S-Methylmethionine Effectively Alleviates Stress in Szarvasi-1 Energy Grass by Reducing Root-to-Shoot Cadmium Translocation
Abstract
1. Introduction
2. Results
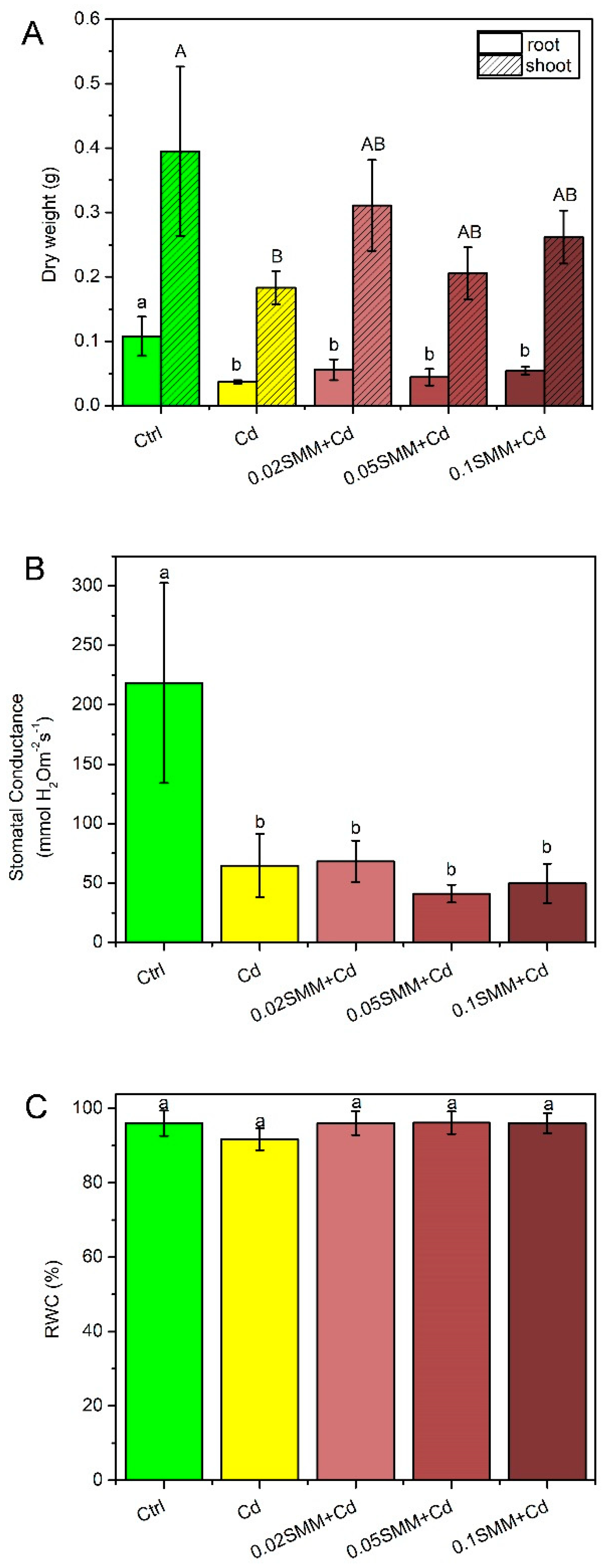
2.1. Effect of S-Methylmetionine on the Plants
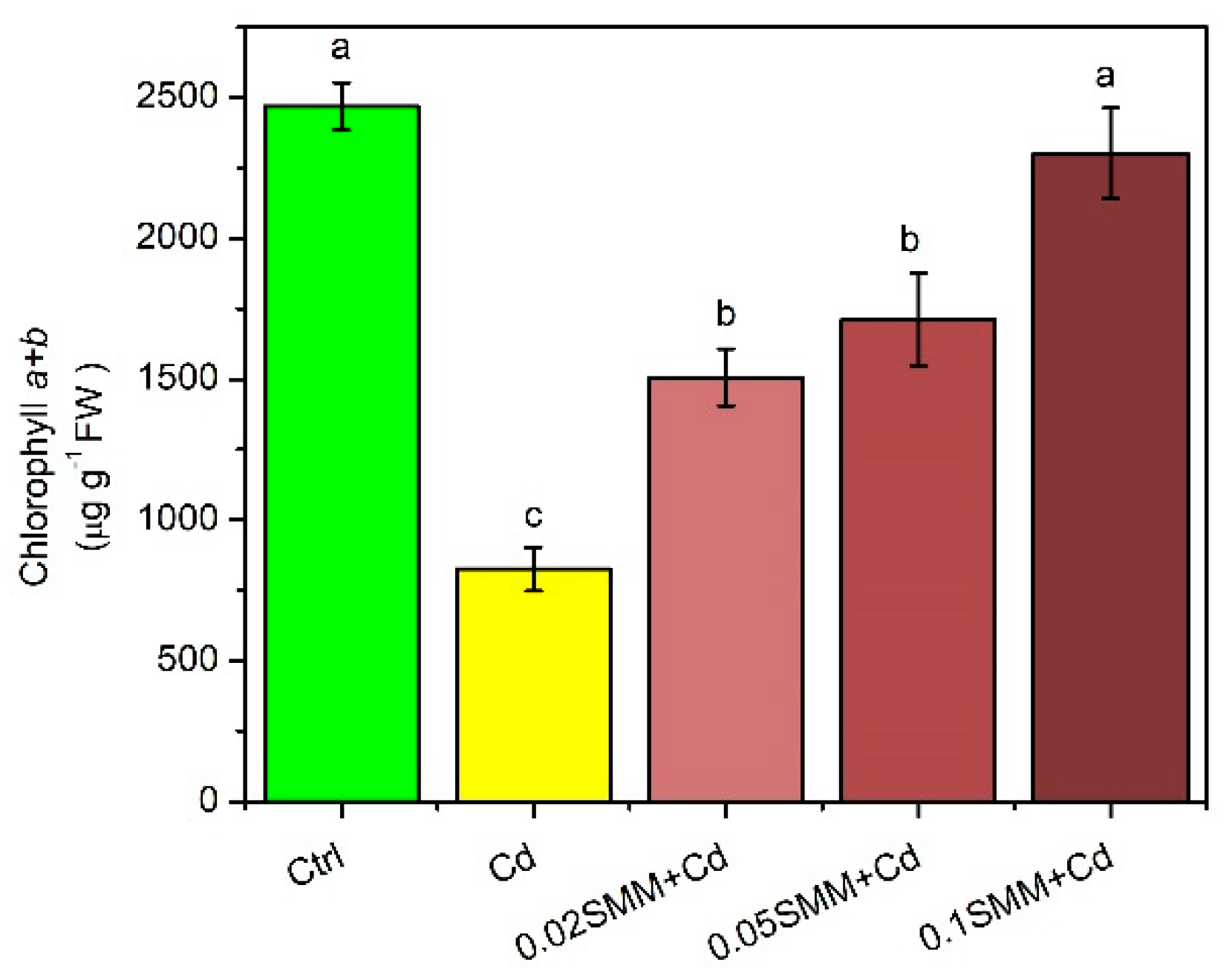
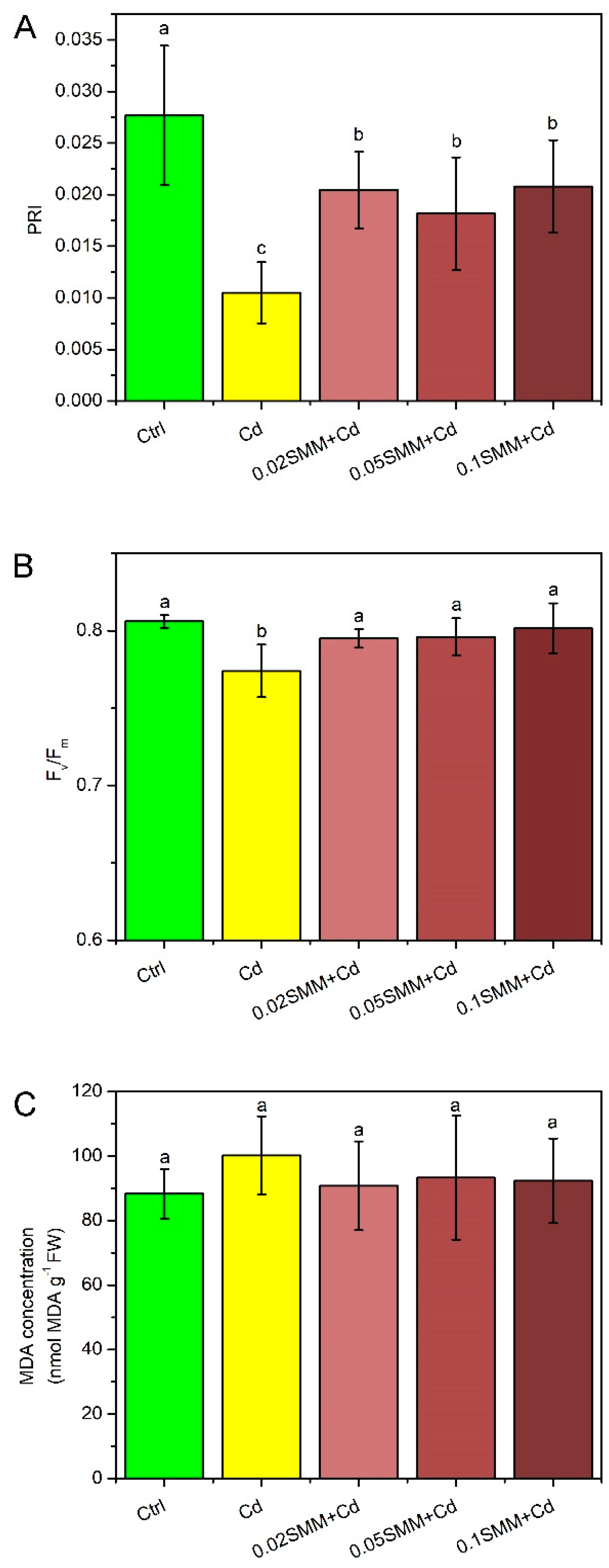
2.2. Physiological Responses of Szarvasi-1 to Cd Treatment and Priming
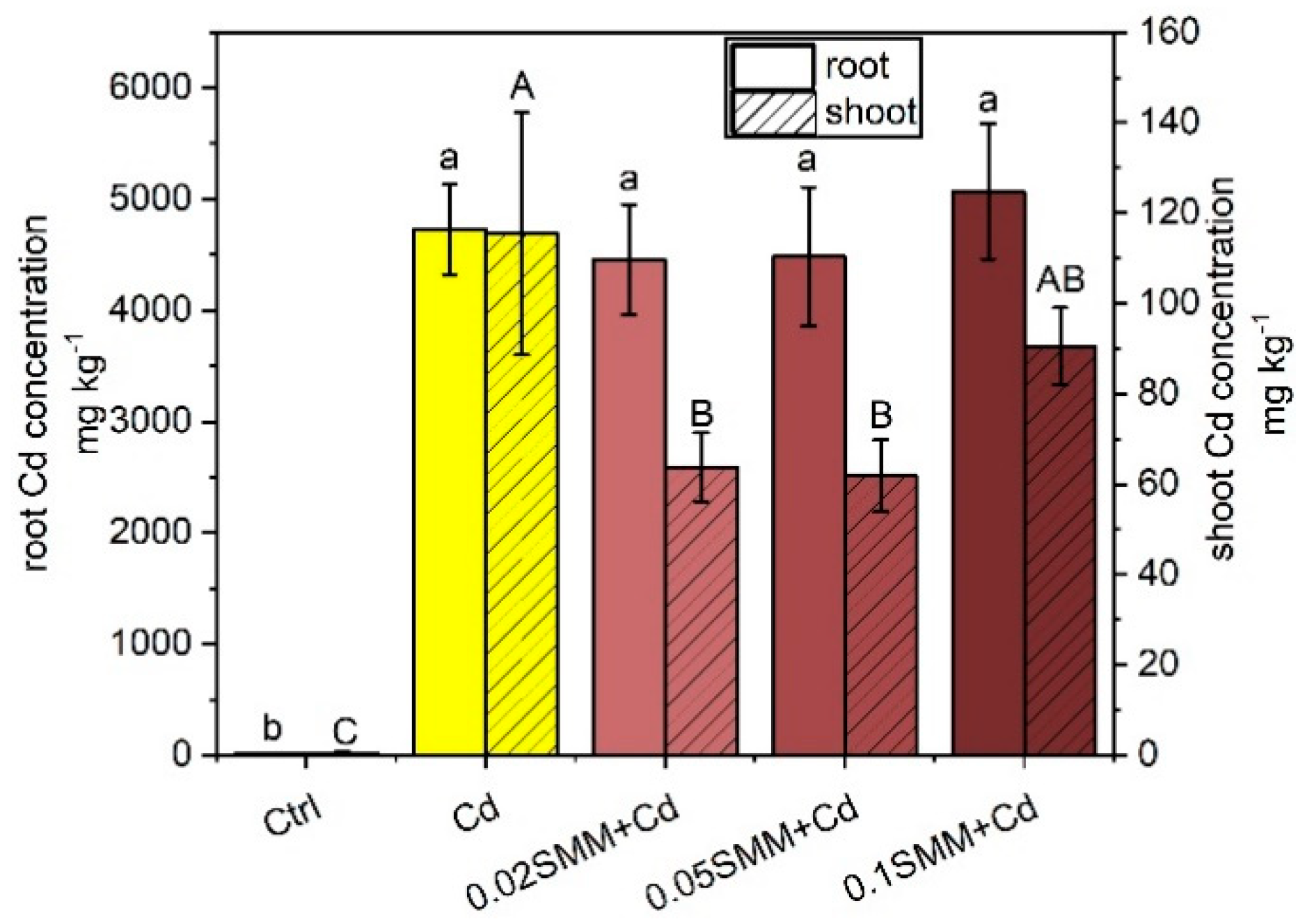
2.3. Changes in the Ionomic Patterns
2.3.1. Cd Accumulation and Its Effect on Element Distributions
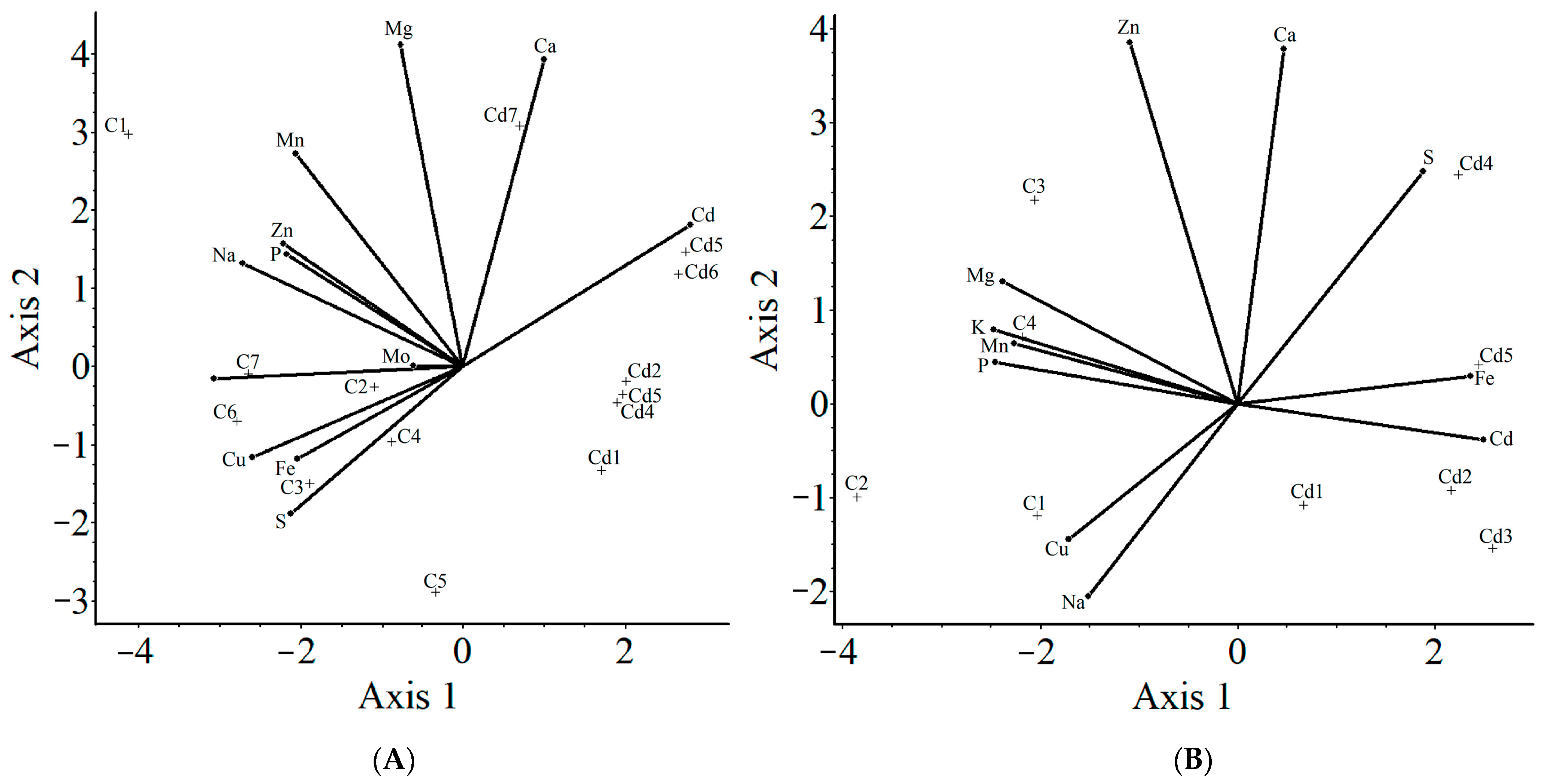
2.3.2. A Multivariate Data (Cluster) Analysis
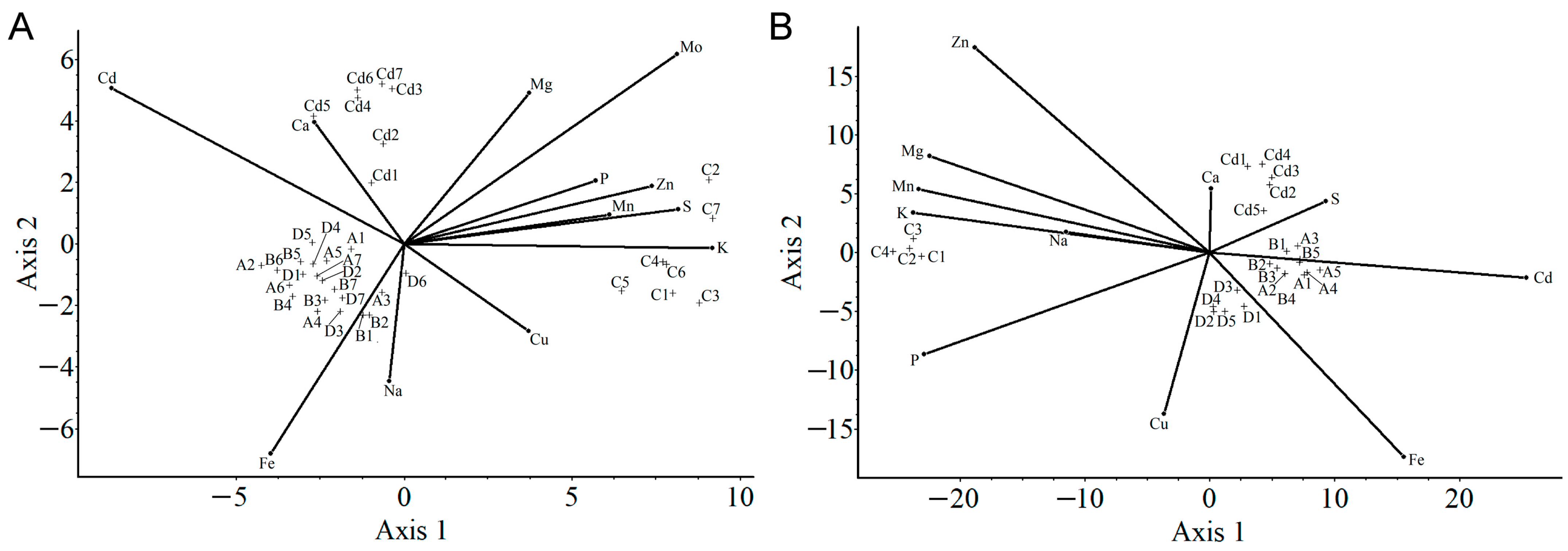
2.3.3. Principal Component Analysis
2.3.4. Canonical Analysis of Variance
3. Discussion
3.1. Physiological Responses of Szarvasi-1 to Cd Treatment and Priming with SMM
3.2. Ionomic Background of the Induced Tolerance to Cd
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Determination of Relative Water Content
4.3. Determination of Chlorophyll Content
4.4. Measurement of Malondialdehyde Content
4.5. Determination of Stomatal Conductance
4.6. Chlorophyll a Fluorescence Induction
4.7. Photochemical Reflectance Index
4.8. Determination of Element Concentration
4.9. Translocation Factor and Bioconcentration Factor
4.10. Statistical Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling Cadmium Toxicity and Tolerance in Plants: Insight into Regulatory Mechanisms. Environ. Exp. Bot. 2012, 83, 3346. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The Effect of Excess Copper on Growth and Physiology of Important Food Crops: A Review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Yousaf, B.; Liu, G.; Wang, R.; Imtiaz, M.; Zia-ur-Rehman, M.; Munir, M.A.M.; Niu, Z. Bioavailability Evaluation, Uptake of Heavy Metals and Potential Health Risks via Dietary Exposure in Urban-Industrial Areas. Environ. Sci. Pollut. Res. 2016, 23, 22443–22453. [Google Scholar] [CrossRef]
- Sanità Di Toppi, L.; Gabbrielli, R. Response to Cadmium in Higher Plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Das, P.; Samantaray, S.; Rout, G.R. Studies on Cadmium Toxicity in Plants: A Review. Environ. Pollut. 1997, 98, 29–36. [Google Scholar] [CrossRef]
- Zorrig, W.; Rouached, A.; Shahzad, Z.; Abdelly, C.; Davidian, J.C.; Berthomieu, P. Identification of Three Relationships Linking Cadmium Accumulation to Cadmium Tolerance and Zinc and Citrate Accumulation in Lettuce. J. Plant Physiol. 2010, 167, 1239–1247. [Google Scholar] [CrossRef]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Yoshida, A.; Kyozuka, J.; Ishikawa, S.; Fujiwara, T. Low-Affinity Cation Transporter (OsLCT1) Regulates Cadmium Transport into Rice Grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef]
- Fan, J.L.; Hu, Z.Y.; Ziadi, N.; Xia, X.; Wu, C.Y.H. Excessive Sulfur Supply Reduces Cadmium Accumulation in Brown Rice (Oryza sativa L.). Environ. Pollut. 2010, 158, 409–415. [Google Scholar] [CrossRef]
- Mori, M.; Kotaki, K.; Gunji, F.; Kubo, N.; Kobayashi, S.; Ito, T.; Itabashi, H. Suppression of Cadmium Uptake in Rice Using Fermented Bark as a Soil Amendment. Chemosphere 2016, 148, 487–494. [Google Scholar] [CrossRef]
- Santillan Medrano, J.; Jurinak, J.J. The Chemistry of Lead and Cadmium in Soil: Solid Phase Formation. Soil Sci. Soc. Am. Proc. 1975, 39, 851–856. [Google Scholar] [CrossRef]
