashem, E.D.M.; Ahmed, S. Soil contamination with heavy metals and its effect on growth, yield and physiological responses of vegetable crop plants (turnip and lettuce). J. Stress Physiol. Biochem. 2013, 9, 145–162. [Google Scholar]
- Muhlemann, J.K.; Younts, T.L.; Muday, G.K. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11188–E11197. [Google Scholar] [CrossRef]
- Chebrolu, K.K.; Fritschi, F.B.; Ye, S.; Krishnan, H.B.; Smith, J.R.; Gillman, J.D. Impact of heat stress during seed development on soybean seed metabolome. Metabolomics 2016, 12, 28. [Google Scholar] [CrossRef]
- Rezanejad, F. Air pollution effects on flavonoids in pollen grains of some ornamental plants. Turk. J. Bot. 2012, 36, 49–54. [Google Scholar] [CrossRef]
- Puckette, M.C.; Tang, Y.; Mahalingam, R. Transcriptomic changes induced by acute ozone in resistant and sensitive Medicago truncatulaaccessions. BMC Plant Biol. 2008, 8, 46. [Google Scholar] [CrossRef]
- He, B.; Cui, X.; Wang, H.; Chen, A. Drought: The most important physical stress of terrestrial ecosystems. Acta Ecol. Sin. 2014, 34, 179–183. [Google Scholar] [CrossRef]
- Naderi, M.M.; Mirchi, A.; Bavani, A.R.M.; Goharian, E.; Madani, K. System dynamics simulation of regional water supply and demand using a food-energy-water nexus approach: Application to Qazvin Plain, Iran. J. Environ. Manag. 2021, 280, 111843. [Google Scholar] [CrossRef] [PubMed]
- Kränzlein, M.; Geilfus, C.M.; Franzisky, B.L.; Zhang, X.; Wimmer, M.A.; Zörb, C. Physiological responses of contrasting maize (Zea mays L.) hybrids to repeated drought. J. Plant Grow Regul. 2022, 41, 2708–2718. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Burritt, D.J.; Tran, L.S.P. The use of metabolomic quantitative trait locus mapping and osmotic adjustment traits for the improvement of crop yields under environmental stresses. Semin. Cell Dev. Biol. 2018, 83, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Aliniaeifard, S.; Shomali, A.; Seifikalhor, M.; Lastochkina, O. Calcium signaling in plants under drought. In Salt and Drought Stress Tolerance in Plants; Signaling and Communication in Plants; Hasanuzzaman, M., Tanveer, M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 259–298. [Google Scholar] [CrossRef]
- Shomali, A.; Aliniaeifard, S.; Didaran, F.; Lotfi, M.; Mohammadian, M.; Seif, M.; Strobel, W.R.; Sierka, E.; Kalaji, H.M. Synergistic effects of melatonin and Gamma-Aminobutyric Acid on protection of photosynthesis system in response to multiple abiotic stressors. Cells 2021, 10, 1631. [Google Scholar] [CrossRef]
- Shomali, A.; Aliniaeifard, S. Overview of signal transduction in plants under salt and drought stresses. In Salt and Drought Stress Tolerance in Plants; Signaling and Communication in Plants; Hasanuzzaman, M., Tanveer, M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 231–258. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Chakraborty, S. Effect of hydrogen sulfide on osmotic adjustment of plants under different abiotic stresses. In Hydrogen Sulfide and Plant Acclimation to Abiotic Stresses; Khan, M.N., Siddiqui, M.H., Alamri, S., Corpas, F.J., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 73–85. [Google Scholar] [CrossRef]
- Niu, X.; Zhai, N.; Yang, X.; Su, M.; Liu, C.; Wang, L.; Qu, P.; Liu, W.; Yuan, Q.; Pei, X. Identification of Drought-Resistant Genes in Shanlan Upland Rice. Agriculture 2022, 12, 150. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K. Responses of Camellia sinensis to drought and rehydration. Biol. Plant. 2004, 48, 597–600. [Google Scholar] [CrossRef]
- Docimo, T.; De Stefano, R.; Cappetta, E.; Piccinelli, A.L.; Celano, R.; De Palma, M.; Tucci, M. Physiological, biochemical, and metabolic responses to short and prolonged saline stress in two cultivated cardoon genotypes. Plants 2020, 9, 554. [Google Scholar] [CrossRef]
