Abstract
(1) Rhododendron is one of the top ten traditional flowers in China, with both high ornamental and economic values. However, with the change of the environment, Rhododendron suffers from various biological stresses. The WRKY transcription factor is a member of the most crucial transcription factor families, which plays an essential regulatory role in a variety of physiological processes and developmental stresses. (2) In this study, 57 RsWRKYs were identified using genome data and found to be randomly distributed on 13 chromosomes. Based on gene structure and phylogenetic relationships, 57 proteins were divided into three groups: I, II, and III. Multiple alignments of RsWRKYs with Arabidopsis thaliana homologous genes revealed that WRKY domains in different groups had different conserved sites. RsWRKYs have a highly conserved domain, WRKYGQK, with three variants, WRKYGKK, WRKYGEK, and WRKYGRK. Furthermore, cis-acting elements analysis revealed that all of the RsWRKYs had stress and plant hormone cis-elements, with figures varying by group. Finally, the expression patterns of nine WRKY genes treated with gibberellin acid (GA), methyl jasmonate (MeJA), heat, and drought in Rhododendron were also measured using quantitative real-time PCR (qRT-PCR). The results showed that the expression levels of the majority of RsWRKY genes changed in response to multiple phytohormones and abiotic stressors. (3) This current study establishes a theoretical basis for future studies on the response of RsWRKY transcription factors to various hormone and abiotic stresses as well as a significant foundation for the breeding of new stress-tolerant Rhododendron varieties.
1. Introduction
The WRKY gene family is the largest transcription factor family in higher plants [1]. The WRKY protein is distinguished by the presence of a highly conserved WRKY DNA binding domain of 60 amino acids and a signature amino acid residue “WRKYGQK” at the N-terminal, followed by a C2H2-type or C2HC-type zinc finger structure [2,3]. Although the majority of the WRKY gene family in plants is relatively conservative, deletion and replacement of N-terminal conserved amino acid residues as well as loss of zinc structure in the C-terminal have been founded in some plants in recent years [4]. WRKY transcription factors are classified into three types based on the number of WRKY-conserved domains and the structure of zinc finger motifs. Group I WRKY proteins typically have two WRKY domains, whereas Group II and Group III proteins only have one WRKY domain and a zinc finger structure C2H2(CX4-5CX22-23HXH) or C2HC(CX7CX23HXC) [5]. The WRKY protein also contains nuclear localization signal peptides (NLS) that regulate target genes. Some WRKY proteins also have a leucine zippers domain that can bind to the W-box element ((C/T) TGAC (T/C)) or the cis-acting element SURE in a promoter to promote or inhibit gene expression [6].
Plants have evolved defense mechanisms to resist varieties of biotic and abiotic stresses throughout their lives. WRKY transcription factors are also crucial regulatory components in plant growth and development, dormancy, lignin biosynthesis, and stress tolerance [7,8]. Identifying and deciphering the key genes that respond to plant abiotic stress could provide the foundation for breeding crops that are resistant to abiotic stresses [9,10]. SPF1, the first WRKY-encoding gene, was discovered in Ipomoea batatas Lamin 1994 [11], and since then, the WRKY gene from Arabidopsis, rice, cucumber, apple, peach, and other plants has been gradually cloned [4,12,13,14,15]. In Arabidopsis, 80% of WRKY genes respond to bacterial infection. AtWRKY25 and AtWRKY33 regulate salt tolerance through interactions with upstream and downstream target genes [16,17]. Overexpression OsWRKY30 in rice increased tolerance to rice fungus Rhizoctonia solani and fungus Magnaporthe grisea [18,19]. Previous studies have also shown that HvWRKY38, OsWRKY12, TaWRKY19, and TaWRKY2 provide resistance under drought stress [20,21,22]. Furthermore, increasing studies have documented that WRKY proteins are involved in signal transduction processes mediated by plant hormones such as salicylic acid (SA), jasmonic acid [23], and others. For example, AtWRKY50 and AtWRKY51 promoted SA biosynthesis in Arabidopsis [24]; and JA treatment increased the expression of AtWRKY17 and AtWRKY33 [25]. Therefore, in this study, we analyzed the expression of RsWRKY genes in response to GA and JA hormones to learn more about their role in the resistance to abiotic stress in Rhododendron. It is known that the WRKY family in Arabidopsis consists of 72 members, while the WRKY family in rice consists of 109 members. The expansion of genes is one of the leading causes of gene family growth. The Group I and II WRKY families appeared before monocot and dicot differentiation, while the Group III WRKY genes appeared later. Some studies have shown that gene expression patterns among different copies of genes are also dissimilar after gene duplication, but the specific mechanism is still unclear [26].
R. simsii is an essential ornamental plant in Ericaceae; a total of 7 subgenera and 720 species of wild Rhododendrons were found in China [27]. To screen Rhododendron varieties with strong drought resistance, 36 varieties of Rhododendron were selected to study the changes of physicochemical characteristics in Rhododendron leaves under simulated drought stress by PEG-6000 [28]. Since soil pH, moisture, high temperature, and resistance to insects are difficulties in Rhododendron cultivation. Identifying the functions of RsWRKY genes plays a crucial significance in Rhododendron. However, the detailed information of the WRKY family in R. simsii and their effect under several abiotic stresses was still unclear. In the present study, WRKY genes from R. simsii were identified, and it may give a novel insight into studying the mechanism of Rhododendron’s coping with abiotic stress.
2. Results
2.1. Identification and Physicochemical Properties Analysis of RsWRKY Gene Family Members
Sixty-three potential candidate genes were searched in NCBI by BLASTX to predict conserved domains, reconfirmed by PFAM and SMART, and four genes without a complete WRKY domain were eliminated. Moreover, 2 (RhsimUnG0199600, Rhsim10G0148800) of these 59 were repetitive sequences, which were manually removed. The remaining 57 RsWRKYs were identified and designated as RsWRKY1–RsWRKY57. Detailed information about RsWRKY genes is shown in Table S1. Proteins encoded by 57 RsWRKY genes contained 115 (RsWRKY40) to 1661 (RsWRKY43) amino acids, with an average of 405 amino acids. Their predicted molecular weight varied from 12866.11 (RsWRKY40) to 190287.09 (RsWRKY43) Da, and the isoelectric point ranged from 4.94 (RsWRKY9) to 10.12 (RsWRKY32). Furthermore, the threshold values of the aliphatic index varied from RsWRKY17 to RsWRKY56. The grand average of hydropathicity values of all were negative, indicating that all RsWRKYs were predominantly hydrophilic proteins. Subcellular localization results revealed that WRKY proteins were all distributed in the nucleus.
2.2. Chromosomal Location of RsWRKY Gene Family Members
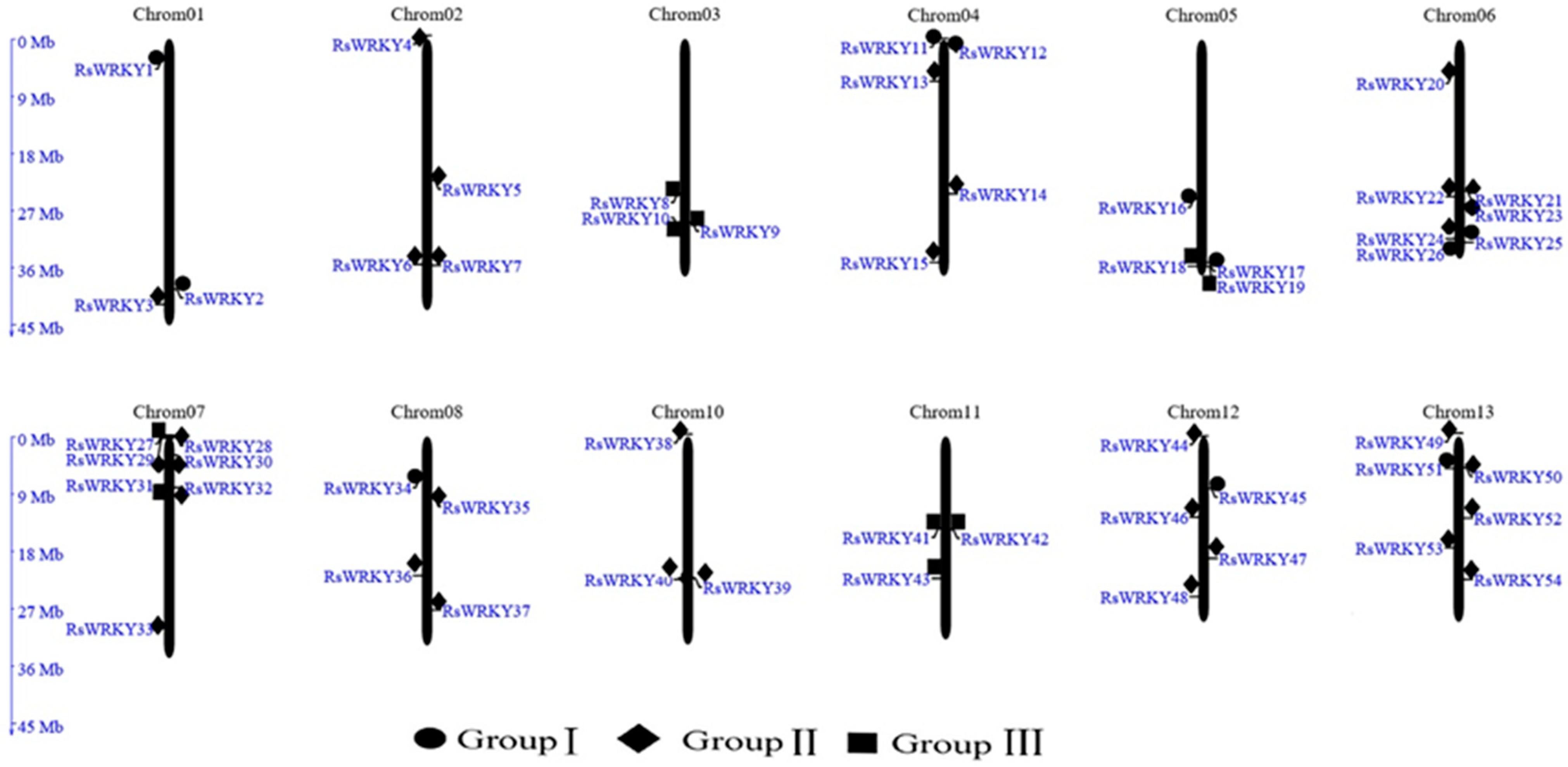
Chromosomal localization analysis revealed that 57 of the candidate genes were unevenly distributed across all 13 chromosomes of the R. simsii genome, with the exception of chromosome 9. The remaining three (RsWRKY55–RsWRKY57), meanwhile, could not be mapped to any chromosomes (Figure 1). From the perspective of a single chromosome, the highest number of genes (seven) were located onchromosomes 6 and 7. Notably, three WRKY genes were mapped on each of chromosomes 1, 3, 10, and 11.