- Bataillard, P.; Cambier, P.; Picot, C. Short-Term Transformations of Lead and Cadmium Compounds in Soil after Contamination. Eur. J. Soil Sci. 2003, 54, 365–376. [Google Scholar] [CrossRef]
- Pinto, A.P.; Mota, A.M.; De Varennes, A.; Pinto, F.C. Influence of Organic Matter on the Uptake of Cadmium, Zinc, Copper and Iron by Sorghum Plants. Sci. Total Environ. 2004, 326, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, M.B. Cadmium in Plants on Polluted Soils: Effects of Soil Factors, Hyperaccumulation, and Amendments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; García-Sánchez, A. Sepiolite as a Feasible Soil Additive for the Immobilization of Cadmium and Zinc. Sci. Total Environ. 2003, 305, 1–12. [Google Scholar] [CrossRef]
- Tezotto, T.; Favarin, J.L.; Azevedo, R.A.; Alleoni, L.R.F.; Mazzafera, P. Coffee Is Highly Tolerant to Cadmium, Nickel and Zinc: Plant and Soil Nutritional Status, Metal Distribution and Bean Yield. Field Crops Res. 2012, 125, 25–34. [Google Scholar] [CrossRef]
- Szolnoki, Z.; Farsang, A.; Puskás, I. Cumulative Impacts of Human Activities on Urban Garden Soils: Origin and Accumulation of Metals. Environ. Pollut. 2013, 177, 106–115. [Google Scholar] [CrossRef]
- Sterckeman, T.; Perriguey, J.; Caël, M.; Schwartz, C.; Morel, J.L. Applying a Mechanistic Model to Cadmium Uptake by Zea mays and Thlaspi caerulescens: Consequences for the Assessment of the Soil Quantity and Capacity Factors. Plant Soil 2004, 262, 289–302. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Mechanisms to Cope with Arsenic or Cadmium Excess in Plants. Curr. Opin. Plant Biol. 2009, 12, 364–372. [Google Scholar] [CrossRef]
- Jabeen, R.; Ahmad, A.; Iqbal, M. Phytoremediation of Heavy Metals: Physiological and Molecular Mechanisms. Bot. Rev. 2009, 75, 339–364. [Google Scholar] [CrossRef]
- Wang, F.; Wang, M.; Liu, Z.; Shi, Y.; Han, T.; Ye, Y.; Gong, N.; Sun, J.; Zhu, C. Different Responses of Low Grain-Cd-Accumulating and High Grain-Cd-Accumulating Rice Cultivars to Cd Stress. Plant Physiol. Biochem. 2015, 96, 261–269. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, J.; Shang, D.; Ning, J.; Zhai, Y.; Sheng, X.; Ding, H. Subcellular Distribution and Chemical Forms of Cadmium in the Edible Seaweed, Porphyra yezoensis. Food Chem. 2015, 168, 48–54. [Google Scholar] [CrossRef]
- Mwamba, T.M.; Li, L.; Gill, R.A.; Islam, F.; Nawaz, A.; Ali, B.; Farooq, M.A.; Lwalaba, J.L.; Zhou, W. Differential Subcellular Distribution and Chemical Forms of Cadmium and Copper in Brassica napus. Ecotoxicol. Environ. Saf. 2016, 134, 239–249. [Google Scholar] [CrossRef]
- Lasat, M.M. Phytoextraction of Toxic Metals: A Review of Biological Mechanisms Soil Microorganisms and Metal Phytoextraction. J. Environ. Qual. 2002, 31, 109–120. [Google Scholar]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root Responses to Cadmium in the Rhizosphere: A Review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef]
- Guerinot, M.L. The ZIP Family of Metal Transporters. Biochim. Biophys. Acta Biomembr. 2000, 1465, 190–198. [Google Scholar]
- Nishida, S.; Mizuno, T.; Obata, H. Involvement of Histidine-Rich Domain of ZIP Family Transporter TjZNT1 in Metal Ion Specificity. Plant Physiol. Biochem. 2008, 46, 601–606. [Google Scholar] [CrossRef]