- Bian, X.H.; Li, W.; Niu, C.F.; Wei, W.; Hu, Y.; Han, J.Q.; Lu, X.; Tao, J.; Jin, M.; Qin, H.; et al. A class B heat shock factor selected for during soybean domestication contributes to salt tolerance by promoting flavonoid biosynthesis. New Phytol. 2020, 225, 268–283. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H.; et al. NtMYB4 and NtCHS1 are critical factors in the regulation of flavonoid biosynthesis and are involved in salinity responsiveness. Front. Plant Sci. 2019, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.M.; Chapman, J.M.; Muday, G.K. Abscisic acid-induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiol. 2017, 175, 1807–1825. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Lv, Z.; Zhang, G.; Li, J.; Zhang, J.; He, C. An ABA–flavonoid relationship contributes to the differences in drought resistance between different sea buckthorn subspecies. Tree Physiol. 2021, 41, 744–755. [Google Scholar] [CrossRef]
- Zhu, M.; Assmann, S.M. Metabolic signatures in response to abscisic acid (ABA) treatment in Brassica napus guard cells revealed by metabolomics. Sci. Rep. 2017, 7, 12875. [Google Scholar] [CrossRef]
- Peer, W.A.; Murphy, A.S. Flavonoids as signal molecules: Targets of flavonoid action. In The Science of Flavonoids; Grotewold, E., Ed.; Springer: New York, NY, USA, 2006; pp. 239–268. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Chen, D.; Li, Z.; Peng, R.; Yao, Q. A grape bHLH transcription factor gene, VvbHLH1, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell Tissue Organ Cult. 2016, 125, 387–398. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustaka, J.; Ouzounidou, G.; Moustakas, M. Leaf age-dependent photosystem II photochemistry and oxidative stress responses to drought stress in Arabidopsis thaliana are modulated by flavonoid accumulation. Molecules 2021, 26, 4157. [Google Scholar] [CrossRef]
- Jayaraman, K.; Sevanthi, A.M.; Sivakumar, S.R.; Viswanathan, C.; Mohapatra, T.; Mandal, P.K. Stress-inducible expression of chalcone isomerase2 gene improves accumulation of flavonoids and imparts enhanced abiotic stress tolerance to rice. Environ. Exp. Bot. 2021, 190, 104582. [Google Scholar] [CrossRef]
- Yang, L.; Shi, Y.; Ruan, X.; Wu, Q.; Qu, A.; Yu, M.; Qian, X.; Li, Z.; Ke, Z.; He, L.; et al. Salt interferences to metabolite accumulation, flavonoid biosynthesis and photosynthetic activity in Tetrastigma hemsleyanum. Environ. Exp. Bot. 2022, 194, 104765. [Google Scholar] [CrossRef]
- Stefanov, M.; Yotsova, E.; Gesheva, E.; Dimitrova, V.; Markovska, Y.; Doncheva, S.; Apostolova, E.L. Role of flavonoids and proline in the protection of photosynthetic apparatus in Paulownia under salt stress. S. Afr. J. Bot. 2021, 139, 246–253. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.N.V.; Sytar, O. Lead toxicity, defense strategies and associated indicative biomarkers in Talinum triangulare grown hydroponically. Chemosphere 2012, 89, 1056–1065. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Semchuk, N.M. Tocopherol biosynthesis: Chemistry, regulation and effects of environmental factors. Acta Physiol. Plant. 2012, 34, 1607–1628. [Google Scholar] [CrossRef]
- Yusuf, M.; Fariduddin, Q.; Varshney, P.; Ahmad, A. Salicylic acid minimizes nickel and/or salinity-induced toxicity in Indian mustard (Brassica juncea) through an improved antioxidant system. Environ. Sci. Pollut. Res. 2012, 19, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Manan, F.A.; Mamat, D.D.; Samad, A.A.; Ong, Y.S.; Ooh, K.F.; Chai, T.T. Heavy metal accumulation and antioxidant properties of Nephrolepis biserrata growing in heavy metal-contaminated soil. Glob. NEST J. 2015, 17, 544–554. [Google Scholar]
- Li, J.; Lu, H.; Liu, J.; Hong, H.; Yan, C. The influence of flavonoid amendment on the absorption of cadmium in Avicennia marina roots. Ecotoxicol. Environ. Saf. 2015, 120, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Darbar, S.; Chatterjee, T.; Das, M.; Polley, N.; Bhattacharyya, M.; Bhattacharya, S.; Pal, D.; Pal, S.K. Spectroscopic studies on dual role of natural flavonoids in detoxification of lead poisoning: Bench-to-bedside preclinical trial. ACS Omega 2018, 3, 15975–15987. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Natural variation in flavonol and anthocyanin metabolism during cold acclimation in Arabidopsis thaliana accessions. Plant Cell Environ. 2015, 38, 1658–1672. [Google Scholar] [CrossRef]