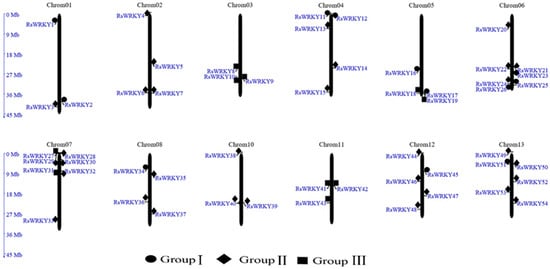
Figure 1.
Chromosomal locations of the RsWRKY gene family in R. simsii. The 57 putative WRKY genes were renamed from RsWRKY1 to RsWRK57 according to their chromosomal locations. Three WRKY genes that could not be mapped to any chromosome were named RsWRKY55–RsWRKY57, respectively.
2.3. Phylogenetic Analysis and Classification of RsWRKY Proteins
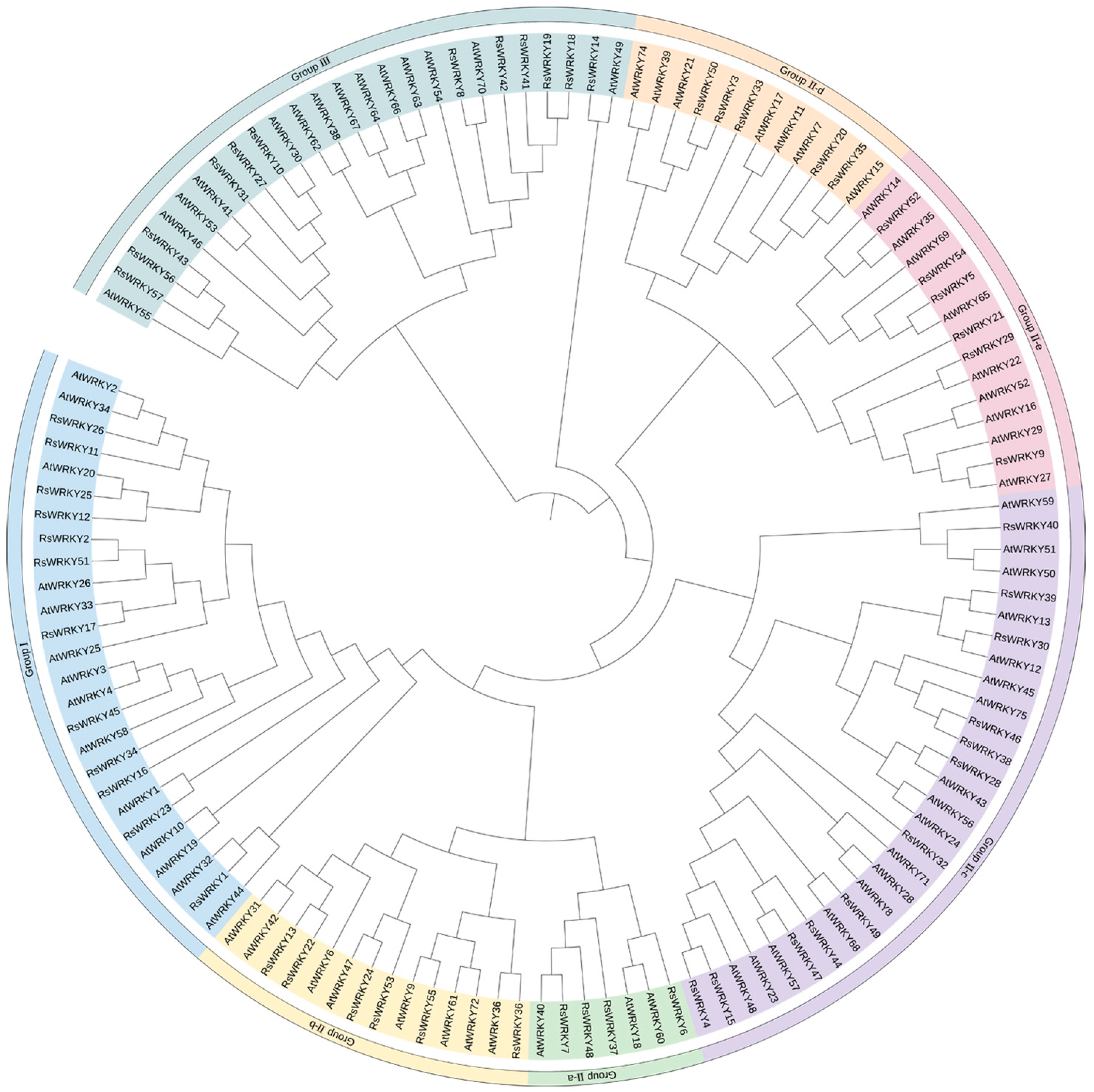
To further analyze the evolutionary relationship of the RsWRKY family, 57 RsWRKY proteins and 72 AtWRKY proteins were examined by MEGA6.0 software (Figure 2). Seven AtWRKYs were selected as representatives of A. thaliana and made multiple sequence alignments with RsWRKYs (Figure 3). The RsWRKYs proteins were classified into three main groups based on the group features of the WRKY superfamily in Arabidopsis [3]. With the exception of RsWRKY16, which originated from Group I but lost one WRKY domain, the 12 RsWRKYs that comprised two WRKY domains and a C2H2 zinc finger motif (C-X4-C-X22-23-HXH) were assigned into Group I, an N-terminal WRKY domain (NTWD), and a C-terminal WRKY domain (CTWD). The same situation occurred in Arabidopsis as well [29]: the deletion of the WRKY domain in the N-terminal is found in Arabidopsis, but the deletion in the C-terminal is found in the RsWRKY family. Group II is the largest group, which is classified into five subgroups with a single WRKY domain and a zinc finger motif. A total of 4, 6, 13, 5, and 6 RsWRKYs clustered in II-a, II-b, II-c, II-d, and II-e, respectively. Among them, II-a and II-b clustered in one clade, while subgroups II-d and II-e clustered in another clade. RsWRKY14 is a member of Group II-c but is more similar to Group III from the same branch based on the phylogenetic analysis and so clustered into II-c when multiple sequences were aligned. The WRKY domain (WRKYGQK, Motif 1) was highly conserved among the 55 proteins, and only two of them contained variations. Thirty-four RsWRKYs harbored the motif 1 sequence, while RsWRKY40 and RsWRKY21 had the WRKYGKK and WRKYGRK sequences instead. The remaining 11 RsWRKYs were distributed into Group III.
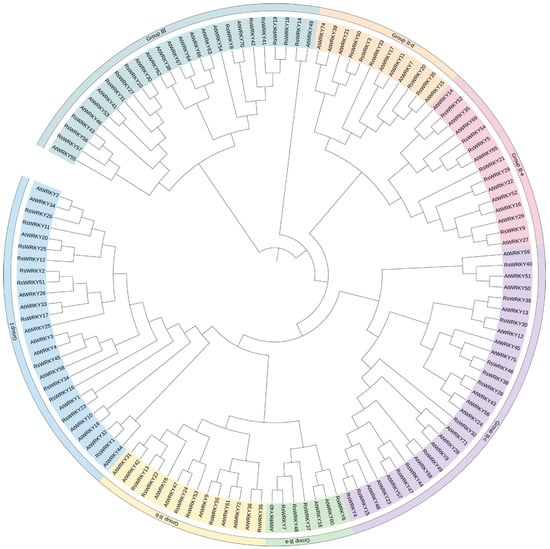
Figure 2.
Phylogenetic analysis among the identified WRKY-conserved proteins in A. thaliana and R. simsii. The 72 A. thaliana and 57 R. simsii WRKY sequences were aligned using Muscle; the phylogenetic tree was constructed by MEGA6.0 with the maximum likelihood method, and the bootstrap value was set at 1000 repetitions. The colorful regions represent different subfamilies.