- Milner, M.J.; Seamon, J.; Craft, E.; Kochian, L.V. Transport Properties of Members of the ZIP Family in Plants and Their Role in Zn and Mn Homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef]
- Korshunova, Y.O.; Eide, D.; Clark, W.G.; Guerinot, M.L.; Pakrasi, H.B. The IRT1 Protein from Arabidopsis thaliana Is a Metal Transporter with a Broad Substrate Range. Plant Mol. Biol. 1999, 40, 37–44. [Google Scholar]
- Nakanishi, H.; Ogawa, I.; Ishimaru, Y.; Mori, S.; Nishizawa, N.K. Iron Deficiency Enhances Cadmium Uptake and Translocation Mediated by the Fe2+ Transporters OsIRT1 and OsIRT2 in Rice. Soil Sci. Plant Nutr. 2006, 52, 464–469. [Google Scholar] [CrossRef]
- Nishida, S.; Tsuzuki, C.; Kato, A.; Aisu, A.; Yoshida, J.; Mizuno, T. AtIRT1, the Primary Iron Uptake Transporter in the Root, Mediates Excess Nickel Accumulation in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 1433–1442. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Senoura, T.; Shimo, H.; Ishikawa, S.; Arao, T.; Nakanishi, H.; Nishizawa, N.K. The OsNRAMP1 Iron Transporter Is Involved in Cd Accumulation in Rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef]
- Ogo, Y.; Kakei, Y.; Itai, R.N.; Kobayashi, T.; Nakanishi, H.; Takahashi, H.; Nakazono, M.; Nishizawa, N.K. Spatial Transcriptomes of Iron-Deficient and Cadmium-Stressed Rice. New Phytol. 2014, 201, 781–794. [Google Scholar] [CrossRef]
- Kaur, R.; Das, S.; Bansal, S.; Singh, G.; Sardar, S.; Dhar, H.; Ram, H. Heavy Metal Stress in Rice: Uptake, Transport, Signaling, and Tolerance Mechanisms. Physiol. Plant. 2021, 173, 430–448. [Google Scholar] [CrossRef]
- Ram, H.; Kaur, A.; Gandass, N.; Singh, S.; Deshmukh, R.; Sonah, H.; Sharma, T.R. Molecular Characterization and Expression Dynamics of MTP Genes under Various Spatio-Temporal Stages and Metal Stress Conditions in Rice. PLoS ONE 2019, 14, e0217360. [Google Scholar] [CrossRef]
- Mihucz, V.G.; Csog, Á.; Fodor, F.; Tatár, E.; Szoboszlai, N.; Silaghi-Dumitrescu, L.; Záray, G. Impact of Two Iron(III) Chelators on the Iron, Cadmium, Lead and Nickel Accumulation in Poplar Grown under Heavy Metal Stress in Hydroponics. J. Plant Physiol. 2012, 169, 561–566. [Google Scholar] [CrossRef]
- Hussain, M.M.; Saeed, A.; Khan, A.A.; Javid, S.; Fatima, B. Differential Responses of One Hundred Tomato Genotypes Grown under Cadmium Stress. Genet. Mol. Res. 2015, 14, 13162–13171. [Google Scholar]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium Minimization in Wheat: A Critical Review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium Stress in Rice: Toxic Effects, Tolerance Mechanisms, and Management: A Critical Review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar]
- Ci, D.; Jiang, D.; Dai, T.; Jing, Q.; Cao, W. Effects of Cadmium on Plant Growth and Physiological Traits in Contrast Wheat Recombinant Inbred Lines Differing in Cadmium Tolerance. Chemosphere 2009, 77, 1620–1625. [Google Scholar]
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Jamil, A. Effect of Cadmium on Seed Germination and Seedling Growth of Four Wheat (Triticum aestivum L.) Cultivars. Pak. J. Bot. 2012, 44, 1569–1574. [Google Scholar]