- Yang, C.; Yang, H.; Xu, Q.; Wang, Y.; Sang, Z.; Yuan, H. Comparative metabolomics analysis of the response to cold stress of resistant and susceptible Tibetan hulless barley (Hordeum distichon). Phytochemistry 2020, 174, 112346. [Google Scholar] [CrossRef]
- Ahmed, N.U.; Park, J.I.; Jung, H.J.; Hur, Y.; Nou, I.S. Anthocyanin biosynthesis for cold and freezing stress tolerance and desirable color in Brassica rapa. Funct. Integr. Genom. 2015, 15, 383–394. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, Y.R.; Hossain, M.A.; Hong, S.W.; Lee, B.H.; Lee, H. A mutation in ELA1, an age-dependent negative regulator of PAP1/MYB75, causes UV-and cold stress-tolerance in Arabidopsis thaliana seedlings. Plant Sci. 2009, 176, 678–686. [Google Scholar] [CrossRef]
- Pawlikowska-Pawlęga, B.; Dziubińska, H.; Król, E.; Trębacz, K.; Jarosz-Wilkołazka, A.; Paduch, R.; Gawron, A.; Gruszecki, W.I. Characteristics of quercetin interactions with liposomal and vacuolar membranes. Biochim. Biophys. Acta 2014, 1838, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Flavonoids are determinants of freezing tolerance and cold acclimation in Arabidopsis thaliana. Sci. Rep. 2016, 6, srep34027. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.U.; Park, J.I.; Jung, H.J.; Yang, T.J.; Hur, Y.; Nou, I.S. Characterization of dihydroflavonol 4-reductase (DFR) genes and their association with cold and freezing stress in Brassica rapa. Gene 2014, 550, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Hughes, N.M.; Carpenter, K.L.; Cannon, J.G. Estimating contribution of anthocyanin pigments to osmotic adjustment during winter leaf reddening. J. Plant Physiol. 2013, 170, 230–233. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Zuo, W.T.; Gao, Y.R.; Li, R.Z.; Yu, C.X.; Liu, Z.Y.; Zheng, Y.; Shen, Y.Y.; Duan, L.S. Integration of metabolome and transcriptome studies reveals flavonoids, abscisic acid, and nitric oxide comodulating the freezing tolerance in Liriope spicata. Front. Plant Sci. 2021, 12, 764625. [Google Scholar] [CrossRef]
- Ashrestaghi, T.; Aliniaeifard, S.; Shomali, A.; Azizinia, S.; Abbasi Koohpalekani, J.; Moosavi-Nezhad, M.; Gruda, N.S. Light intensity: The role player in cucumber response to cold stress. Agronomy 2022, 12, 201. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Li, M.; Fu, D.; Wu, S.; Li, J.; Gong, Z.; Liu, H.; Yang, S. The CRY2–COP1–HY5–BBX7/8 module regulates blue light-dependent cold acclimation in Arabidopsis. Plant Cell 2021, 33, 3555–3573. [Google Scholar] [CrossRef]
- Khan, A.L.; Kang, S.M.; Dhakal, K.H.; Hussain, J.; Adnan, M.; Kim, J.G.; Lee, I.J. Flavonoids and amino acid regulation in Capsicum annuum L. by endophytic fungi under different heat stress regimes. Sci. Hortic. 2013, 155, 1–7. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, A.; Wu, X.; Zhu, Z.; Yang, Z.; Zhu, Y.; Zha, D. Transcriptome analysis revealed expression of genes related to anthocyanin biosynthesis in eggplant (Solanum melongena L.) under high-temperature stress. BMC Plant Biol. 2019, 19, 387. [Google Scholar] [CrossRef]
- Lin-Wang, K.U.I.; Micheletti, D.; Palmer, J.; Volz, R.; Lozano, L.; Espley, R.; Hellens, R.P.; Chagne, D.; Rowan, D.D.; Troggio, M.; et al. High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex. Plant Cell Environ. 2011, 34, 1176–1190. [Google Scholar] [CrossRef]
- Movahed, N.; Pastore, C.; Cellini, A.; Allegro, G.; Valentini, G.; Zenoni, S.; Cavallini, E.; D’Incà, E.; Tornielli, G.B.; Filippetti, I. The grapevine VviPrx31 peroxidase as a candidate gene involved in anthocyanin degradation in ripening berries under high temperature. J. Plant Res. 2016, 129, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Brossa, R.; Casals, I.; Pintó-Marijuan, M.; Fleck, I. Leaf flavonoid content in Quercus ilex L. resprouts and its seasonal variation. Trees 2009, 23, 401–408. [Google Scholar] [CrossRef]