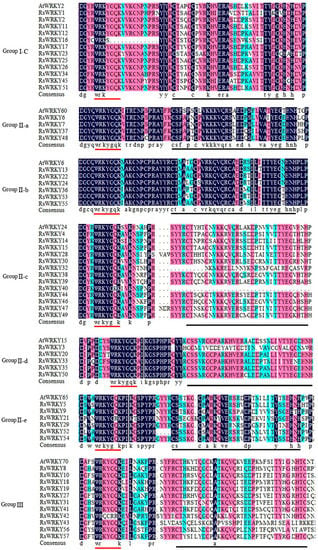
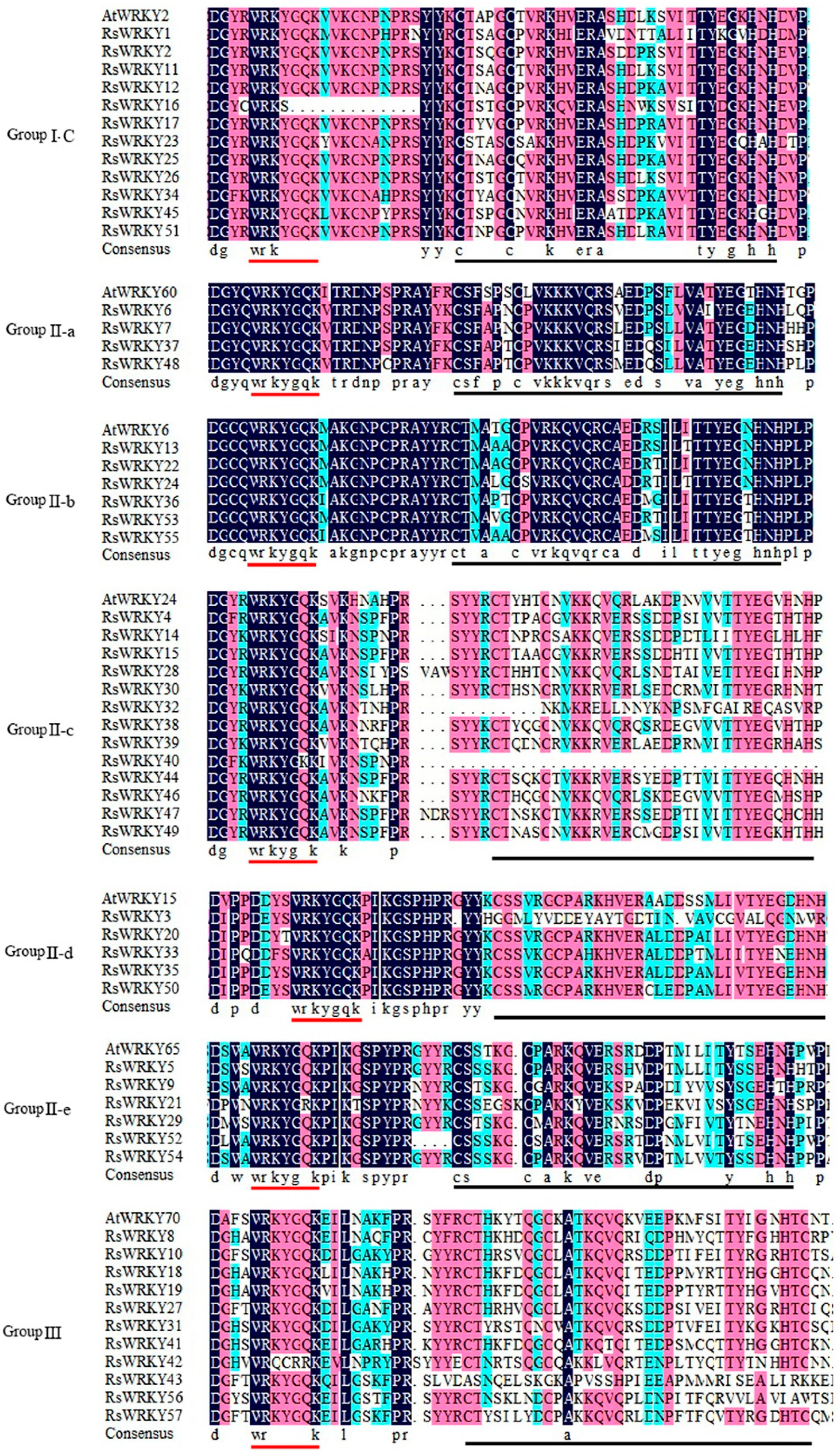
Figure 3.
Multiple alignments of RsWRKY proteins and selected AtWRKY amino acid sequences. “-N” and “-C” indicate the N-terminal and C-terminal WRKY domains of Group I WRKY members, respectively. The red solid line represents the highly conserved WRKYGQK heptapeptide, and the black solid line represents the zinc finger domain.
2.4. Gene Structure and Motif Composition of RsWRKY Gene Family
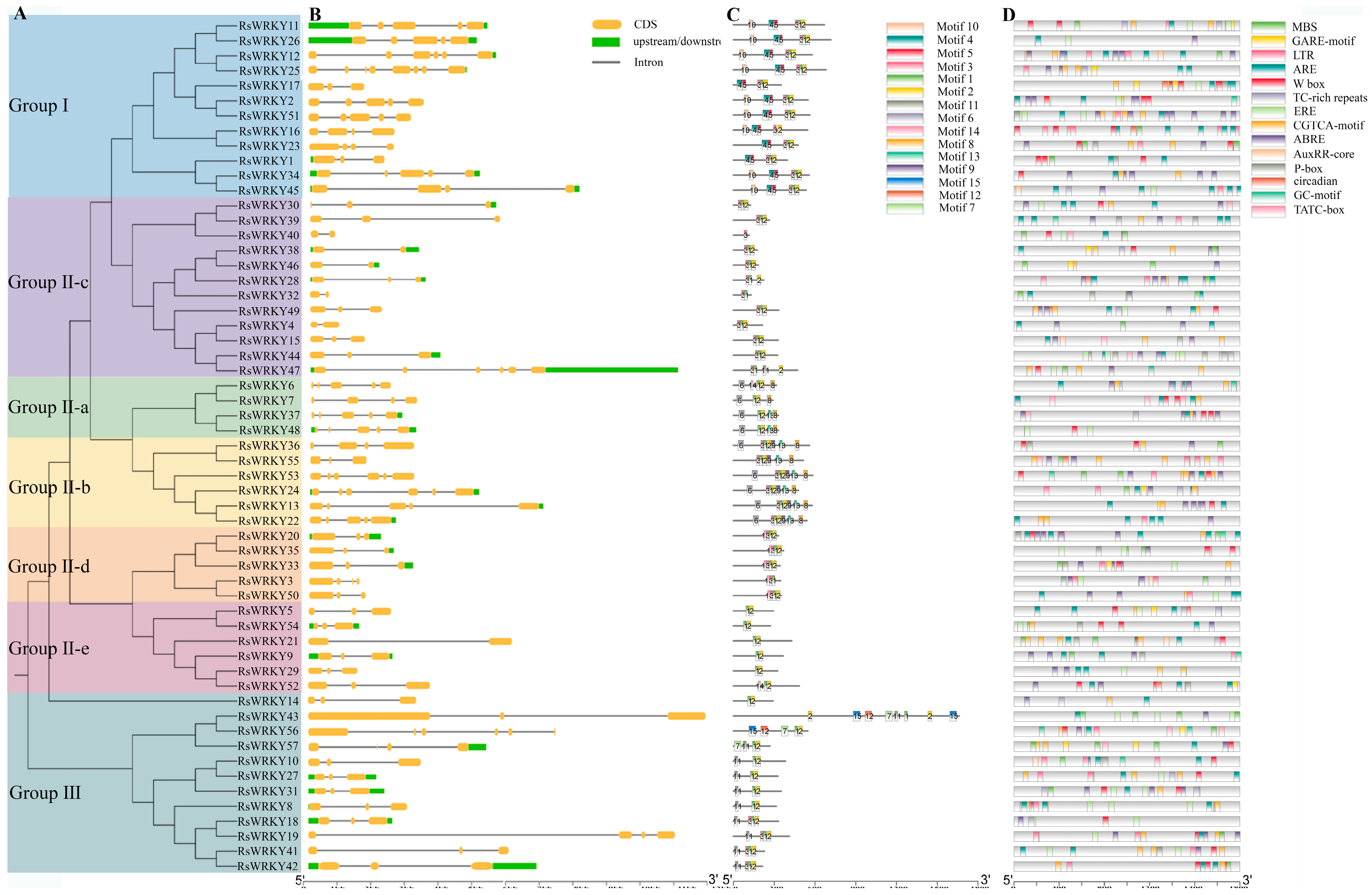
MEGA6.0 was used to construct an evolutionary tree (Figure 4A), the result was consistent with the evolution of Arabidopsis thaliana (Figure 2), which revealed that WRKY proteins were highly conserved in evolution. RsWRKY family members are mainly distributed in several different branches, which have differences and similarities in evolution. All RsWRKY genes possessed one to seven introns. Overall, 26 (45.6%) genes contained two introns, 24 (42%) had three to six introns, while seven introns were found in RsWRKY25 (Figure 4B).
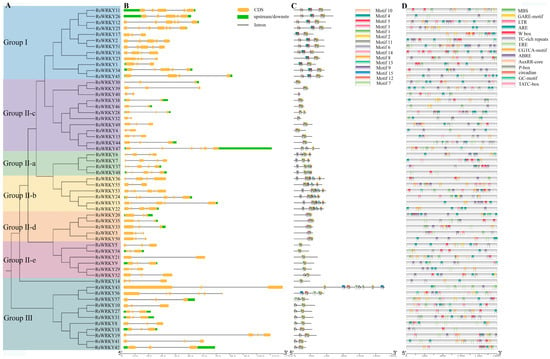
Figure 4.
Phylogenetic tree, gene structure, distribution of conserved motif, and cis-regulatory elements of RsWRKY gene family. (A) Phylogenetic tree was constructed by MEGA6.0 software and shown on the left. (B) Schematic of gene structure was displayed by the gene structure display server, CDSs, UTRs, and introns are represented by orange boxes, green boxes, and black lines. (C) The conserved motifs of RsWRKY proteins and different motifs are represented by different color boxes, and numbers. (D) Cis-acting element analysis of promoter region of RsWRKY gene family.
To examine the number and types of motifs contained in RsWRKY proteins and their homologous protein pairs in different groups, 57 WRKY protein members were analyzed by the online software MEME, and the maximum number of motifs was set at 15. The length of 15 motifs ranged roughly between 21 and 50 amino acids, as shown in (Figure S1). The heptapeptide stretch WRKYGQK, which was considered as an essential characteristic of the WRKY family, was present in motif 1 and motif 4. Proteins in the same group had similar numbers and types of motifs. Except for the RsWRKY16, motifs 1, 2, 3, 4, and 5 were found in Group I. Motif 6 was identified as nuclear localization signals (NLS), which was observed in II-a and II-b; meanwhile, motif 8 was unique to these two groups, and motif 11 was only present in Group III (Figure 4C).
Cis-acting elements on promoters are functional elements that regulate gene expression. We downloaded the 2 kb promoter region sequence upstream of the initiation codons of the RsWRKY genes and used PlantCARE to identify the cis-regulatory elements in there (Figure 4D). Interestingly, in addition to a large number of basic TATA-box, CAAT-box core elements, and light-response elements, there are numerous hormones, stress response, and regulation of growth and development elements. Elements that are involved in defense and stress response include TC-rich repeats, the MYB binding site involved in drought induction (MBS), low-temperature-response element (LTR), essential regulator element for anaerobic induction (ARE), and hypoxia-specific induction-related element (GC-motif). Other types of cis-elements, such as auxin-reaction element (AUXRR-core), abscisic-acid-reaction element (ABRE), estrogen-response element (ERE), gibberellin-reaction element (TATC-box, P-box, and GARE-motif), and methyl-jasmonate-reaction elements (CGTCA-motif), are hormone-related elements. Cis-acting element analysis indicated that WRKY family genes of R. simsii may be closely related to biological stress, abiotic stress response, hormone induction, and plant growth and development.