- Yourtchi, M.S.; Bayat, H. Effect of Cadmium Toxicity on Growth, Cadmium Accumulation and Macronutrient Content of Durum Wheat (Dena CV.). Int. J. Agric. Crop Sci. 2013, 6, 1099–1103. [Google Scholar]
- Wang, M.; Zou, J.; Duan, X.; Jiang, W.; Liu, D. Cadmium Accumulation and Its Effects on Metal Uptake in Maize (Zea mays L.). Bioresour. Technol. 2007, 98, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ouzounidou, G.; Moustakas, M.; Eleftheriou, E.P. Physiological and Ultrastructural Effects of Cadmium on Wheat (Triticum aestivum L.) Leaves. Arch. Environ. Contam. Toxicol. 1997, 32, 154–160. [Google Scholar]
- Ci, D.; Jiang, D.; Wollenweber, B.; Dai, T.; Jing, Q.; Cao, W. Cadmium Stress in Wheat Seedlings: Growth, Cadmium Accumulation and Photosynthesis. Acta Physiol. Plant. 2010, 32, 365–373. [Google Scholar] [CrossRef]
- Vaculík, M.; Pavlovič, A.; Lux, A. Silicon Alleviates Cadmium Toxicity by Enhanced Photosynthetic Rate and Modified Bundle Sheath’s Cell Chloroplasts Ultrastructure in Maize. Ecotoxicol. Environ. Saf. 2015, 120, 66–73. [Google Scholar]
- Farooq, M.; Ullah, A.; Lee, D.J.; Alghamdi, S.S. Terminal Drought-Priming Improves the Drought Tolerance in Desi and Kabuli Chickpea. Int. J. Agric. Biol. 2018, 20, 1129–1136. [Google Scholar]
- Younis, U.; Malik, S.A.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Shah, M.H.R.; Rehman, R.A.; Ahmad, N. Biochar Enhances the Cadmium Tolerance in Spinach (Spinacia oleracea) through Modification of Cd Uptake and Physiological and Biochemical Attributes. Environ. Sci. Pollut. Res. 2016, 23, 21385–21394. [Google Scholar] [CrossRef]
- Hernández, L.E.; Carpena-Ruiz, R.; Gárate, A. Alterations in the Mineral Nutrition of Pea Seedlings Exposed to Cadmium. J. Plant Nutr. 1996, 19, 1581–1598. [Google Scholar]
- Jalil, A.; Selles, F.; Clarke, J.M. Effect of Cadmium on Growth and the Uptake of Cadmium and Other Elements by Durum Wheat. J. Plant Nutr. 1994, 17, 1839–1858. [Google Scholar] [CrossRef]
- Clemens, S.; Palmgren, M.G.; Krämer, U. A Long Way Ahead: Understanding and Engineering Plant Metal Accumulation. Trends Plant Sci. 2002, 7, 309–315. [Google Scholar] [CrossRef]
- Rochayati, S.; Du Laing, G.; Rinklebe, J.; Meissner, R.; Verloo, M. Use of Reactive Phosphate Rocks as Fertilizer on Acid Upland Soils in Indonesia: Accumulation of Cadmium and Zinc in Soils and Shoots of Maize Plants. J. Plant Nutr. Soil Sci. 2011, 174, 186–194. [Google Scholar] [CrossRef]
- Kovács, K.; Kuzmann, E.; Vértes, A.; Lévai, L.; Cseh, E.; Fodor, F. Effect of Cadmium on Iron Uptake in Cucumber Roots: A Mössbauer-Spectroscopic Study. Plant Soil 2010, 327, 49–56. [Google Scholar] [CrossRef]
- Solti, Á.; Sárvári, É.; Tóth, B.; Basa, B.; Lévai, L.; Fodor, F. Cd Affects the Translocation of Some Metals Either Fe-like or Ca-like Way in Poplar. Plant Physiol. Biochem. 2011, 49, 494–498. [Google Scholar] [CrossRef]
- Solti, Á.; Gáspár, L.; Mészáros, I.; Szigeti, Z.; Lévai, L.; Sárvári, É. Impact of Iron Supply on the Kinetics of Recovery of Photosynthesis in Cd-Stressed Poplar (Populus glauca). Ann. Bot. 2008, 102, 771–782. [Google Scholar] [CrossRef]
- Solti, Á.; Sárvári, É.; Szöllosi, E.; Tóth, B.; Mészáros, I.; Fodor, F.; Szigeti, Z. Stress Hardening under Long-Term Cadmium Treatment Is Correlated with the Activation of Antioxidative Defence and Iron Acquisition of Chloroplasts in Populus. Z. Naturforsch. Sect. C J. Biosci. 2016, 71, 323–334. [Google Scholar] [CrossRef]