- Giampaoli, P.; Fernandes, F.F.; Tavares, A.R.; Domingos, M.; Cardoso-Gustavson, P. Fluorescence emission spectra of target chloroplast metabolites (flavonoids, carotenoids, lipofuscins, pheophytins) as biomarkers of air pollutants and seasonal tropical climate. Environ. Sci. Pollut. Res. 2020, 27, 25363–25373. [Google Scholar] [CrossRef]
- Shahbani, N.S.; Ismail, H.A.; Ramaiya, S.D.; Saupi, N.; Zakaria, M.H.; Awang, M.A. Effect of Haze on Fruit Development, Pigmentation and Productivity of Passiflora quadrangularis L. (Giant Granadilla Passion Fruit). In Emerging Trends of Plant Physiology in Changing Environment; Husin, N.M.C., Roseli, A.N.M., Sekeli, R., Othman, R., Osman, N., Zan, N.M., Hassan, S.A., Ahmad, S.H., Yusoff, M.M., Sukiran, N.L., et al., Eds.; Malaysian Society of Plant Physiology: Serdang, Selangor, Malaysia, 2021; pp. 29–35. [Google Scholar]
- Giraud, E.; Ivanova, A.; Gordon, C.S.; Whelan, J.; Considine, M.J. Sulphur dioxide evokes a large scale reprogramming of the grape berry transcriptome associated with oxidative signalling and biotic defence responses. Plant Cell Environ. 2012, 35, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Montesinos-Pereira, D.; Barrameda-Medina, Y.; Baenas, N.; Moreno, D.A.; Sanchez-Rodriguez, E.; Blasco, B.; Ruiz, J.M. Evaluation of hydrogen sulfide supply to biostimulate the nutritive and phytochemical quality and the antioxidant capacity of Cabbage (Brassica oleracea L. “Bronco”). J. Appl. Bot. Food Qual. 2016, 89, 290–298. [Google Scholar]
- Cai, Z.; Kastell, A.; Speiser, C.; Smetanska, I. Enhanced resveratrol production in Vitis vinifera cell suspension cultures by heavy metals without loss of cell viability. Appl. Biochem. Biotechnol. 2013, 171, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Zhang, J.; Wang, Y.; Yang, Y.; Chen, X.; Wang, Y. How does Malus crabapple resist ozone? Transcriptomics and metabolomics analyses. Ecotoxicol. Environ. Saf. 2020, 201, 110832. [Google Scholar] [CrossRef]
- Hu, K.D.; Tang, J.; Zhao, D.L.; Hu, L.Y.; Li, Y.H.; Liu, Y.S.; Jones, R.; Zhang, H. Stomatal closure in sweet potato leaves induced by sulfur dioxide involves H2S and NO signaling pathways. Biol. Plant. 2014, 58, 676–680. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Aliniaeifard, S.; Shomali, A.; Didaran, F. Interaction of Light Intensity and CO Concentration Alters Biomass Partitioning in Chrysanthemum. J. Hortic. Res. 2021, 29, 45–56. [Google Scholar] [CrossRef]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021, 48, 515–528. [Google Scholar] [CrossRef]
- Esmaeili, S.; Aliniaeifard, S.; Dianati Daylami, S.; Karimi, S.; Shomali, A.; Didaran, F.; Telesiński, A.; Sierka, E.; Kalaji, H.M. Elevated light intensity compensates for nitrogen deficiency during chrysanthemum growth by improving water and nitrogen use efficiency. Sci. Rep. 2022, 12, 10002. [Google Scholar] [CrossRef] [PubMed]
- Yari Kamrani, Y.; Shomali, A.; Aliniaeifard, S.; Lastochkina, O.; Moosavi-Nezhad, M.; Hajinajaf, N.; Talar, U. Regulatory Role of Circadian Clocks on ABA Production and Signaling, Stomatal Responses, and Water-Use Efficiency under Water-Deficit Conditions. Cells 2022, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Määttä-Riihinen, K.; Kärenlampi, S.; Hohtola, A. Activation of flavonoid biosynthesis by solar radiation in bilberry (Vaccinium myrtillus L.) leaves. Planta 2004, 218, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.; Linatoc, A.C.; Bakar, M.F.A.; Ibrahim, Z.T.; Audu, Y. Effect of light quality and quantity on the accumulation of flavonoid in plant species. J. Sci. Technol. 2018, 10, 32–45. [Google Scholar] [CrossRef]
- dos Santos Nascimento, L.B.; Tattini, M. Beyond Photoprotection: The Multifarious Roles of Flavonoids in Plant Terrestrialization. Int. J. Mol. Sci. 2022, 23, 5284. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, L.; Pang, S.; Jia, Z.; Wang, L.; Li, W.; Jin, B. UV-B promotes flavonoid synthesis in Ginkgo biloba leaves. Ind. Crops Prod. 2020, 151, 112483. [Google Scholar] [CrossRef]