2.5. Expression Profile of RsWRKY Genes under GA and MeJA Treatment
A phylogenetic tree was constructed between the RsWRKY family and the AtWRKY family in our studies to screen candidate genes associate with abiotic stress for subsequent analysis (Figure 2). For qRT-PCR, we selected the RsWRKY genes that clustered with the reported AtWRKY resistance-related genes. Following cis-acting element analysis, it was discovered that some RsWRKYs contain gibberellin-reaction elements (TATC-box, P-box, and GARE-motif) as well as methyl-jasmonate-reaction elements (CGTCA-motif). To investigate the relationship between RsWRKYs and phytohormone-signaling molecules, the expression levels of RsWRKYs were analyzed by qRT-PCR under GA and MeJA treatment.
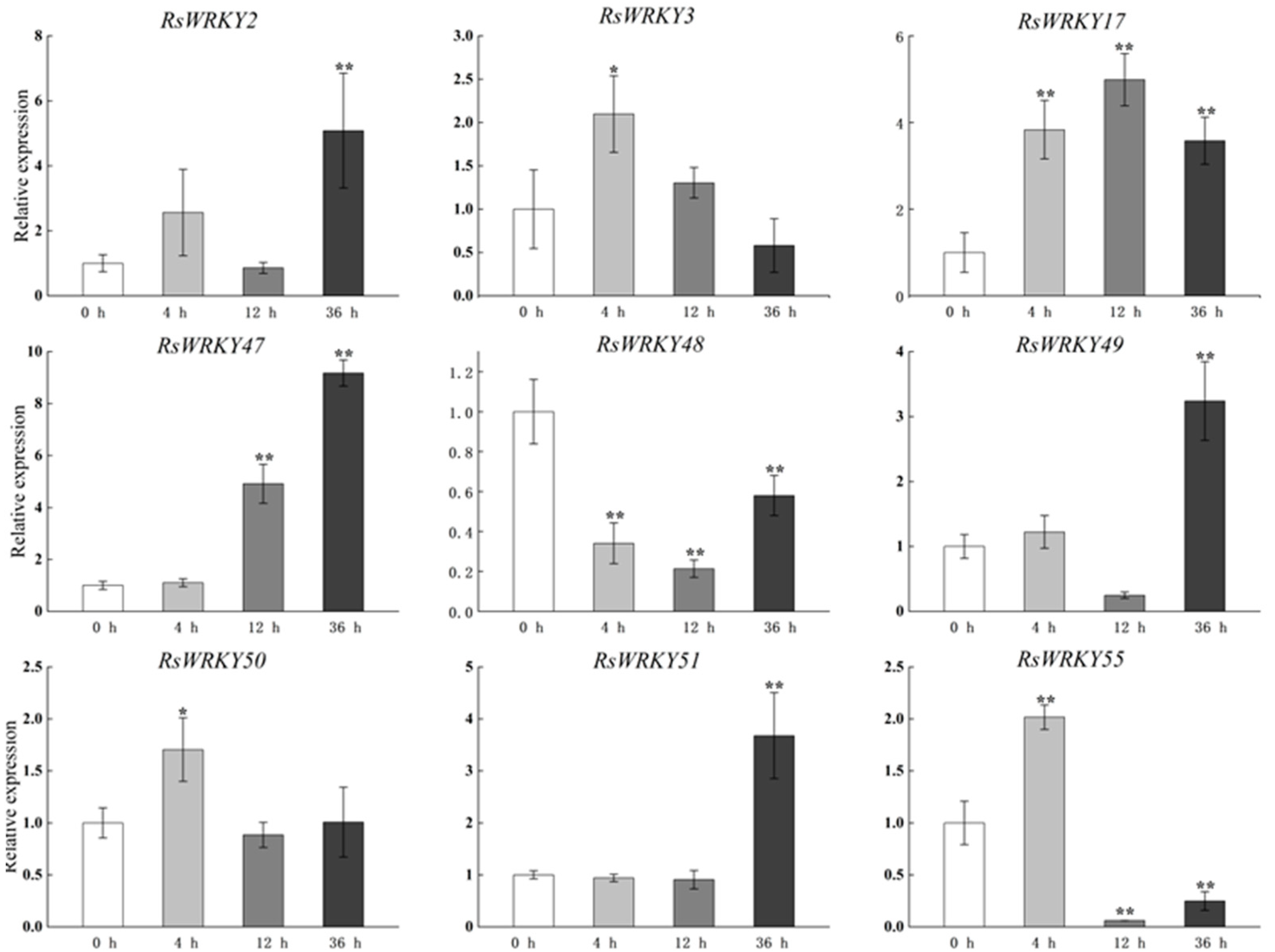
Other genes were expressed after GA treatment, as shown in Figure 5, with the exception of RsWRKY48, which was down-regulated. Obviously, the expression levels of RsWRKY2, RsWRKY47, RsWRKY49, and RsWRKY51 reached the highest value at 36 h after GA hormone treatment, which was much higher than that of the control. RsWRKY3, RsWRKY50, and RsWRKY55 reached their highest point after 4 h GA treatment. However, RsWRKY17 was significantly expressed throughout the treatment period.
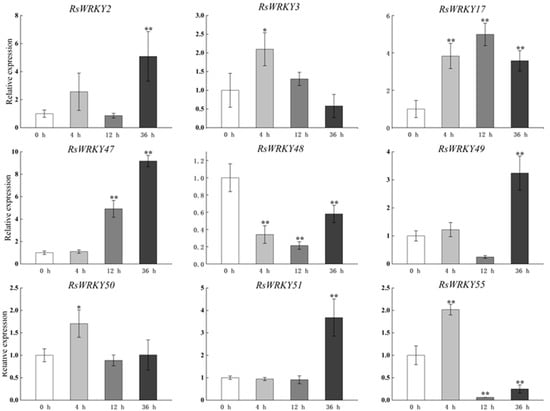
Figure 5.
Expression profiles of RsWRKY genes under 100 µM GA treatment. Gene expression at 0 h was normalized as “1.” Data are mean ± standard deviation (SD), calculated from three biological replicates, and vertical lines represent the standard deviation. * and ** mean significant difference at p < 0.05 and p < 0.01, respectively.
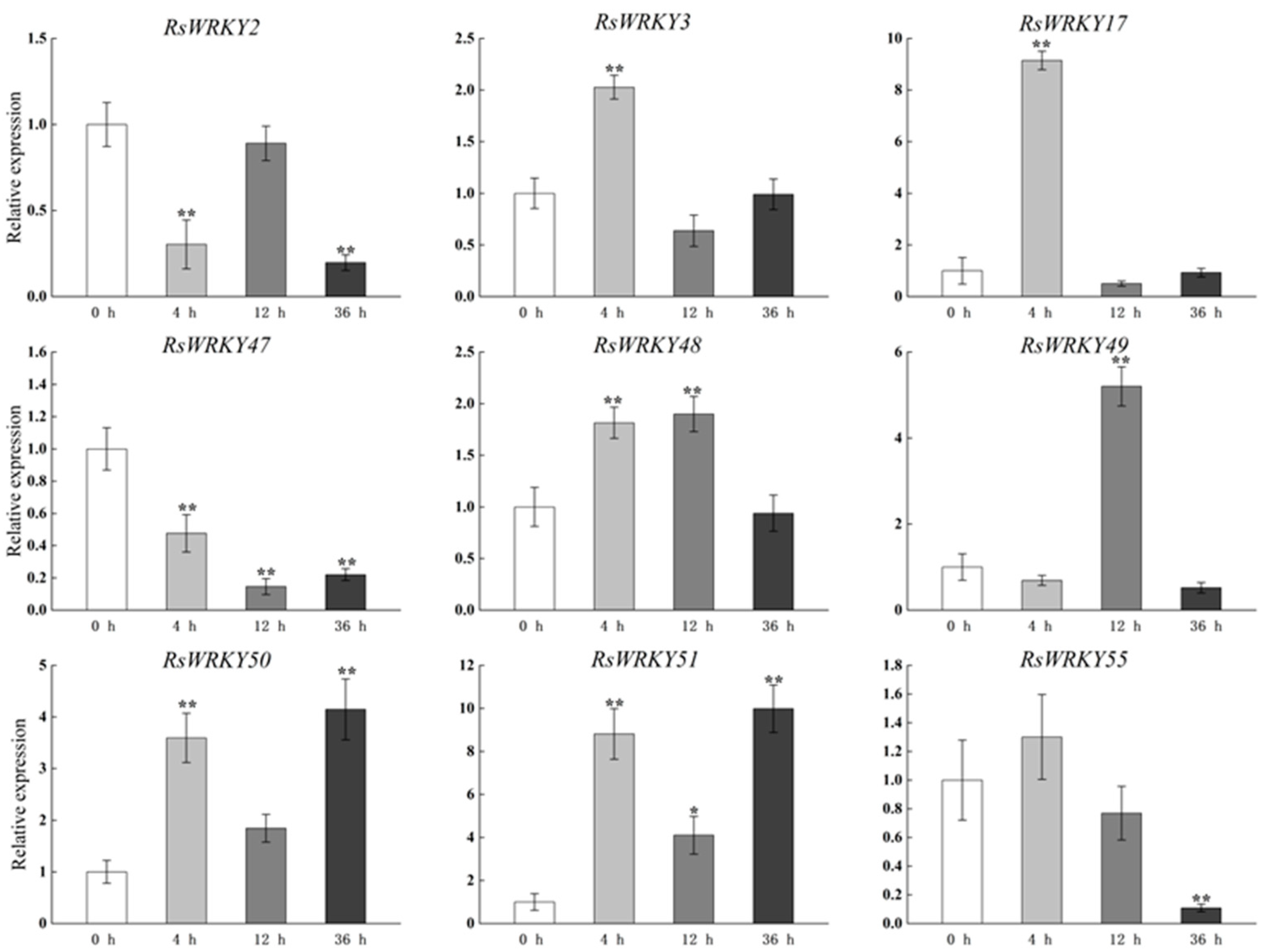
RsWRKY3, RsWRKY17, RsWRKY48, RsWRKY50, and RsWRKY51 all responded to MeJA treatment with similar expression patterns, and their expression levels were all up-regulated after 4 h treatment. However, after MeJA treatment, RsWRKY2, RsWRKY47, and RsWRKY55 showed an obvious decreasing trend (Figure 6).