- Éva, C.; Solti, Á.; Oszvald, M.; Tömösközi-Farkas, R.; Nagy, B.; Horváth, G.V.; Tamás, L. Improved Reactive Aldehyde, Salt and Cadmium Tolerance of Transgenic Barley Due to the Expression of Aldo–Keto Reductase Genes. Acta Physiol. Plant. 2016, 38, 1–10. [Google Scholar] [CrossRef]
- Zoghlami, L.B.; Djebali, W.; Abbes, Z.; Hediji, H.; Maucourt, M.; Moing, A.; Brouquisse, R.; Chaïbi, W. Metabolite Modifications in Solanum lycopersicum Roots and Leaves under Cadmium Stress. Afr. J. Biotechnol. 2011, 10, 567–579. [Google Scholar]
- Zhang, Z.C.; Chen, B.X.; Qiu, B.S. Phytochelatin Synthesis Plays a Similar Role in Shoots of the Cadmium Hyperaccumulator Sedum alfredii as in Non-Resistant Plants. Plant Cell Environ. 2010, 33, 1248–1255. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and Metallothioneins: Roles in Heavy Metal Detoxification and Homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular Mechanisms for Heavy Metal Detoxification and Tolerance. J. Exp. Bot. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.G.; Jobe, T.O.; Hauser, F.; Schroeder, J.I. Long-Distance Transport, Vacuolar Sequestration, Tolerance, and Transcriptional Responses Induced by Cadmium and Arsenic. Curr. Opin. Plant Biol. 2011, 14, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Cosio, C.; Martinoia, E.; Keller, C. Hyperaccumulation of Cadmium and Zinc in Thlaspi caerulescens and Arabidopsis halleri at the Leaf Cellular Level. Plant Physiol. 2004, 134, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Korenkov, V.; Hirschi, K.; Crutchfield, J.D.; Wagner, G.J. Enhancing Tonoplast Cd/H Antiport Activity Increases Cd, Zn, and Mn Tolerance, and Impacts Root/Shoot Cd Partitioning in Nicotiana tabacum L. Planta 2007, 226, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals-Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and Opportunities in the Phytoremediation of Heavy Metals Contaminated Soils: A Review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, I.; Schmidt, U.; Londo, M.; Faaij, A. The Economic Value of the Phytoremediation Function—Assessed by the Example of Cadmium Remediation by Willow (Salix Ssp). Agric. Syst. 2006, 89, 68–89. [Google Scholar] [CrossRef]
- Csete, S.; Stranczinger, S.; Szalontai, B.; Farkas, A.W.R.; Salamon-Albert, E.; Kocsis, M.; Tovari, P.; Vojtela, T.; Dezso, J.; Walcz, I.; et al. Tall Wheatgrass Cultivar Szarvasi-1 (Elymus elongatus Subsp. Ponticus cv. Szarvasi-1) as a Potential Energy Crop for Semi-Arid Lands of Eastern Europe. In Sustainable Growth and Applications in Renewable Energy Sources; Nayeripour, M., Kheshti, M., Eds.; IntechOpen: London, UK, 2011; pp. 269–294. [Google Scholar]
- Sipos, G.; Solti, Á.; Czech, V.; Vashegyi, I.; Tóth, B.; Cseh, E.; Fodor, F. Heavy Metal Accumulation and Tolerance of Energy Grass (Elymus elongatus Subsp. Ponticus cv. Szarvasi-1) Grown in Hydroponic Culture. Plant Physiol. Biochem. 2013, 68, 96–103. [Google Scholar]
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A. Seed Priming and Transgenerational Drought Memory Improves Tolerance against Salt Stress in Bread Wheat. Plant Physiol. Biochem. 2017, 118, 362–369. [Google Scholar] [CrossRef]