- Eichholz, I.; Rohn, S.; Gamm, A.; Beesk, N.; Herppich, W.B.; Kroh, L.W.; Ulrichs, C.; Huyskens-Keil, S. UV-B-mediated flavonoid synthesis in white asparagus (Asparagus officinalis L.). Food Res. Int. 2012, 48, 196–201. [Google Scholar] [CrossRef]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB Plants 2018, 10, ply052. [Google Scholar] [CrossRef]
- Palma, C.F.F.; Castro-Alves, V.; Morales, L.O.; Rosenqvist, E.; Ottosen, C.; Strid, Å. Spectral composition of light affects sensitivity to UV-B and photoinhibition in cucumber. Front. Plant Sci. 2020, 11, 2016. [Google Scholar] [CrossRef]
- Palma, C.F.F.; Castro-Alves, V.; Rosenqvist, E.; Ottosen, C.O.; Strid, Å.; Morales, L.O. Effects of UV radiation on transcript and metabolite accumulation are dependent on monochromatic light background in cucumber. Physiol. Plant. 2021, 173, 750–761. [Google Scholar] [CrossRef]
- Markus, C.; Pecinka, A.; Merotto, A. Insights into the role of transcriptional gene silencing in response to herbicide-treatments in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 3314. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Eno, R.F.; Freitag-Pohl, S.; Coxon, C.R.; Straker, H.E.; Wortley, D.J.; Hughes, D.J.; Mitchell, G.; Moore, J.; Cummins, I.; et al. Flavonoid-based inhibitors of the Phi-class glutathione transferase from black-grass to combat multiple herbicide resistance. Org. Biomol. Chem. 2021, 19, 9211–9222. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zou, Q.; Guo, Q.; Yang, F.; Wu, L.; Zhang, W. Widely targeted metabolomics analysis reveals the effect of flooding stress on the synthesis of flavonoids in Chrysanthemum morifolium. Molecules 2019, 24, 3695. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, L.C.; Yang, J.; Qian, Z.H.; He, Y.X.; Li, M.W. Physiological and transcriptional changes provide insights into the effect of root waterlogging on the aboveground part of Pterocarya stenoptera. Genomics 2021, 113, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Nanjo, Y.; Maruyama, K.; Yasue, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Komatsu, S. Transcriptional responses to flooding stress in roots including hypocotyl of soybean seedlings. Plant Mol. Biol. 2011, 77, 129–144. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Liu, Y.; Cao, B. Regulation of flavanone 3-hydroxylase gene involved in the flavonoid biosynthesis pathway in response to UV-B radiation and drought stress in the desert plant, Reaumuria soongorica. Plant Physiol. Biochem. 2013, 73, 161–167. [Google Scholar] [CrossRef]
- Greenberg, B.M.; Wilson, M.I.; Huang, X.-D.; Duxbury, C.L.; Gerhardt, K.E.; Gensemer, R.W. The effects of ultraviolet-B radiation on higher plants. In Plants for Environmental Studies; CRC Press LLC: Boca Raton, FL, USA, 1997; pp. 1–36. [Google Scholar]
- Markham, K.R.; Ryan, K.G.; Bloor, S.J.; Mitchell, K.A. An increase in the luteolin: Apigenin ratio in Marchantia polymorpha on UV-B enhancement. Phytochemistry 1998, 48, 791–794. [Google Scholar] [CrossRef]
- Olsen, K.M.; Slimestad, R.; Lea, U.S.; Brede, C.; Løvdal, T.; Ruoff, P.; Verheul, M.; Lillo, C. Temperature and nitrogen effects on regulators and products of the flavonoid pathway: Experimental and kinetic model studies. Plant Cell Environ. 2009, 32, 286–299. [Google Scholar] [CrossRef]
- Berli, F.J.; Moreno, D.; Piccoli, P.; Hespanhol-Viana, L.; Silva, M.F.; Bressan-Smith, R.; Bottini, R. Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ. 2010, 33, 1–10. [Google Scholar] [CrossRef]
- Bharti, P.; Mahajan, M.; Vishwakarma, A.K.; Bhardwaj, J.; Yadav, S.K. AtROS1 overexpression provides evidence for epigenetic regulation of genes encoding enzymes of flavonoid biosynthesis and antioxidant pathways during salt stress in transgenic tobacco. J. Exp. Bot. 2015, 66, 5959–5969. [Google Scholar] [CrossRef]
