Physiological Responses of Cigar Tobacco Crop to Nitrogen Deficiency and Genome-Wide Characterization of the NtNPF Family Genes
Abstract
1. Introduction
2. Results
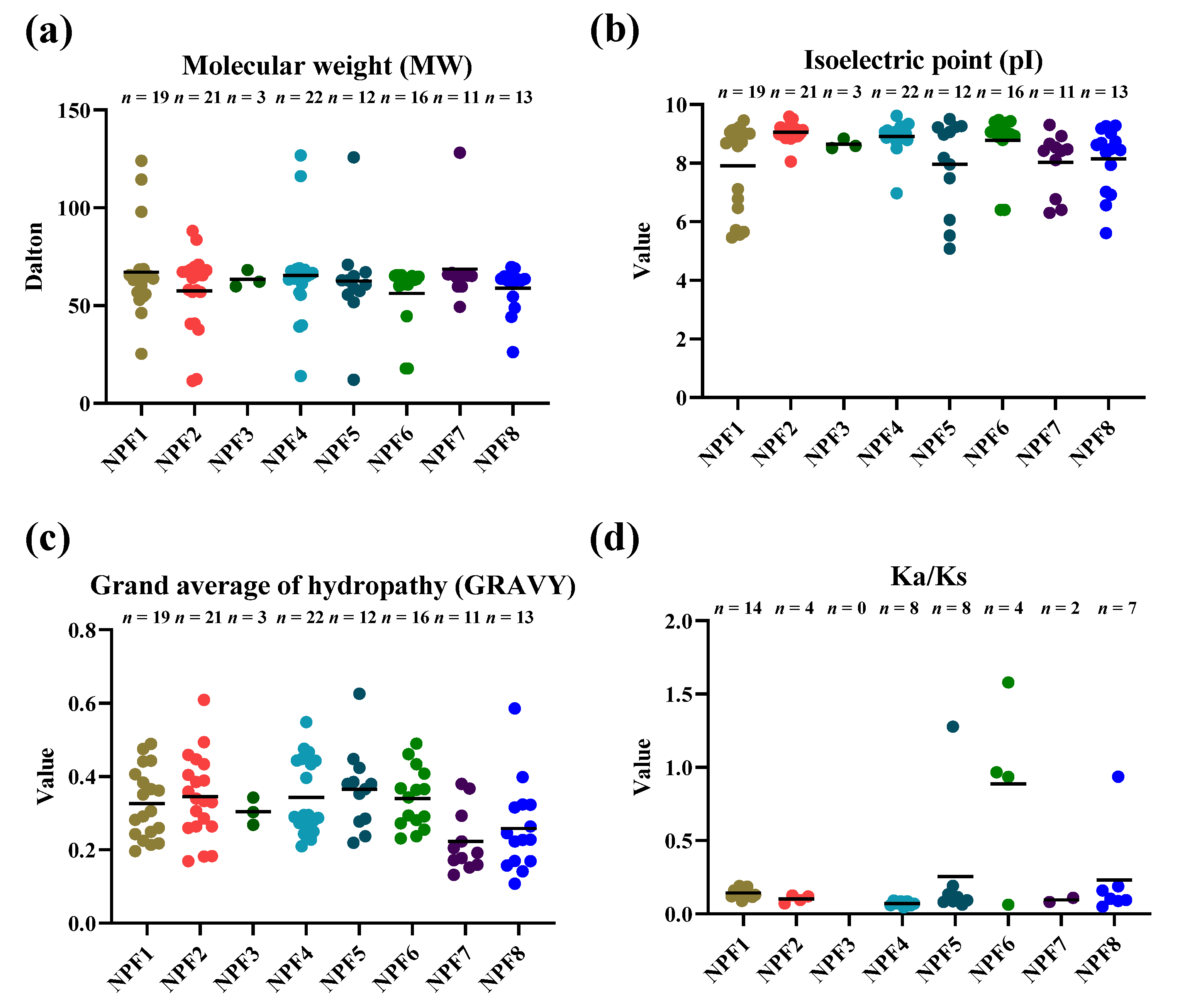
2.1. Genome-Wide Identification and Characterization of the NPF Genes in N. tabacum
2.2. Phylogenetic Analysis of the NPFs in N. tabacum
2.3. Gene Structure and Conserved Motif Analysis of the NPFs in N. tabacum
2.4. Chromosomal Location and Syntenic Analysis of the NPFs in N. tabacum
2.5. Analysis of Cis-Elements in NPF Promoters in N. tabacum
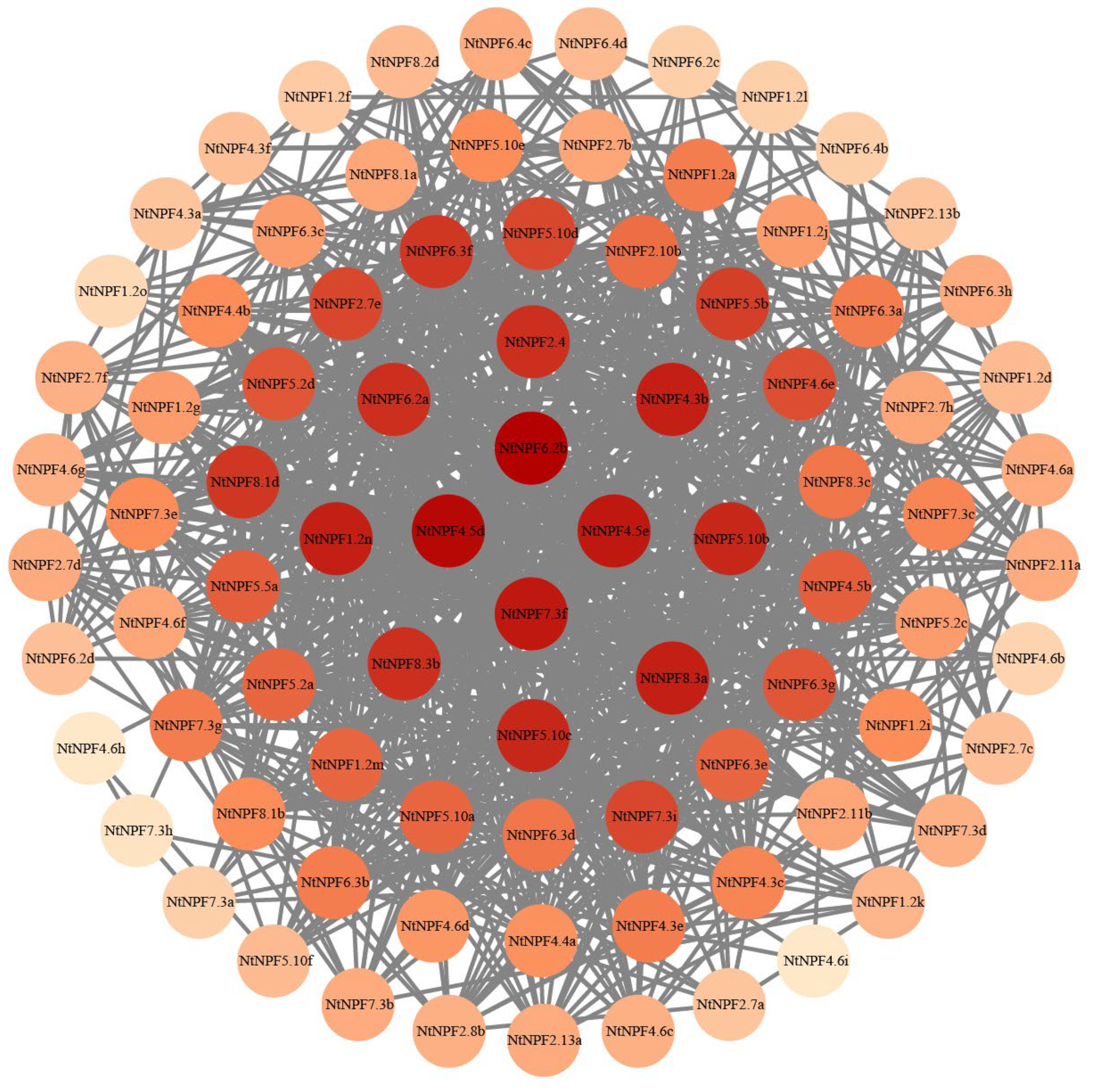
2.6. Transcriptional Profiles of the NtNPFs under N Stress and Co-Expression Network Analysis
2.7. 3D Protein Structure, Protein Transmembrane Domain, and Cis-Regulatory Element Analysis of the Hub NtNPFs in N. tabacum
2.8. The Physiological Responses of Two Cigar Tobaccos to N Stress
2.9. The Identification of Key Genes in Nitrate Efficient Uptake and Utilization in Cigar Tobacco
3. Discussion
3.1. Composition of the NPF Gene Family in Tobacco
3.2. Evolutionary and Structural Characteristics of the Tobacco NPF Family Genes
3.3. NtNPF Genes Function Critically in Nitrogen Uptake and Utilization of Cigar Tobacco
4. Materials and Methods
4.1. Identification of NPF Family Genes in Tobacco
4.2. Gene Structure, Conserved Motif, and Phylogenetic Analysis
4.3. Protein Biophysical Property and Evolutionary Pressure Analysis
4.4. Synteny Analysis and Chromosome Localization
4.5. Cis-Regulatory Elements Analysis
4.6. Heatmap and Co-Expression Network Analysis
4.7. Prediction of 3D Protein Structure
4.8. Plant Materials and Stress Treatments
4.9. Phenotypic Measurements
4.10. Gene Expression Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Chapter 6—Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 135–189. [Google Scholar]
- Mus, F.; Crook, M.B.; Garcia, K.; Garcia Costas, A.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.H.; Oldroyd, G.; Poole, P.S.; et al. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.A. Systems biology for enhanced plant nitrogen nutrition. Science 2012, 336, 1673–1675. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Environ. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Wang, G.; Ding, G.; Li, L.; Cai, H.; Ye, X.; Zou, J.; Xu, F. Identification and characterization of improved nitrogen efficiency in interspecific hybridized new–type Brassica napus. Ann. Bot. 2014, 114, 549–559. [Google Scholar] [CrossRef]