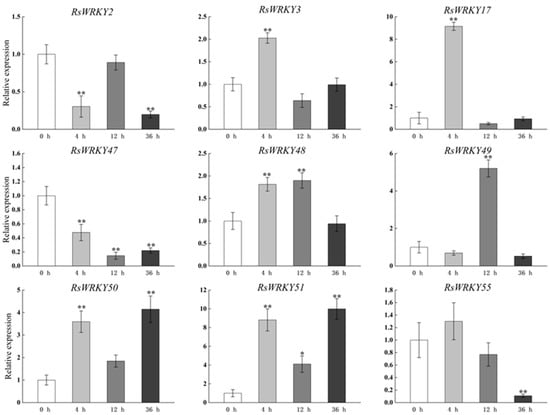
Figure 6.
Expression profiles of RsWRKY genes under 200 µM MeJA treatment. Gene expression at 0 h was normalized as “1.” Data are mean ± standard deviation (SD), calculated from three biological replicates, and vertical lines represent the standard deviation. * and ** mean significant difference at p < 0.05 and p < 0.01, respectively.
2.6. Expression Profile of RsWRKY Genes under Heat and Drought Treatment
Heat and drought are increasingly limiting factors for plant growth and development as a result of global warming. Therefore, it is urgent to explore Rhododendron stress-resistance genes for Rhododendron breeding and application.
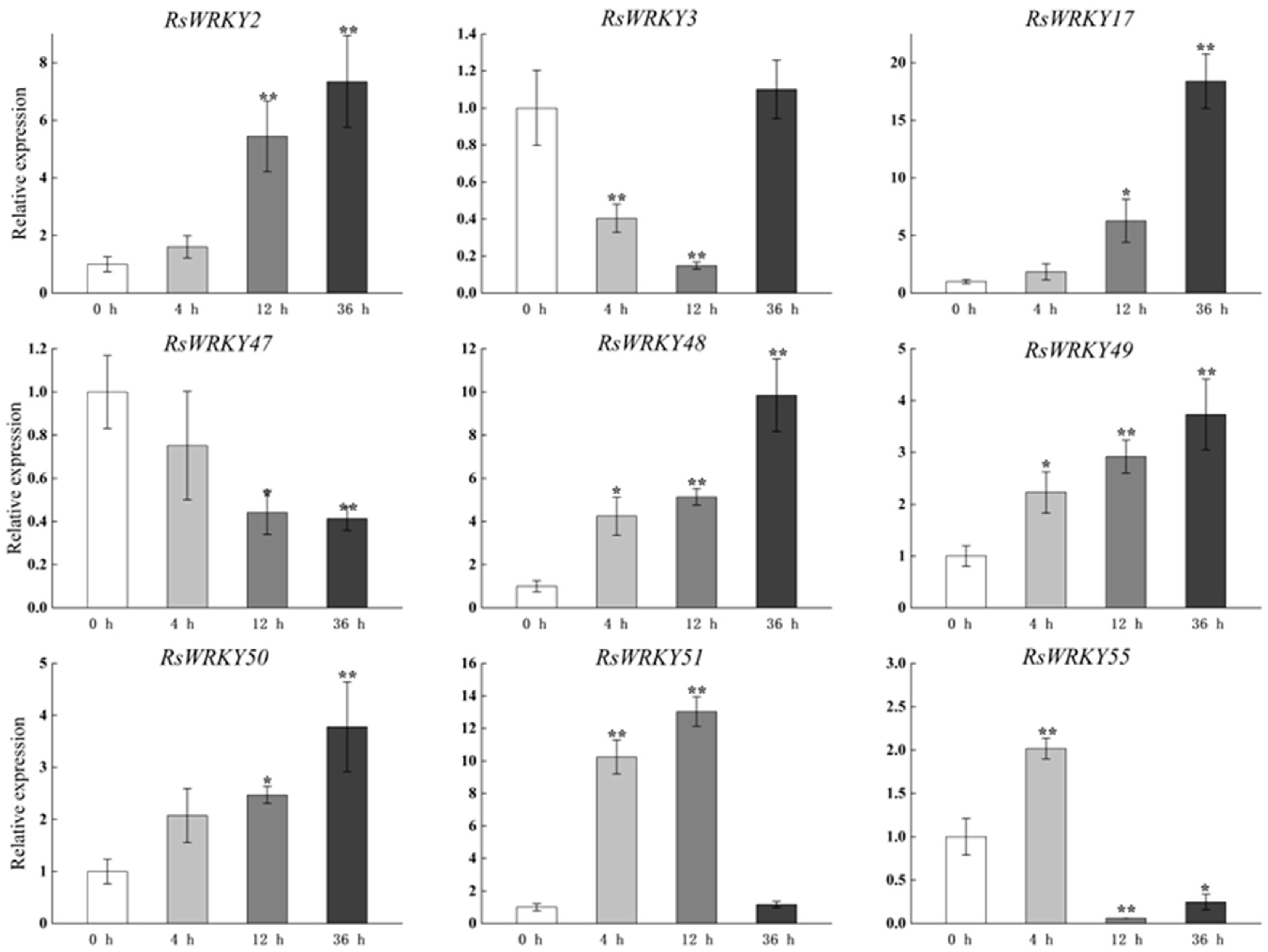
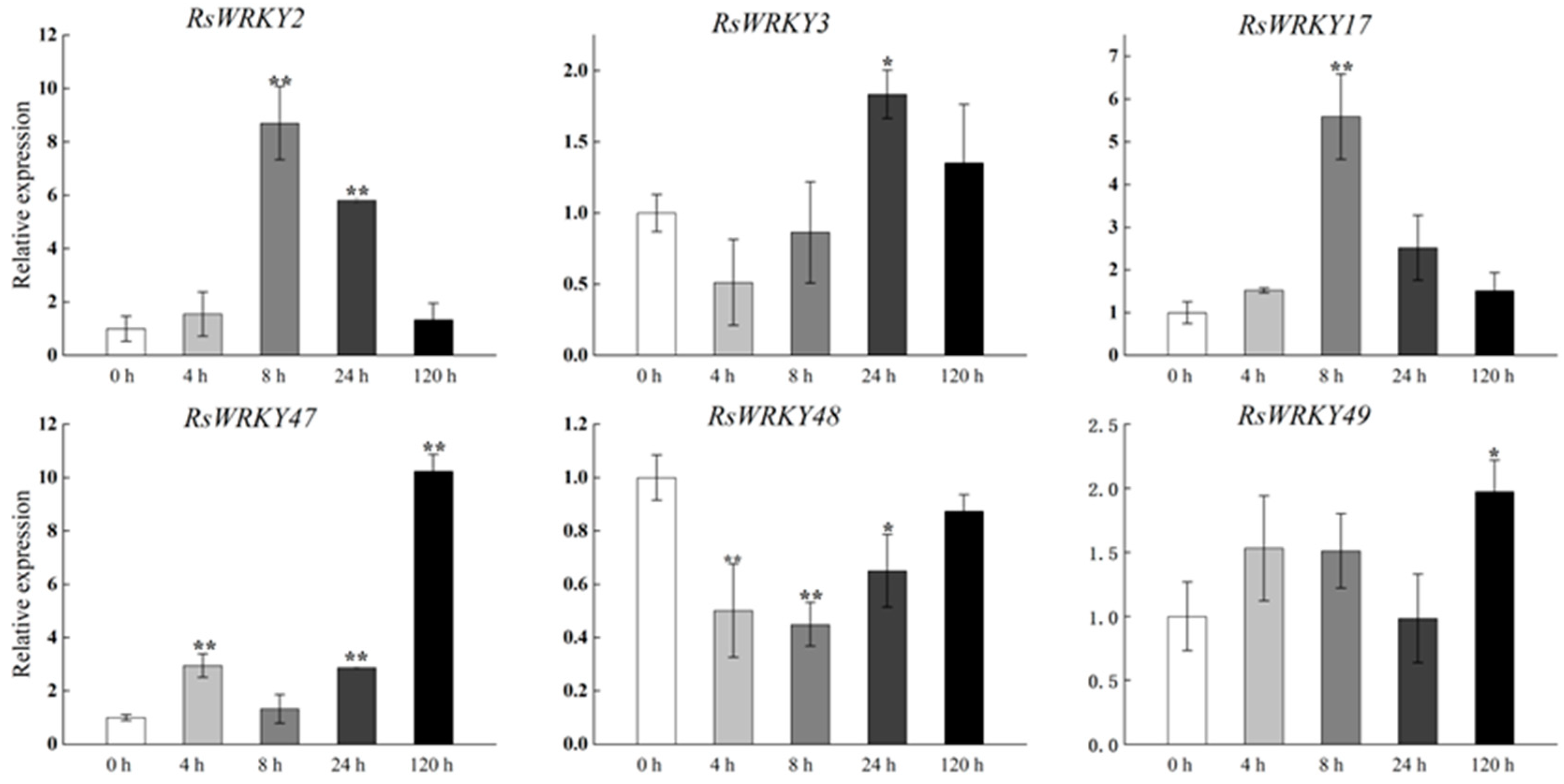
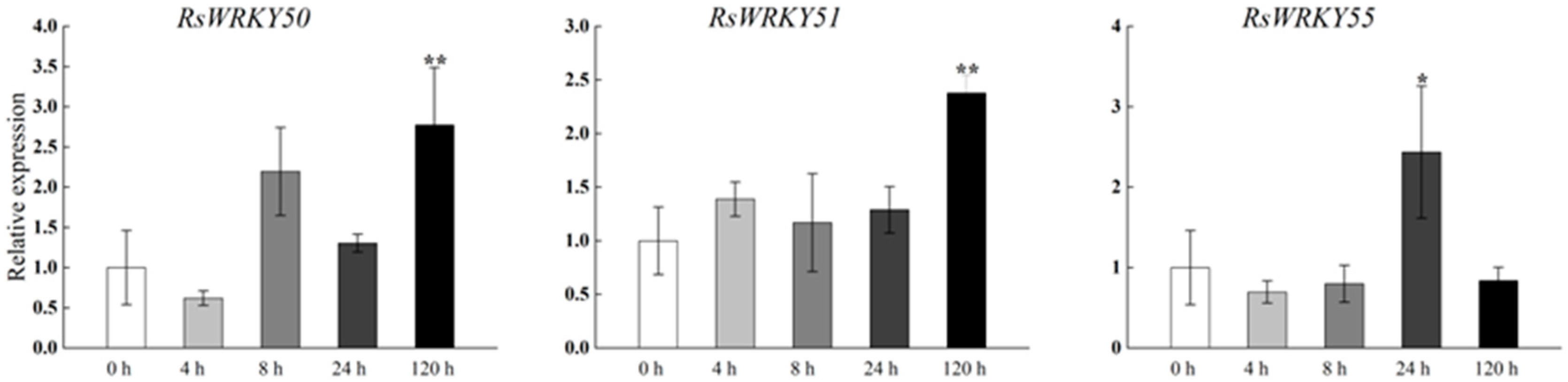
Under high-temperature treatment, RsWRKY2, RsWRKY17, RsWRKY48, RsWRKY49, and RsWRKY50 were up-regulated at all five time points. RsWRKY51 and RsWRKY55 up-regulated first and then dropped rapidly, while RsWRKY3 showed an opposite response to high temperature (Figure 7). At the same time, the drought treatment of Rhododendrons was carried out by withholding the irrigation. RsWRKY2 and RsWRKY17 showed noticeable up-regulation at 8 h, and the expression level of RsWRKY2 was over seven-fold higher at 8 h drought treatment. RsWRKY47, RsWRKY49, RsWRKY50, and RsWRKY51 genes were mainly induced on the fifth day of drought treatment; notably, RsWRKY3 and RsWRKY55 first showed a downward trend at the initial stage but began to reach the highest value on the first day of drought (Figure 8).