- Yang, X.; Lu, C. Photosynthesis Is Improved by Exogenous Glycine betaine in Salt-Stressed Maize Plants. Physiol. Plant. 2005, 124, 343–352. [Google Scholar] [CrossRef]
- Vannier, N.; Mony, C.; Bittebière, A.K.; Vandenkoornhuyse, P. Epigenetic Mechanisms and Microbiota as a Toolbox for Plant Phenotypic Adjustment to Environment. Front. Plant Sci. 2015, 6, 1159. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [PubMed]
- McRorie, R.A.; Sutherland, G.L.; Lewis, M.S.; Barton, A.D.; Glazener, M.R.; Shive, W. Isolation and Identification of a Naturally Occurring Analog of Methionine1a. J. Am. Chem. Soc. 1954, 76, 115–118. [Google Scholar] [CrossRef]
- Ranocha, P.; McNeil, S.D.; Ziemak, M.J.; Li, C.; Tarczynski, M.C.; Hanson, A.D. The S-Methylmethionine Cycle in Angiosperms: Ubiquity, Antiquity and Activity. Plant J. 2001, 25, 575–584. [Google Scholar] [CrossRef]
- Ludmerszki, E.; Páldi, K.; Rácz, I.; Szigeti, Z.; RudnóY, S. The Promising Role of Exogenous Smethylmethionine in Agriculture, in the Case of Maize Cultivation. Appl. Ecol. Environ. Res. 2014, 12, 777–785. [Google Scholar] [CrossRef]
- Páldi, K.; Rácz, I.; Szigeti, Z.; Rudnóy, S. S-Methylmethionine Alleviates the Cold Stress by Protection of the Photosynthetic Apparatus and Stimulation of the Phenylpropanoid Pathway. Biol. Plant. 2014, 58, 189–194. [Google Scholar] [CrossRef]
- Gondor, O.K.; Janda, T.; Soós, V.; Pál, M.; Majláth, I.; Adak, M.K.; Balázs, E.; Szalai, G. Salicylic Acid Induction of Flavonoid Biosynthesis Pathways in Wheat Varies by Treatment. Front. Plant Sci. 2016, 7, 1447. [Google Scholar] [CrossRef]
- Janda, T.; Khalil, R.; Tajti, J.; Pál, M.; Szalai, G.; Rudnóy, S.; Rácz, I.; Kátay, G.; Molnár, A.B.; Lejmel, M.A.; et al. The Newly Synthesized Plant Growth Regulator S-Methylmethionine Salicylate May Provide Protection against High Salinity in Wheat. Plant Growth Regul. 2018, 85, 305–315. [Google Scholar] [CrossRef]
- Antoniou, C.; Savvides, A.; Christou, A.; Fotopoulos, V. Unravelling chemical priming machinery in plants: The role of reactive oxygen–nitrogen–sulfur species in abiotic stress tolerance enhancement. Curr. Opin. Plant Biol. 2016, 33, 101–107. [Google Scholar] [CrossRef]
- Canellas, L.P.; Canellas, N.O.A.; da S Irineu, L.E.S.; Olivares, F.L.; Piccolo, A. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 2020, 7, 12. [Google Scholar] [CrossRef]
- Janda, T.; Pál, M.; Darkó, É.; Szalai, G. Use of Salicylic Acid and Related Compounds to Improve the Abiotic Stress Tolerance of Plants: Practical Aspects. In Salicylic Acid: A Multifaceted Hormone; Nazar, R., Iqbal, N., Khan, N., Eds.; Springer: Singapore, 2017; pp. 35–46. [Google Scholar]
- Prasad, M.N.V.; Strzałka, K. Impact of Heavy Metals on Photosynthesis. In Heavy Metal Stress in Plants; Prasad, M.N.V., Strzałka, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 117–138. [Google Scholar]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-ur-Rehman, M.; Farid, M.; Asma, M. Effect of Zinc-Lysine on Growth, Yield and Cadmium Uptake in Wheat (Triticum aestivum L.) and Health Risk Assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef]