- Li, Q.; Ding, G.; Yang, N.; White, P.J.; Ye, X.; Cai, H.; Lu, J.; Shi, L.; Xu, F. Comparative genome and transcriptome analysis unravels key factors of nitrogen use efficiency in Brassica napus L. Plant Cell Environ. 2020, 433, 712–731. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J.N. An Earth–system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of fertilizers for enhanced nitrogen use efficiency—Trends and perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef]
- Gramma, V.; Kontbay, K.; Wahl, V. Crops for the future: On the way to reduce nitrogen pollution. Am. J. Bot. 2020, 107, 1211–1213. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Ann. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Kiba, T.; Krapp, A. Plant nitrogen acquisition under low availability: Regulation of uptake and root architecture. Plant Cell Physiol. 2016, 57, 707–714. [Google Scholar] [CrossRef]
- Fan, X.; Naz, M.; Fan, X.; Xuan, W.; Miller, A.J.; Xu, G. Plant nitrate transporters: From gene function to application. J. Exp. Bot. 2017, 68, 2463–2475. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef]
- Vidal, E.A.; Alvarez, J.M.; Araus, V.; Riveras, E.; Brooks, M.D.; Krouk, G.; Ruffel, S.; Lejay, L.; Crawford, N.M.; Coruzzi, G.M.; et al. Nitrate in 2020: Thirty years from transport to signaling networks. Plant Cell. 2020, 32, 2094–2119. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.; Orsel, M.; Smith, S.J.; Wells, D.M. Nitrate transport and signaling. J. Exp. Bot. 2007, 58, 2297–2306. [Google Scholar] [CrossRef]
- Tsay, Y.F.; Chiu, C.C.; Tsai, C.B.; Ho, C.H.; Hsu, P.K. Nitrate transporters and peptide transporters. FEBS Lett. 2007, 581, 2290–2300. [Google Scholar] [CrossRef]
- Léran, S.; Varala, K.; Boyer, J.C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B.; et al. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci. 2014, 19, 5–9. [Google Scholar] [CrossRef]
- Tsay, Y.F.; Schroeder, J.I.; Feldmann, K.A.; Crawford, N.M. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate–inducible nitrate transporter. Cell 1993, 72, 705–713. [Google Scholar] [CrossRef]
- Liu, K.H.; Huang, C.Y.; Tsay, Y.F. CHL1 is a dual–affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 1999, 11, 865–874. [Google Scholar] [CrossRef]
- Ho, C.H.; Lin, S.H.; Hu, H.C.; Tsay, Y.F. CHL1 functions as a nitrate sensor in plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Kuo, H.F.; Canivenc, G.; Lin, C.S.; Lepetit, M.; Hsu, P.K.; Tillard, P.; Lin, H.L.; Wang, Y.Y.; Tsai, C.B.; et al. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root to–shoot nitrate transport. Plant Cell 2008, 20, 2514–2528. [Google Scholar] [CrossRef] [PubMed]