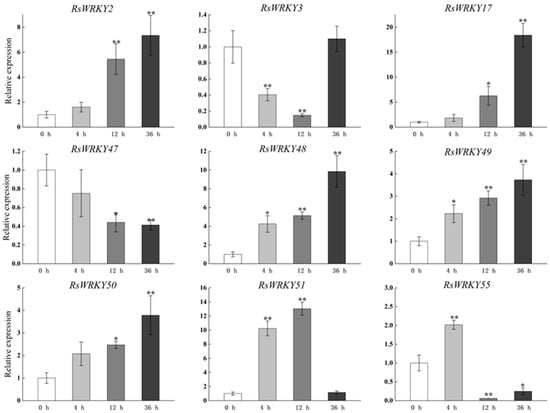
Figure 7.
Expression profiles of RsWRKY genes under heat treatment. Gene expression at 0 h was normalized as “1.” Data are mean ± standard deviation (SD), calculated from three biological replicates, and vertical lines represent the standard deviation. * and ** mean significant difference at p < 0.05 and p < 0.01, respectively.
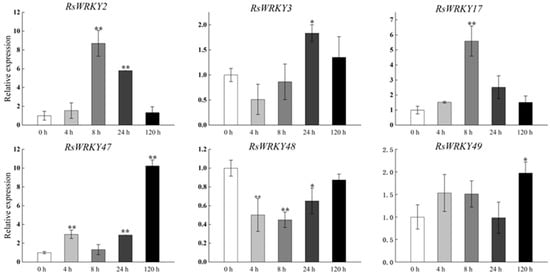
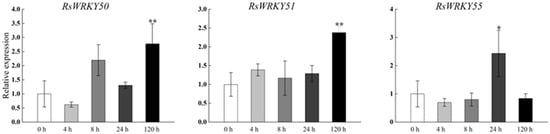
Figure 8.
Expression profiles of RsWRKY genes under drought treatment. Gene expression at 0 h was normalized as “1.” Data are meant ± standard deviation (SD), calculated from three biological replicates, and vertical lines represent the standard deviation. * and ** mean significant difference at p < 0.05 and p < 0.01, respectively.
In summary, we identified some RsWRKY genes that may potentially play a vital role in heat- and drought-stress resistance. This study can provide a meaningful reference for Rhododendron breeding improvement.
3. Discussion
Transcription factors, also known as trans-acting factors [29], exist in the form of a gene superfamily and play a critical regulatory role in the growth and development of plants and their response to the external environment. Thus far, the research on the identification and analysis of transcription factor families from the whole genome is increasing, which has become one of the focuses of genomics research [30], aiming to provide a reference for further study. The WRKY family is one of the ten transcription factor families in higher plants, and WRKY is famous for its highly conserved sequence (WRKYGQK), which is essential for WRKY transcription factor recognition and binding to the W-box element at the target gene promoter [3,7,31]. WRKY transcription factors are involved in a wide range of plant biological processes, including plant growth and development, stress-response mechanism, and secondary metabolite synthesis [32]. With the publication and improvement of plant genome data, many plant WRKY gene family members have been identified at the genome-wide level, and WRKY families were identified in Arabidopsis (72), tomato (104), cucumber (61), Populus trichocarpa (122), and rice (102) [1,2,33,34,35].
In this study, 57 WRKY genes (RsWRKY1–RsWRKY57) were identified in the genome data of R. simsii (Table S1). The phylogenetic tree was constructed with 57 RsWRKY genes and 72 AtWRKY genes, and genes with similar structures were clustered into Groups I, II, and III, respectively (Figure 2). Thirty-four members of Group II were divided into five subgroups: II-a, II-b, II-c, II-d, and II-e. This is consistent with the grouping of model plants Arabidopsis thaliana [10]. In the conserved heptapeptide structure, RsWRKYs had three types of variations (Figure 3). These variations may cause the protein to fail to bind to downstream genes or even lose their gene function. For example, in soybean, GmWRKY6 and GmWRKY21 fail to bind to the downstream gene W-box due to structural changes in the heptapeptide [36]. In terms of protein quantity, proteins of II-c were the largest, accounting for 22.8% of all proteins, followed by proteins of I. Except for RsWRKY16, other members of the Group I all had two WRKY-conserved domains, suggesting that RsWRKY16 may have lost one WRKY-conserved domain during evolution. The loss of the WRKY domain also occurred in the evolution of Arabidopsis, as AtWRKY10 in Group I only had one WRKY domain [14]. Group I is the original ancestor of Group II and Group III in the evolution of the plant WRKY gene family, which are mainly found in lower plants [5]. The gene function of Group I was more conserved than that of Group II and III [37,38]. In terms of evolution, RsWRKYs of Group II are more active than AtWRKYs of Arabidopsis thaliana Group II, which is the main driving force of the expansion of the RsWRKY gene family. It is speculated that members of this group may play specific functions in Rhododendron. Chromosomal mapping analysis showed that RsWRKYs were found on the 13 chromosomes except for chromosome 9 (Figure 1), and this similar phenomenon was also found in grape [39]. It is supposed that the sequencing accuracy and assembly of the genome are not perfect in R. simsii, RsWRKY55–RsWRKY57 sequences cannot be located, and they may be distributed on any chromosome.
A conserved domain motif 1 was discovered in all members of the RsWRKY family through the results of conserved motif analysis and gene structure analysis, which is likely to be the core conserved domain of the RsWRKY family (Figure 4C). Previous studies suggested that the gene encoding one WRKY domain evolved from the gene encoding two WRKY domains; the WRKY domain at the C-terminal is highly conserved, which plays a leading role in determining the binding process of DNA [40]. This explains why the number of proteins containing two WRKY domains is much smaller than that containing one WRKY domain. Cis-acting elements are important molecular switches that regulate gene expression. Comprehensive analysis of the type and number of cis-acting elements can better elucidate the expression patterns and potential biological functions of genes [41,42,43]. We predicted cis-acting elements in these RsWRKY genes and found a variety of cis-elements (Figure 4D), including light-response element, hormone-response element, and stress-response element. We speculate that optical signals, hormones, and biotic and abiotic stress may activate RsWRKY genes for expression, which directly or indirectly regulates various biological processes. Numerous studies have demonstrated that WRKYs can increase resistance to biotic and abiotic stresses, such as osmotic, drought, salt, cold, heat, and UV-B stress [44,45,46,47,48,49]. To further identify candidate genes involved in hormone signaling pathways or abiotic stress responses, we used the qRT-PCR method to measure their expression levels in response to the hormone, drought, and high-temperature treatments. Hormones play a crucial role in plant growth and development and abiotic stress responses. For example, AtWRKY11, AtWRKY17, and AtWRKY70 are essential components of the antagonistic action between JA and SA [25,50]. OsWRKY3 and OsWRKY12 are up-regulated in response to JA, SA, benzothiadiazole, and so on [51]. Furthermore, overexpression of AtWRKY39 in Arabidopsis can improve its tolerance to heat stress, and expression of AtWRKY39 is induced by SA and MeJA and enhances plant heat tolerance [48]. In Camellia sinensis, CsWRKY2 is involved in ABA downstream signaling pathways and drought tolerance [52]. In this study, RsWRKYs responded to exogenous GA and MeJA, and nine RsWRKYs can respond to one of them. RsWRKY49 and RsWRKY51 exhibited a similar trend under GA treatment; moreover, RsWRKY50 and RsWRKY51 were also mainly expressed at 4 h and 12 h treatment (Figure 5 and Figure 6). There are many abiotic stresses that can cause osmotic stress in plants, such as drought, high-salt, and high-temperature stress. The specific expression of a gene usually reflects its corresponding function. In Vitis vinifera, the expressions of VvWRKY24 and VvWRKY49 increased under drought stress, while other genes did not change significantly [53]. Therefore, high temperature and drought were treated as abiotic stresses in this study. RsWRKY17, RsWRKY49, RsWRKY50, and RsWRKY51 were expressed under all treatments; RsWRKY17 was the most responsive to high-temperature treatment (Figure 7). RsWRKY2 and RsWRKY17 were significantly up-regulation at 8 h of drought stress, while RsWRKY47, RsWRKY49, RsWRKY50, and RsWRKY51 genes were mainly expressed on the fifth days (Figure 8). qRT-PCR results showed that differential expression of some RsWRKY genes under hormone and abiotic stress treatments highlight the extensive involvement of WRKY genes in environmental adaptation. We speculated that RsWRKYs may enhance its resistance to drought and heat stress. Whether a single RsWRKY acts alone or multiple RsWRKYs act together under drought stress needs to be further studied. The above findings suggest that transcription factors can act through their involvement in hormone synthesis or hormone-signaling networks. In summary, our findings provide a valuable resource for select candidate RsWRKY genes, which can facilitate the further functional studies of RsWRKYs involved in various biological stresses. Currently, since genetic transformation systems have not been established in most plants, it is necessary to explore the function of genes in enhancing plant resistance by performing functional validation in model plants and analyzing the effects of genes on plant phenotype and physiology [54,55]. Interestingly, the specific regulatory mechanisms in Rhododendron needs to be further studied.