- Ismail, H.; Maksimović, J.D.; Maksimović, V.; Shabala, L.; Živanović, B.D.; Tian, Y.; Shabala, S. Rutin, a flavonoid with antioxidant activity, improves plant salinity tolerance by regulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans. Funct. Plant Biol. 2015, 43, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Cao, A.; Wang, F.; Chen, X.; Xie, S.; Shen, H.; Jin, X.; Li, H. Calcium-dependent protein kinase genes in Glycyrrhiza Uralensis appear to be involved in promoting the biosynthesis of glycyrrhizic acid and flavonoids under salt stress. Molecules 2019, 24, 1837. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Kim, N.; Lee, S.-H.; Khan, M.A.; Asaf, S.; Lubna; Park, J.-R.; Asif, S.; Lee, I.-J.; Kim, K.-M. Enhanced Flavonoid Accumulation Reduces Combined Salt and Heat Stress Through Regulation of Transcriptional and Hormonal Mechanisms. Front. Plant Sci. 2021, 12, 796956. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kong, W.; Wong, G.; Fu, L.; Peng, R.; Li, Z.; Yao, Q. AtMYB12 regulates flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis thaliana. Mol. Genet. Genom. 2016, 291, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, F.; Jin, C.; Tong, Y.; Wang, T. A R2R3-MYB transcription factor VvMYBF1 from grapevine (Vitis vinifera L.) regulates flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis. J. Hortic. Sci. Biotechnol. 2020, 95, 147–161. [Google Scholar] [CrossRef]
- Wang, F.; Ren, X.; Zhang, F.; Qi, M.; Zhao, H.; Chen, X.; Ye, Y.; Yang, J.; Li, S.; Zhang, Y. A R2R3-type MYB transcription factor gene from soybean, GmMYB12, is involved in flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis. Plant Biotechnol. Rep. 2019, 13, 219–233. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Kong, W.; Peng, R.; Liu, Q.; Yao, Q. The Antirrhinum AmDEL gene enhances flavonoids accumulation and salt and drought tolerance in transgenic Arabidopsis. Planta 2016, 244, 59–73. [Google Scholar] [CrossRef]
- Waseem, M.; Li, Z. Overexpression of tomato SlbHLH22 transcription factor gene enhances fruit sensitivity to exogenous phytohormones and shortens fruit shelf-life. J. Biotechnol. 2019, 299, 50–56. [Google Scholar] [CrossRef]
- Wang, F.; Ren, G.; Li, F.; Qi, S.; Xu, Y.; Wang, B.; Yang, Y.; Ye, Y.; Zhou, Q.; Chen, X. A chalcone synthase gene AeCHS from Abelmoschus esculentus regulates flavonoid accumulation and abiotic stress tolerance in transgenic Arabidopsis. Acta Physiol. Plant. 2018, 40, 97. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Zheng, X.-T.; Sun, B.-Y.; Peng, C.-L.; Chow, W.S. Over-expression of the CHS gene enhances resistance of Arabidopsis leaves to high light. Environ. Exp. Bot. 2018, 154, 33–43. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Zhu, C.; Yao, X.; Zheng, Z.; Tian, Z.; Cai, X. EkFLS overexpression promotes flavonoid accumulation and abiotic stress tolerance in plant. Physiol. Plant. 2021, 172, 1966–1982. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.-Q.; Sun, Y.; Guo, T.; Shi, C.-L.; Zhang, Y.-M.; Kan, Y.; Xiang, Y.-H.; Zhang, H.; Yang, Y.-B.; Li, Y.-C. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 2629. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, X.; Ma, B.; Wu, Z.; Zheng, L.; Qi, Z.; Wang, Y. A leucoanthocyanidin dioxygenase gene (RtLDOX2) from the feral forage plant Reaumuria trigyna promotes the accumulation of flavonoids and improves tolerance to abiotic stresses. J. Plant Res. 2021, 134, 1121–1138. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Yang, J.; Yuan, Y.; Huang, L.; Chen, P. Overexpression of two R2R3-MYB genes from Scutellaria baicalensis induces phenylpropanoid accumulation and enhances oxidative stress resistance in transgenic tobacco. Plant Physiol. Biochem. 2015, 94, 235–243. [Google Scholar] [CrossRef]
- Wang, N.; Qu, C.; Jiang, S.; Chen, Z.; Xu, H.; Fang, H.; Su, M.; Zhang, J.; Wang, Y.; Liu, W. The proanthocyanidin-specific transcription factor Md MYBPA 1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples. Plant J. 2018, 96, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Q.; Lv, W.; Yu, X.; Zhang, Z. Ethylene positively regulates Cd tolerance via reactive oxygen species scavenging and apoplastic transport barrier formation in rice. Environ. Pollut. 2022, 302, 119063. [Google Scholar] [CrossRef]