- Almagro, A.; Lin, S.H.; Tsay, Y.F. Characterization of the Arabidopsis nitrate transporter NRT1.6 reveals a role of nitrate in early embryo development. Plant Cell 2008, 20, 3289–3299. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutiérrez, R.A. Nitrate transport, sensing, and responses in plants. Mol. Plant. 2016, 9, 837–856. [Google Scholar] [CrossRef]
- Léran, S.; Garg, B.; Boursiac, Y.; Corratgé-Faillie, C.; Brachet, C.; Tillard, P.; Gojon, A.; Lacombe, B. AtNPF5.5, a nitrate transporter affecting nitrogen accumulation in Arabidopsis embryo. Sci. Rep. 2015, 5, 7962. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, W.; Ou, S.; Tang, J.; Li, H.; Che, R.; Zhang, Z.; Chai, X.; Wang, H.; Wang, Y.; et al. Variation in NRT1.1B contributes to nitrate–use divergence between rice subspecies. Nat. Genet. 2015, 47, 834–838. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Zhang, N.; Hu, B.; Jin, T.; Xu, H.; Qin, Y.; Yan, P.; Zhang, X.; Guo, X.; et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field–grown rice. Nat. Biotechnol. 2019, 37, 676–684. [Google Scholar] [CrossRef]
- Corratge-Faillie, C.; Lacombe, B. Substrate (un)specificity of Arabidopsis NRT1/PTR FAMILY (NPF) proteins. J. Exp. Bot. 2017, 68, 3107–3113. [Google Scholar] [CrossRef]
- Jorgensen, M.E.; Xu, D.; Crocoll, C.; Ramirez, D.; Motawia, M.S.; Olsen, C.E.; Nour–Eldin, H.H.; Halkier, B.A. Origin and evolution of transporter substrate specificity within the NPF family. Elife 2017, 6, e19466. [Google Scholar] [CrossRef]
- Kanstrup, C.; Nour-Eldin, H.H. The emerging role of the nitrate and peptide transporter family: NPF in plant specialized metabolism. Curr. Opin. Plant 2022, 68, 102243. [Google Scholar] [CrossRef]
- Boursiac, Y.; Léran, S.; Corratgé-Faillie, C.; Gojon, A.; Krouk, G.; Lacombe, B. ABA transport and transporters. Trends Plant Sci. 2013, 18, 325–333. [Google Scholar] [CrossRef]
- Tal, I.; Zhang, Y.; Jorgensen, M.E.; Pisanty, O.; Barbosa, I.C.R.; Zourelidou, M.; Regnault, T.; Crocoll, C.; Olsen, C.E.; Weinstain, R.; et al. The Arabidopsis NPF3 protein is a GA transporter. Nat. Commun. 2016, 7, 11486–11496. [Google Scholar] [CrossRef]
- Nour-Eldin, H.H.; Andersen, T.G.; Burow, M.; Madsen, S.R.; Jørgensen, M.E.; Olsen, C.E.; Dreyer, I.; Hedrich, R.; Geiger, D.; Halkier, B.A. NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature 2012, 488, 531–534. [Google Scholar] [CrossRef]
- Zhao, X.B.; Huang, J.Y.; Yu, H.H. Genomic survey, characterization and expression profile analysis of the peptide transporter family in rice (Oryza sativa L.). BMC Plant Biol. 2010, 10, 92. [Google Scholar] [CrossRef]