4. Materials and Methods
4.1. Plant Materials and Growth Condition
The Rhododendron “FengGuan” was used throughout the study, which was planted in an artificial climate incubator with a 14 h light/10 h dark photoperiod, 600 μmol m−2 s−1 light intensity, 25 ℃ day/18 ℃ night, and 75% humidity at the JIYANG College of Zhejiang A&F University, Zhejiang, China (29°44′51″ N, 120°15′17″ E). Plants of uniform size were subjected to the hormone, drought, and heat stresses in this experiment. Gibberellic acid (Sigma, Darmstadt, Germany) was dissolved in 1 ml ethanol, then diluted with deionized water, obtaining a final solution with ethanol:water(v/v) ratio of 1:2000 in a solution containing 0.02% Tween-20. Methyl jasmonate (Aladdin, Shanghai, China) solution was prepared in the same way as gibberellin. Hormone treatments were carried out by spraying leaves with 100 µM GA and 200 µM MeJA, respectively; leaves were sampled at 0 h, 4 h, 8 h, 12 h, and 36 h. For drought treatment, irrigation was stopped for 5 days and sampled after 0 h, 4 h, 12 h, 24 h, and 120 h of treatment. For temperature stress, plantlets were exposed to 43 °C and sampled as above for hormone treatment. The collected leaf samples were frozen in liquid nitrogen and stored at −80 °C.
4.2. Identification and Physicochemical Properties of WRKY Genes from R. simsii
Putative RsWRKY gene sequences were downloaded from R. simsii genome data [56], and the Rhododendron genome database (http://bioinfor.kib.ac.cn/RPGD/index.html, accessed on 10 June 2022) has been comprehensively described [57]. All sequences were sent to NCBI (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 10 June 2022) [58] to ensure whether they contained a WRKY-conservative domain; conservative domain prediction software PFAM (http://pfam.xfam.org/, accessed on 10 June 2022) [59] and SMART (http://smart.embl-heidelberg.de/, accessed on 10 June 2022) [60] were used to eliminate duplicate and incomplete sequences. We used PlantRegMap to identify WRKY family members in the R. simsii genome and obtained 63 potential genes that were in accord with the genes listed in the supplementary table by Yang [56]. Four genes (Rhsim10G0148700, Rhsim07G0087600, Rhsim13G0004700, and Rhsim11G0062400) were discovered to lack the WRKYGQK-conserved domain following motif analysis of these 63 putative genes utilizing multiple sequence alignment and online software MEME. Therefore, we removed the aforementioned genes. Additionally, we discovered that Rhsim10G0148800 and RsWRKY40 (Rhsim10G0148600) share a 91.3% similarity in their nucleotide and amino acid sequences. Analogously, RsWRKY55 (RhsimUnG0056300) and RhsimUnG0199600 are comparable, with the comparison similarity reaching 93.12%. The definition of tandem repeat genes by Huang [2] stated that a gene is classified as tandem repeat if the physical distance between two or more homologous genes on the chromosome is less than 100 kb. In view of the similarity and the chromosome, we manually deleted these two repetitive sequences. The physicochemical properties of RsWRKYs members were analyzed by the ProtParam tool on ExPASY (https://web.expasy.org/protparam/, accessed on 10 June 2022) [61]. Including the number of amino acids, molecular weight, theoretical isoelectric point, aliphatic index, and grand average of hydropathicity, the subcellular localization of RsWRKYs was predicted by WoLF PSORT (https://wolfpsort.hgc.jp/, accessed on 10 June 2022) and PLOC (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc, accessed on 10 June 2022).
4.3. Chromosomal Location of RsWRKY Genes in R. simsii
After obtaining the mapping information of the RsWRKY gene family on chromosomes from the DNA annotation file of the R. simsii genome database [57], the online tool MG2C (http://mg2c.iask.in/mg2c_v2.0/, accessed on 10 June 2022) was used to visualize the distribution of RsWRKY genes on barely 13 chromosomes.
4.4. Multiple Sequence Alignment and Phylogenetic Analysis of RsWRKYs
The WRKY proteins of Arabidopsis thaliana were retrieved from TAIR (http://www.arabidopsis.org/, accessed on 10 June 2022). Multiple sequence alignments between RsWRKYs and AtWRKYs were first performed using DNAMAN, and the MEGA6 software was used to construct a phylogenetic tree using the maximum likelihood (ML) method [62], with partial deletion of 1000 bootstraps and a WAG model. iTOL (https://itol.embl.de/, accessed on 10 June 2022) [63] was used to visualize and beautify the phylogenetic tree.
4.5. Analysis of the Gene Structure, Conserved Motifs, and Cis-Acting Elements of RsWRKYs
The website Gene Structure Display Server (GSDS) (http://gsds.cbi.pku.edu.cn/, accessed on 10 June 2022) was employed to determine the exon-intron distribution from DNA sequences and the coding domain sequences (CDS). A common motif in a gene family is likely to be a key sequence that performs the corresponding function or an essential sequence in the gene family. The conserved motifs of 57 RsWRKYs were detected and analyzed by MEME (http://meme-suite.org/tools/meme, accessed on 10 June 2022) [64]. The number of motif software parameters was set to 15, and the default values for other parameters were used. Fifty-seven promoter sequences corresponding to WRKY upstream 2 kb were extracted from R. simsii chromosome genome data; then, we used PlantCARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 10 June 2022) [65] to identify transcription factor binding sites. To analyze promoter specificity under multiple abiotic stresses, low temperature, heat stress, drought, hormones responses, and salt response, motifs were selected.
4.6. Expression Profile Analysis of RsWRKY Genes by qRT-PCR
Total RNA was isolated by FastPure Plant Total RNA Isolation Kit (Polysaccharides and Polyphenolics-rich) (Vazyme, Nanjing, China); then, reverse 1 μg DNA-free RNA was transcribed into cDNA using HiScript III All-in-one RT SuperMix Perfect for qPCR (Vazyme). Gene-specific primers (Table S2) were designed using Primer Premier5 and synthesized by Tsingke (Beijing, China). Individual qRT-PCR reactions contained 5 μL 2 × ChamQ Universal SYBR qPCR Master Mix, 2 μL cDNA of 25-times diluted cDNA, and 0.2 μL forward and reverse primers and was filled with deionized water to 10 μL. The reaction was carried out as follows: 30 s at 95 °C for denaturation, followed by 40 cycles of 10 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as an internal control [66] to normalize the measured gene expression levels based on the standard curves, and the Ct values were automatically calculated by LightCycler® 480 II (Roche Diagnostics, Germany) software. Finally, the relative expression of the target gene was calculated according to the 2−ΔΔCT method [67]. SPSS 20.0 software (IBM Corporation, Armonk, NY, USA) was used to perform one-way ANOVA for all of the experimental data, and Duncan’s test was used to count the significant difference among all samples (p < 0.05). Then, all data were visualized via Origin 9.0 (Northampton, MA, USA). Three biological replicates were performed for each sample to ensure reliability.
5. Conclusions
In this paper, we identified 57 RsWRKY transcription factors at the genome level. Physicochemical properties, gene structure, motif composition, cis-acting elements, chromosomal localization, phylogenetic relationship, and differential gene expression under four abiotic stresses were demonstrated in this study. Phylogenetic tree analysis showed that RsWRKY family members were divided into seven groups (I, II a–e, and III) with similar motif-distribution patterns. Promoter analysis revealed that RsWRKYs contained cis-regulatory elements related to hormones and stress response. RsWRKYs were highly conserved in the evolutionary process, and most of them were distributed on 13 chromosomes of R. simsii. We found that RsWRKYs in this family were highly conserved at gene structure and evolutional levels. Expression profiles of RsWRKY genes responding to hormone and abiotic stresses were observed. Our results may contribute to selecting appropriate candidate genes involved in hormones and stress responses in Rhododendron species and to lay a foundation for mechanism research and practical application of the WRKY transcription factor in Rhododendron through the combined efforts of our group.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11212967/s1, Figure S1: The specific sequence information of 15 motifs. The letters represent amino acids, the color does not represent any meaning, which are automatically synthesized by the MEME, and the height of the letters represents the motifs conservation, Table S1: Physicochemical properties of WRKY transcription factors in Rhododendron simsii, Table S2: Primers of the RsWRKY genes for qRT-PCR.