- Jia, X.; Gong, X.; Jia, X.; Li, X.; Wang, Y.; Wang, P.; Huo, L.; Sun, X.; Che, R.; Li, T.; et al. Overexpression of MdATG8i Enhances Drought Tolerance by Alleviating Oxidative Damage and Promoting Water Uptake in Transgenic Apple. Int. J. Mol. Sci. 2021, 22, 5517. [Google Scholar] [CrossRef]
- Wang, M.; Ren, T.; Huang, R.; Li, Y.; Zhang, C.; Xu, Z. Overexpression of an Apocynum venetum flavonols synthetase gene confers salinity stress tolerance to transgenic tobacco plants. Plant Physiol. Biochem. 2021, 162, 667–676. [Google Scholar] [CrossRef]
- Yuan, Y.; Qi, L.; Yang, J.; Wu, C.; Liu, Y.; Huang, L. A Scutellaria baicalensis R2R3-MYB gene, SbMYB8, regulates flavonoid biosynthesis and improves drought stress tolerance in transgenic tobacco. Plant Cell Tissue Organ Cult. 2015, 120, 961–972. [Google Scholar] [CrossRef]

| Genes, Transcript | Method | Plant Species | Stress | Results | Reference |
|---|---|---|---|---|---|
| GuCPKs | Induced expression of GuCPKs gene | Glycyrrhiza uralensis | NaCl (30 mM) and CaCl2 (2.5 mM) | Improved the accumulation of flavonoids biosynthesis and glycyrrhizic acid. | [175] |
| flavanol 3-hydroxylase. | Induced expression of flavanol 3-hydroxylase gene | Rice | Salinity (150 mM) and heat stress (28–30 °C, light 16/8 h) | Improved biosynthesis of quercetin and kaempferol. Increased oxidative damage, which was mitigated with the accumulation of flavonoids content. | [176] |
| AtMYB12 | Overexpression of AtMYB12 | Arabidopsis | Drought (25% PEG6000 for 2 weeks) and salinity stress (300 mM once every 2 days for 4 weeks) | Increased the flavonoids agglomeration by the upregulation of genes actively involved in flavonoid biosynthesis | [177] |
| VvMyBF1 | VvMyBF1 gene cloned from grapevine induced into Arabidopsis | Arabidopsis | Drought (25% PEG6000 for 2 weeks) and salt stress (200 mM NaCl for 2 weeks) | Increased the accumulation of flavonoids. Higher activities of SOD, POD, pyrroline-5-carboxylate synthase, dihydroflavonol reductase, FLS, CHI, and PAL, as well as a significant reduction of MDA and H2O2 content. | [178] |
| GmMyB12 | Overexpression of GmMyB12 | Arabidopsis | Salinity (200 mM NaCl, 2 weeks) and drought stress (25% PEG6000, 2 weeks) | Increased the downstream flavonoids by improving the expression of flavonoid biosynthesis-related genes. Increased the pyrroline-5-carboxylate synthase, SOD, and POD. | [179] |
| Basic helix-loop-helix (bHLH) | Transcription factor gene of (bHLH) antirrhinum (AmDEL) induced in Arabidopsis | Arabidopsis | Drought (25% PEG6000 for 2 weeks) and salinity stress (300 mM 2 days for 4 week) | Higher activities of pyrrline-5-carboxylate synthase, dihydroflavonol reductase, chalcone isomerase, and phenylalanine ammonia lyase (PAL) in transgenic plants. Upregulated flavonoids biosynthesis genes. | [180] |
| SIbHLH22 | Overexpression of SIbHLH22 | Tomato | Drought (100 mM mannitol) and slat stress (200 mM NaCl) | Transgenic plants showed enhanced vigor by improving ROS scavenging system. Showed small leaves, short height, and higher accumulation of flavonoids. | [181] |
| Chalcone isomerase 2 (OsCHI2) | Induction of OsCHI2 | Rice | Heat (40 °C for 3 days), cold stress (2 °C; 16 h light/8 h dark for 12 days), salinity stresses (150 mM NaCl for 7 days), and drought stress (withholding water 7 days at 9 to 10 leave stage). | Abundant structural genes of flavonoid biosynthesis and modulation of flavonoid metabolism. | [118] |
| AeCHS o in Arabidopsis plants | AeCHS gene isolated from Abelmosschus esculentus and induced in Arabidopsis. | Arabidopsis | Osmotic (300 mM mannitol for a week) and salt stress (200 mM NaCl for a week) | Increased flavonoid biosynthesis and abiotic stress tolerance. | [182] |
| CHS gene by | Overexpression of CHS gene in Arabidopsis | Arabidopsis | High light stress (200 µmol m−2 s−1) | Increased the synthesis of anthocyanins that enhance the adaptability of plants against light stress. | [183] |