- Qin, S.; Liu, H.; Nie, Z.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of Cadmium and Its Competition with Mineral Nutrients for Uptake by Plants: A Review. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface between Stress Perception and Physiological Responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Pandhair, V.; Sekhon, B.S. Reactive Oxygen Species and Antioxidants in Plants: An Overview. J. Plant Biochem. Biotechnol. 2006, 15, 71–78. [Google Scholar] [CrossRef]
- Shaheen, S.; Ahmad, R.; Mahmood, O.; Mubarak, H.; Mirza, N.; Hayat, M.T. Physiology and selected genes expression under cadmium stress in Arundo donax L. Int. J. Phytorem. 2018, 20, 1162–1167. [Google Scholar] [CrossRef]
- Rév, A.; Tóth, B.; Solti, Á.; Sipos, G.; Fodor, F. Responses of Szarvasi-1 Energy Grass to Sewage Sludge Treatments in Hydroponics. Plant Physiol. Biochem. 2017, 118, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Xuebin, Q.; Riaz, L.; Yasin, G.; Shah, A.N.; Shahzad, U.; Jahan, M.S.; Ditta, A.; Bashir, M.A.; Rehim, A.; et al. The Interactive Effect of PH Variation and Cadmium Stress on Wheat (Triticum aestivum L.) Growth, Physiological and Biochemical Parameters. PLoS ONE 2021, 16, e0253798. [Google Scholar] [CrossRef]
- Rácz, I.; Páldi, E.; Szalai, G.; Janda, T.; Pál, M.; Lásztity, D. S-Methylmethionine Reduces Cell Membrane Damage in Higher Plants Exposed to Low-Temperature Stress. J. Plant Physiol. 2008, 165, 1483–1490. [Google Scholar] [CrossRef]
- Fodorpataki, L.; Molnar, K.; Tompa, B.; Bartha, C. Exogenous S-Methylmethionine Alleviates Salinity Stress by Modulation of Physiological Processes in Canola (Brassica napus). Int. J. Agric. Biol. 2021, 25, 11–19. [Google Scholar] [CrossRef]
- Zhang, R.H.; Li, J.; Guo, S.R.; Tezuka, T. Effects of Exogenous Putrescine on Gas-Exchange Characteristics and Chlorophyll Fluorescence of NaCl-Stressed Cucumber Seedlings. Photosynth. Res. 2009, 100, 155–162. [Google Scholar] [CrossRef]
- Kósa, E.; Szegő, D.; Horváth, E.; Rácz, I.; Szigeti, Z.; Lásztity, D.; Páldi, E. Effect of S-methylmethionine on the photosynthesis in maize at different chilling temperatures. Cent. Eur. J. Biol. 2011, 6, 75–83. [Google Scholar] [CrossRef]
- Balassa, G.; Oláh, C.; Balassa, K.; Rácz, I.; Kátay, G.; Kalapos, B.; Boldizsár, I.; Sárvári, É.; Solti, Á.; Pál, M.; et al. Physiological and Molecular Background of Maize Cold-Tolerance Enhancement with S-methylmethionine Salicylate. J. Plant Growth Regul. 2022, 41, 2073–2091. [Google Scholar] [CrossRef]
- Cohen, A.S.; Popovic, R.B.; Zalik, S. Effects of Polyamines on Chlorophyll and Protein Content, Photochemical Activity, and Chloroplast Ultrastructure of Barley Leaf Discs during Senescence. Plant Physiol. 1979, 64, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Sghayar, S.; Ferri, A.; Lancilli, C.; Lucchini, G.; Abruzzese, A.; Porrini, M.; Ghnaya, T.; Nocito, F.F.; Abdelly, C.; Sacchi, G.A. Analysis of Cadmium Translocation, Partitioning and Tolerance in Six Barley (Hordeum vulgare L.) Cultivars as a Function of Thiol Metabolism. Biol. Fertil. Soils 2015, 51, 311–320. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Fukuoka, H.; Arao, T.; Ohyama, A.; Nunome, T.; Miyatake, K.; Negoro, S. Gene Expression Analysis in Cadmium-Stressed Roots of a Low Cadmium-Accumulating Solanaceous Plant, Solanum Torvum. J. Exp. Bot. 2010, 61, 423–437. [Google Scholar] [CrossRef]