- Li, H.; Yu, M.; Du, X.Q.; Wang, Z.F.; Wu, W.H.; Quintero, F.J.; Jin, X.H.; Li, H.D.; Wang, Y. NRT1.5/NPF7.3 functions as a proton–coupled H+/K+ antiporter for K+ Loading into the xylem in Arabidopsis. Plant Cell 2017, 29, 2016–2026. [Google Scholar] [CrossRef]
- Li, B.; Qiu., J.; Jayakannan, M.; Xu, B.; Li, Y.; Mayo, G.M.; Tester, M.; Gilliham, M.; Roy, S.J. AtNPF2.5 modulates chloride (Cl–) efflux from roots of Arabidopsis thaliana. Front. Plant Sci. 2017, 7, 2013. [Google Scholar] [CrossRef]
- Sierro, N.; Battey, J.N.D.; Ouadi, S.; Bakaher, N.; Bovet, L.; Willig, A.; Goepfert, S.; Peitsch, M.C.; Ivanov, N.V. The tobacco genome sequence and its comparison with those of tomato and potato. Nat. Commun. 2014, 5, 3833. [Google Scholar] [CrossRef]
- Sisson, V.A.; Rufty, T.W.; Williamson, R.E. Nitrogen–use efficiency among flue–cured tobacco genotypes. Crop Sci. 1991, 31, 1615–1620. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, K.; He, X.; Chen, Y.; Hu, B.; Hu, X.; Li, J.; Jin, Y.; Zhao, Z.; Zou, C. The response of flue–cured tobacco cultivar K326 to nitrogen fertilizer rate in China. J. Agric. Sci. 2020, 158, 371–382. [Google Scholar] [CrossRef]
- Schmeltz, I.; Hoffmann, D. Nitrogen–containing compounds in tobacco and tobacco smoke. Chem. Rev. 1977, 77, 295–311. [Google Scholar] [CrossRef]
- Teng, W.; Li, W.; Li, C. Comparison of N uptake and internal use efficiency in two tobacco varieties. Crop J. 2015, 3, 80–86. [Google Scholar] [CrossRef]
- Rhoads, F.M. A comparison of ammonium and nitrate nitrogen for cigar wrapper tobacco. Agron. J. 1972, 64, 209–210. [Google Scholar] [CrossRef]
- Liu, L.H.; Fan, T.F.; Shi, D.X.; Li, C.J.; He, M.J.; Chen, Y.Y.; Zhang, L.; Yang, C.; Cheng, X.Y.; Chen, X.; et al. Coding–sequence identification and transcriptional profiling of nine AMTs and four NRTs from tobacco revealed their differential regulation by developmental stages, nitrogen nutrition, and photoperiod. Front. Plant Sci. 2018, 9, 210. [Google Scholar] [CrossRef]
- Nekrutenko, A.; Makova, K.D.; Li, W.-H. The KA/KS ratio test for assessing the protein–coding potential of genomic regions: An empirical and simulation study. Genome Res. 2001, 12, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Thiel, T.; Graner, A.; Waugh, R.; Grosse, I.; Close, T.J.; Stein, N. Evidence and evolutionary analysis of ancient whole–genome duplication in barley predating the divergence from rice. BMC Evol. Biol. 2009, 9, 209. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.; Dong, Q.; Huang, D.; Li, C.; Li, P.; Ma, F. Genome–wide identification and analysis of apple nitrate transporter 1/peptide transporter family (NPF) genes reveals MdNPF6.5 confers high capacity for nitrogen uptake under low–nitrogen conditions. Int. J. Mol. Sci. 2018, 19, 2761. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Shi, M.; Wang, S.; Shi, L.; Xu, F.; Ding, G. Genome–wide systematic characterization of the NPF family genes and their transcriptional responses to multiple nutrient stresses in allotetraploid rapeseed. Int. J. Mol. Sci. 2020, 21, 5947. [Google Scholar] [CrossRef]
- Chao, H.B.; He, J.J.; Cai, Q.Q.; Zhao, W.G.; Fu, H.; Hua, Y.P.; Li, M.T.; Huang, J.Y. The expression characteristics of NPF genes and their response to vernalization and nitrogen deficiency in rapeseed. Int. J. Mol. Sci. 2021, 22, 4944. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, K.; Ruan, L.; Bai, P.; Wu, L.; Wang, L.; Cheng, H. Systematic investigation and expression profiles of the nitrate transporter 1/peptide transporter family (NPF) in tea plant (Camellia sinensis). Int. J. Mol. Sci. 2022, 23, 6663. [Google Scholar] [CrossRef]
- Pellizzaro, A.; Planchet, E.; Limami, A.M.; Alibert, B.; Paven, M.C. Nitrate transporters: An overview in legumes. Planta 2017, 246, 585–595. [Google Scholar] [CrossRef]
- Bai, H.; Euring, D.; Volmer, K.; Janz, D.; Polle, A. The nitrate transporter (Nrt) gene family in Poplar. PLoS ONE 2013, 8, e72126. [Google Scholar] [CrossRef]
- You, H.G.; Liu, Y.M.; Minh, T.N.; Lu, H.R.; Zhang, P.M.; Li, W.F.; Xiao, J.L.; Ding, X.D.; Li, Q. Genome–wide identification and expression analyses of nitrate transporter family genes in wild soybean (Glycine soja). J. Appl. Genet. 2020, 61, 489–501. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Zhu, F.; Ming, R.; Chen, L.-Q. Genome–wide analysis of nitrate transporter (NRT/NPF) family in sugarcane Saccharum spontaneum L. Trop. Plant Boil. 2019, 12, 133–149. [Google Scholar] [CrossRef]
- Wang, H.; Wan, Y.; Buchner, P.; King, R.; Ma, H.; Hawkesford, M.J. Phylogeny and gene expression of the complete nitrate transporter 1/peptide transporter family in Triticum aestivum. J. Exp. Bot. 2020, 71, 4531–4546. [Google Scholar] [CrossRef]
- Léran, S.; Muños, S.; Brachet, C.; Tillard, P.; Gojon, A.; Lacombe, B. Arabidopsis NRT1.1 is a bidirectional transporter involved in root–to–shoot nitrate translocation. Mol. Plant 2013, 6, 1984–1987. [Google Scholar] [CrossRef]
- Bouguyon, E.; Brun, F.; Meynard, D.; Kubeš, M.; Pervent, M.; Leran, S.; Lacombe, B.; Krouk, G.; Guiderdoni, E.; Zažímalová, E.; et al. Multiple mechanisms of nitrate sensing by Arabidopsis nitrate transceptor NRT1.1. Nat. Plants 2015, 1, 15015. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, J.; Han, Y.; Hua, Y.; Guan, C.; Zhang, Z. Low nitrogen enhances nitrogen use efficiency by triggering NO3– uptake and its long–distance translocation. J. Agric. Food Chem. 2019, 67, 6736–6747. [Google Scholar] [CrossRef]