| EkFLS gene | Overexpressed the EkFLS gene in Arabidopsis; isolated from Euphorbia kansui Liou | Arabidopsis | Drought stress (20% PEG600) and salinity stress (200 mM NaCl) | Increased flavonoids biosynthesis and gave a theoretical base for improving the phytoextracts of medicinal plants and their resistance against multiple stresses simultaneously. | [184] |
| GSA1 gene | Overexpression of GSA1 in rice | Rice | Salinity stress (150 mM for 7 days), drought stress (16% PEG8000 for 2 to 3 weeks), and heat stress (42 °C for dozens of hours) | Redirected the metabolic flux from lignin synthesis toward flavonoids synthesis. Accumulated more glycosides and flavonoids. | [185] |
| glycosyltransferase gene (UGT76E11) | Overexpression of UGT76E1 | Arabidopsis | H2O2 (0.4 mM), drought (200 mM mannitol), and salinity (100 mM NaCl for 10 days) stress | Showed substantially enhanced tolerance through producing of higher glucosylate quercetin by modulating flavonoid biosynthesis pathway. | [88] |
| RtLDOX/RtLDOX2 | Expressed leucoanthocyanidin dioxygenase genes (RtLDOX/RtLDOX2) of Reaumuria trigyna in Arabidopsis | Arabidopsis | Drought (150 mM and 300 mM mannitol for 15 days), salinity (75 mM and 100 mM NaCl for 10 days), and ultraviolet-B-stress (30 min per day for 7 days) | Overexpression of RtLDOX2 showed a higher accumulation of flavonols and anthocyanin and converted dihydrokaempferol to kaempferol, scavenging ROS. | [186] |
| UDP-sugar glycosyltransferase gene (CrUGT87A1) | CrUGT87A1 cloned form Carex rigescens in Arabidopsis | Arabidopsis | Salt stress (100 mM and 125 mM NaCl for 7 days) | Higher accumulation of antioxidants and flavonoids. | [61] |
| R2R3-MYB (SbMYB2 and SbMYB7) | Overexpression of R2R3-MYB form Scutellaria baicalensis in tobacco | Tobacco | Salt stress (150 mM NaCl), drought (0.2 M mannitol), and ABA (100 µ M) for 3, 6, and 9 days, respectively | Higher fresh weight, lower flavonoid synthesis gene and antioxidants, and higher phenylpropanoid accumulation. | [187] |
| PA1-type MYB transcription factor (MdMYBPA1) | MdbHLH33 directly binds to the cis element of the MdMYBPA1 responsive to low temperature | Apple (Malus x domestica) | Low temperature (14 °C) | Responded to flavonoid biosynthesis by synthesizing anthocyanin from proanthocyanin. | [188] |
| Ethylene insensitive 2 (EIN2) | Overexpression of EIN2 | Rice | Cd stress (10 µM for 10 days) | Increased flavonoid and phenolics biosynthesis. | [189] |
| Core apple autophagy-related gene (MdATG8i) | Overexpression of MdATG8i | Apple | Drought (withholding water for 6 days) | Higher photosynthesis, amino acids, flavonoids, and antioxidant activities, lower ROS and oxidized and insoluble proteins, higher roots hydraulic conductivity, and improved water uptake. | [190] |
| AvFLS | Apocynum venetum gene overexpression in AvFLS induced in tobacco | Tobacco | Salinity stress (200 mM for 72 h) | Increased flavonoids synthesis, absorbed more K+, maintained Na+/K+ homeostasis, and increased K+/Na+ ratio. | [191] |
| SbMYB8 | Overexpression of R2R3-MYB form Scutellaria baicalensis in tobacco | Tobacco | Salt stress (150 mM NaCl), drought (0.2 M mannitol), and ABA (100 µM) for 3, 6, and 9 days, respectively | Higher flavonoid biosynthesis and antioxidants, and improved tolerance against stress. | [192] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants 2022, 11, 3158. https://doi.org/10.3390/plants11223158
Shomali A, Das S, Arif N, Sarraf M, Zahra N, Yadav V, Aliniaeifard S, Chauhan DK, Hasanuzzaman M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants. 2022; 11(22):3158. https://doi.org/10.3390/plants11223158
Chicago/Turabian StyleShomali, Aida, Susmita Das, Namira Arif, Mohammad Sarraf, Noreen Zahra, Vaishali Yadav, Sasan Aliniaeifard, Devendra Kumar Chauhan, and Mirza Hasanuzzaman. 2022. "Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance" Plants 11, no. 22: 3158. https://doi.org/10.3390/plants11223158
APA StyleShomali, A., Das, S., Arif, N., Sarraf, M., Zahra, N., Yadav, V., Aliniaeifard, S., Chauhan, D. K., & Hasanuzzaman, M. (2022). Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants, 11(22), 3158. https://doi.org/10.3390/plants11223158