- Yokosho, K.; Yamaji, N.; Ueno, D.; Mitani, N.; Ma, J.F. OsFRDL1 is a Citrate Transporter Required for Efficient Transformation of Iron in Rice. Plant Physiol. 2009, 149, 297–305. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium Toxicity in Plants: Impacts and Remediation Strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Ludmerszki, E.; Almási, A.; Rácz, I.; Szigeti, Z.; Solti, Á.; Oláh, C.; Rudnóy, S. S-Methylmethionine Contributes to Enhanced Defense against Maize Dwarf Mosaic Virus Infection in Maize. Rev. Bras. Bot. 2015, 38, 771–782. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron Uptake, Translocation, and Regulation in Higher Plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar]
- Mudd, S.H.; Datko, A.H. The S-Methylmethionine Cycle in Lemna paucicostata. Plant Physiol. 1990, 93, 623–630. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. BBA Bioenerg. 1989, 975, 384–394. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Belkhodja, R.; Morales, F.; Quílez, R.; López-Millán, A.F.; Abadía, A.; Abadía, J. Iron Deficiency Causes Changes in Chlorophyll Fluorescence Due to the Reduction in the Dark of the Photosystem II Acceptor Side. Photosynth. Res. 1998, 56, 265–276. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index:an optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef] [PubMed]
| Treatment | pH in the Fresh Solution | pH at Harvesting Day |
|---|---|---|
| Ctrl | 5.90 | 7.25 ± 0.01 a |
| Cd | 5.85 | 7.18 ± 0.03 b |
| 0.02SMM+Cd | 5.85 | 7.11 ± 0.02 c |
| 0.05SMM+Cd | 5.85 | 7.03 ± 0.01 d |
| 0.1SMM+Cd | 5.85 | 6.98 ± 0.02 d |
| 0.02SMM | 5.99 | |
| 0.05SMM | 5.97 | |
| 0.1SMM | 5.87 |
| Treatment | TF | BCF |
|---|---|---|
| Ctrl | 0.045 ± 0.037 * | |
| Cd | 0.024 ± 0.006 a | 102.66 ± 23.88 a |
| 0.02SMM+Cd | 0.014 ± 0.002 b | 61.36 ± 13.76 b |
| 0.05SMM+Cd | 0.014 ± 0.001 b | 59.72 ± 14.06 b |
| 0.1SMM+Cd | 0.018 ± 0.002 ab | 91.52 ± 24.67 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, D.; Arcoverde Cerveira Sterner, V.; Potluri, A.K.; May, Z.; Müller, B.; Solti, Á.; Rudnóy, S.; Sipos, G.; Gyuricza, C.; Fodor, F. S-Methylmethionine Effectively Alleviates Stress in Szarvasi-1 Energy Grass by Reducing Root-to-Shoot Cadmium Translocation. Plants 2022, 11, 2979. https://doi.org/10.3390/plants11212979
Rana D, Arcoverde Cerveira Sterner V, Potluri AK, May Z, Müller B, Solti Á, Rudnóy S, Sipos G, Gyuricza C, Fodor F. S-Methylmethionine Effectively Alleviates Stress in Szarvasi-1 Energy Grass by Reducing Root-to-Shoot Cadmium Translocation. Plants. 2022; 11(21):2979. https://doi.org/10.3390/plants11212979
Chicago/Turabian StyleRana, Deepali, Vitor Arcoverde Cerveira Sterner, Aravinda Kumar Potluri, Zoltán May, Brigitta Müller, Ádám Solti, Szabolcs Rudnóy, Gyula Sipos, Csaba Gyuricza, and Ferenc Fodor. 2022. "S-Methylmethionine Effectively Alleviates Stress in Szarvasi-1 Energy Grass by Reducing Root-to-Shoot Cadmium Translocation" Plants 11, no. 21: 2979. https://doi.org/10.3390/plants11212979
APA StyleRana, D., Arcoverde Cerveira Sterner, V., Potluri, A. K., May, Z., Müller, B., Solti, Á., Rudnóy, S., Sipos, G., Gyuricza, C., & Fodor, F. (2022). S-Methylmethionine Effectively Alleviates Stress in Szarvasi-1 Energy Grass by Reducing Root-to-Shoot Cadmium Translocation. Plants, 11(21), 2979. https://doi.org/10.3390/plants11212979