- Yang, R.; Yang, J.; Yu, J.; Wang, S.; Yang, C.; Xu, F. Effects of different nitrogen application rates on the quality and metabolomics of cigar tobacco. Agron. J. 2022, 114, 1155–1167. [Google Scholar] [CrossRef]
- Chiu, C.C.; Lin, C.S.; Hsia, A.P.; Su, R.C.; Lin, H.L.; Tsay, Y.F. Mutation of a nitrate transporter, AtNRT1:4, results in a reduced petiole nitrate content and altered leaf development. Plant Cell Physiol. 2004, 45, 1139–1148. [Google Scholar] [CrossRef]
- Hsu, P.K.; Tsay, Y.F. Two phloem nitrate transporters, NRT1.11 and NRT1.12, are important for redistributing xylem–borne nitrate to enhance plant growth. Plant Physiol. 2013, 163, 844–856. [Google Scholar] [CrossRef]
- Bilas, R.; Szafran, K.; Hnatuszko-Konka, K.; Kononowicz, A.K. Cis–regulatory elements used to control gene expression in plants. Plant Cell Tiss. Org. 2016, 127, 269–287. [Google Scholar] [CrossRef]
- Liu, Y.J.; Gao, N.; Ma, Q.J.; Zhang, J.C.; Wang, X.; Lu, J.; Hao, Y.J.; Wang, X.F.; You, C.X. The MdABI5 transcription factor interacts with the MdNRT1.5/MdNPF7.3 promoter to fine–tune nitrate transport from roots to shoots in apple. Hortic. Res. 2021, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.D.; Fernandez-Pozo, N.; Drake–Stowe, K.; Humphry, M.; Evans, A.D.; Bombarely, A.; Allen, F.; Hurst, R.; White, B.; Kernodle, S.P.; et al. A reference genome for Nicotiana tabacum enables map–based cloning of homeologous loci implicated in nitrogen utilization efficiency. BMC Genom. 2017, 18, 448. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. Tbtools—An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 22, 1674. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Gao, S.; Lercher, M.J.; Chen, W.H.; Hu, S. Evolview v2: An online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016, 44, W236–W241. [Google Scholar] [CrossRef]
- He, X.; Zhang, H.; Ye, X.; Hong, J.; Ding, G. Nitrogen Assimilation Related Genes in Brassica napus: Systematic characterization and expression analysis identified hub genes in multiple nutrient stress responses. Plants 2021, 10, 2160. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis–acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Zhou, Y.; Wang, J.; Geng, X.; Shi, H. Transcriptome analysis of tobacco root response to different concentrations of nitrate. Agron. J. 2020, 112, 1111–1125. [Google Scholar] [CrossRef]
- Li, J.; Wei, H.; Zhao, X. DeGNServer: Deciphering genome–scale gene networks through high performance reverse engineering analysis. BioMed. Res. Int. 2013, 2013, 856325. [Google Scholar] [CrossRef]
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering workflow–based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef]
- Geourjon, C.; Deléage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci. 1995, 11, 681. [Google Scholar] [CrossRef]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS–MODEL workspace: A web–based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef]
- Blaine, H.M. Simplifying and enhancing the use of PyMOL with horizontal scripts. Protein Sci. 2016, 25, 1873–1882. [Google Scholar]
- Li, S.; Zhang, H.; Wang, S.L.; Shi, L.; Xu, F.S.; Wang, C.; Cai, H.M.; Ding, G.D. The rapeseed genotypes with contrasting NUE response discrepantly to varied provision of ammonium and nitrate by regulating photosynthesis, root morphology, nutritional status, and oxidative stress response. Plant Physiol. Biochem. 2021, 166, 348–360. [Google Scholar] [CrossRef]
- Volkov, R.A.; Panchuk, I.I.; Schöffl, F. Heat–stress–dependency and developmental modulation of gene expression: The potential of house–keeping genes as internal standards in mRNA expression profiling using real–time RT–PCR. J. Exp. Bot. 2003, 54, 2343–2349. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real–time quantitative PCR and the 2(–Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; He, X.; Zhang, H.; Tan, R.; Yang, J.; Xu, F.; Wang, S.; Yang, C.; Ding, G. Physiological Responses of Cigar Tobacco Crop to Nitrogen Deficiency and Genome-Wide Characterization of the NtNPF Family Genes. Plants 2022, 11, 3064. https://doi.org/10.3390/plants11223064
Guo H, He X, Zhang H, Tan R, Yang J, Xu F, Wang S, Yang C, Ding G. Physiological Responses of Cigar Tobacco Crop to Nitrogen Deficiency and Genome-Wide Characterization of the NtNPF Family Genes. Plants. 2022; 11(22):3064. https://doi.org/10.3390/plants11223064
Chicago/Turabian StyleGuo, Hao, Xuyou He, Hao Zhang, Ronglei Tan, Jinpeng Yang, Fangsen Xu, Sheliang Wang, Chunlei Yang, and Guangda Ding. 2022. "Physiological Responses of Cigar Tobacco Crop to Nitrogen Deficiency and Genome-Wide Characterization of the NtNPF Family Genes" Plants 11, no. 22: 3064. https://doi.org/10.3390/plants11223064
APA StyleGuo, H., He, X., Zhang, H., Tan, R., Yang, J., Xu, F., Wang, S., Yang, C., & Ding, G. (2022). Physiological Responses of Cigar Tobacco Crop to Nitrogen Deficiency and Genome-Wide Characterization of the NtNPF Family Genes. Plants, 11(22), 3064. https://doi.org/10.3390/plants11223064