Author Contributions
Conceptualization, Z.W. and S.J.; methodology, Z.W. and H.C.; validation, X.L. and J.Z.; formal analysis, Z.W. and H.C.; investigation, Z.W., X.L. and Y.C.; resources, Y.X.; data curation, Z.W. and X.L.; writing—original draft preparation, Z.W.; writing—review and editing, Y.X. and S.J.; supervision, Y.C.; project administration, Y.X. and S.J.; funding acquisition, Y.X. and S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China (2019YFE0118900), the National Natural Science Foundation of China (31971641), Shaoxing City Domestic and Overseas Talents Project (RC2022B05), and the research developmental fund of Jiyang College of Zhejiang agriculture and forestry university (RQ2020B15).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ulker, B.; Somssich, I.E. WRKY transcription factors: From DNA binding towards biological function. Curr. Opin. Plant Biol. 2004, 7, 491–498. [Google Scholar] [CrossRef]
- Huang, S.X.; Gao, Y.F.; Liu, J.K.; Peng, X.L.; Niu, X.L.; Fei, Z.J.; Liu, Y.S. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol. Genet. Genom. 2012, 287, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Gu, Y.B.; Ji, Z.R.; Chi, F.M.; Qiao, Z.; Xu, C.N.; Zhang, J.X.; Zhou, Z.S.; Dong, Q.L. Genome-wide identification and expression analysis of the WRKY gene family in peach. Hereditas 2016, 38, 254–270. [Google Scholar] [PubMed]
- Brand, L.H.; Fischer, N.M.; Harter, K.; Kohlbacher, O.; Wanke, D. Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays. Nucleic Acids Res. 2013, 41, 9764–9778. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Hao, Z.N.; Xie, K.; Wu, K.L.; Guo, Z.J. Leucine zipper like structure in rice WRKY89 enhances its affinity for binding with W box elements. Chinese Sci Bull. 2005, 50, 980–989. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wu, J.; Wang, Y.P.; Sun, Q.F. Advances in the function of WRKY transcription factor in plant stress. Mol. Plant Breed. 2020, 18, 7413–7422. [Google Scholar]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, M.; Liu, Q.; Miura, S.; Shinozaki, K. Improving plant drought, salt and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1999, 17, 287–291. [Google Scholar] [CrossRef]
- Valliyodan, B.; Nguyen, H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 2006, 9, 189–195. [Google Scholar] [CrossRef]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and β-amylase from sweet potato. Mol. Gen. Genet. 1994, 244, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Pater, S.D.; Greco, V.; Pham, K.; Memelink, J.; Kijne, J. Characterization of a zinc-dependent transcriptional activator from Arabidopsis. Nucleic Acids Res. 1996, 24, 4624–4631. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Lee, S.H.; Park, H.C.; Bae, C.G.; Cheong, Y.H.; Choi, Y.J.; Han, C.D.; Lee, S.Y.; Lim, C.O.; Cho, M.J. Identification of rice blast fungal elicitor-responsive genes by differential display analysis. Mol. Plant-Microbe Interact. 2000, 13, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Jiang, W.J.; Zhang, Y.; Yu, H.J.; Gu, X.F.; Huang, S.W.; Xie, B.Y. Genome-wide analysis of WRKY gene family in Cucumis sativus. BMC Genom. 2011, 12, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.R.; Zhang, S.Z.; Cao, H.; Su, H.R. Bioinformatics Analysis of WRKY Transcription Factor Genes Family in Apple. Acta Hortic. Sin. 2012, 39, 2049–2060. [Google Scholar]
- Dong, J.X.; Chen, C.H.; Chen, Z.X. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Deyholos, M.K. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol. Biol. 2009, 69, 91–105. [Google Scholar] [CrossRef]
- Peng, X.; Hu, Y.J.; Tang, X.K.; Zhou, P.L.; Deng, X.B.; Wang, H.H.; Guo, Z.J. Constitutive expression of rice WRKY30 gene increases the endogenous jasmonic acid accumulation, PR gene expression and resistance to fungal pathogens in rice. Planta 2012, 236, 1485–1498. [Google Scholar] [CrossRef]
- Vivian, V.E.; Busanello, C.; Da, L.C.; Pegoraro, C. Activation of rice WRKY transcription factors: An army of stress fighting soldiers? Curr. Opin. Plant Biol. 2018, 45, 268–275. [Google Scholar]
- Xiong, X.; James, V.A.; Zhang, H.; Altpeter, F. Constitutive expression of the barley HvWRKY38 transcription factor enhances drought tolerance in turf and forage grass (Paspalum notatum Flugge). Mol. Breed. 2010, 25, 419–432. [Google Scholar] [CrossRef]
- Wu, X.L.; Shiroto, Y.; Kishitani, S.; Ito, Y.; Toriyama, K. Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep. 2009, 28, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.F.; Wei, W.; Zhou, Q.Y.; Tian, A.G.; Hao, Y.J.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, Z.B.; Chen, S.Y. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ. 2012, 35, 1156–1170. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chen, Z. Identification of genes encoding receptor-like protein kinases as possible targets of pathogen- and salicylic acid-induced WRKY DNA-binding proteins in Arabidopsis. Plant J. 2000, 24, 837–847. [Google Scholar] [CrossRef]
- Gao, Q.M.; Venugopal, S.; Navarre, D.; Kachroo, A. Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 proteins. Plant Physiol. 2011, 155, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Journot-catalino, N.; Somssich, I.E.; Roby, D.; Kroj, T. The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell 2006, 18, 3289–3302. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Sun, W.H.; Yang, G.Y.; Sun, J. WRKY transcription factors in legumes. BMC Plant Biol. 2018, 18, 243. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.J.; Li, M.J.; Yuan, T.H. A dataset on wild Rhododendron and geographical distribution information in China. Biodivers. Sci. 2021, 29, 1175–1180. [Google Scholar] [CrossRef]
- Zheng, Y. Drought resistance evaluation of six newly introduced Rhododendron varieties. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2018. [Google Scholar]
- Wu, L.T.; Du, C.F.; Zhang, M.Q.; Zhou, L.; Han, H.S. The structure and function of WRKY transcription factors in abiotic and biotic stress. Mol. Plant Breed. 2013, 11, 634–638. [Google Scholar]
- Cai, B.; Yang, X.H.; Tuskan, G.A.; Cheng, Z.M. MicroSyn: A user-friendly tool for detection of microsynteny in a gene family. BMC Bioinform. 2011, 12, 79. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, L.J. The WRKY transcription factor superfamily: Its origin in eukaryotes and expansion in plants. BMC Evol. Biol. 2005, 5, 1–12. [Google Scholar] [CrossRef]
- Zhao, C.L.; Chang, L.; Lv, Y.T.; Zhang, J.; Dong, J.J.; Liu, S.F.; Jia, X.Y. Cloning and expression analysis of IbWRKY44 from sweet potato. Shanxi Agric. Sci. 2021, 49, 1507–1514. [Google Scholar]
- Chen, C.H.; Chen, X.Q.; Han, J.; Liu, W.L.; Ren, Z.H. Genome-wide analysis of the WRKY gene family in the cucumber genome and transcriptome-wide identification of WRKY transcription factors that respond to biotic and abiotic stresses. BMC Plant Biol. 2020, 20, 443. [Google Scholar] [CrossRef]
- Zhou, J.; Zeng, M.Y.; An, X.M. Identification of Populus trichocarpa WRKY gene family and its’ response to drought stress. Chin. J. Cell Biol. 2019, 41, 2160–2173. [Google Scholar]
- Ross, C.A.; Liu, Y.; Shen, Q.J. The WRKY gene family in rice (Oryza sativa). J. Integr. Plant Biol. 2007, 49, 827–842. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Tian, A.G.; Zou, H.F.; Xie, Z.M.; Lei, G.; Huang, J.; Wang, H.W.; Zhang, J.S.; Chen, S.Y. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008, 5, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Villano, C.; Esposito, S.; Damelia, V.; Garramone, R.; Daniela, A.; Astolfo, Z.; Riccardo, A.; Domenico, C. WRKY genes family study reveals tissue-specific and stress-responsive TFs in wild potato species. Sci. Rep. 2020, 10, 7196. [Google Scholar] [CrossRef]
- Cheng, Y.; Ahammed, G.J.; Yao, Z.P.; Ye, Q.J.; Ruan, M.Y.; Wang, R.Q.; Li, Z.M.; Zhou, G.Z. Comparative genomic analysis reveals extensive genetic variations of WRKYs in Solanaceae and functional variations of CaWRKYs in pepper. Front. Genet. 2019, 10, 492. [Google Scholar] [CrossRef]
- Li, C.H.; Cai, B. Genome-wide analysis of the WRKY transcription factor gene family in grape. Nonwood Forest Res. 2013, 31, 127–131. [Google Scholar]
- Wu, K.L.; Guo, Z.J.; Wang, H.H.; Li, J. The family of transcription factors in rice and Arabidopsis and their origins. DNA Res. 2005, 12, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef]
- Hu, J.F.; Fang, H.C.; Wang, J.; Mao, Z.L.; Zou, Q.; Jiang, H.Y.; Guo, Z.W.; Yu, L.; Feng, T.; Lu, L.; et al. Ultraviolet B-induced MdWRKY72 expression promotes anthocyanin synthesis in apple. Plant Sci. 2020, 292, 110377. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.J.; Ren, Q.Y.; Chen, Y.L.; Xu, G.L.; Qian, Y.X. Genome-wide identification and analysis of WRKY gene family in maize provide insights into regulatory network in response to abiotic stresses. BMC Plant Biol. 2021, 21, 427. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Zhang, L.P.; Yu, D.Q. Wounding-induced WRKY8 is involved in basal defense in Arabidopsis. Mol. Plant-Microbe Interact. 2010, 23, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.X.; Fan, X.X.; Hao, J.; Liu, G.C.; Zhang, Z.; Liu, X. Negative regulation by transcription factor VvWRKY13 in drought stress of Vitis vinifera L. Plant Physiol. Biochem. 2020, 148, 114–121. [Google Scholar] [CrossRef]
- More, P.; Agarwal, P.; Joshi, P.S.; Agarwal, P.K. The JcWRKY tobacco transgenics showed improved photosynthetic efficiency and wax accumulation during salinity. Sci. Rep. 2019, 9, 19617. [Google Scholar] [CrossRef]
- Zou, C.; Jiang, W.; Yu, D. Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. J. Exp. Bot. 2010, 61, 3901–3914. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhou, X.; Chen, L.G.; Huang, W.D.; Yu, D.Q. Functional characterization of Arabidopsis thaliana WRKY39 in heat stress. Mol. Cells 2010, 29, 475–483. [Google Scholar] [CrossRef]
- Wang, H.H.; Hao, J.J.; Chen, X.J.; Hao, Z.N.; Wang, X.; Lou, Y.G.; Peng, Y.L.; Guo, Z.J. Overexpression of rice WRKY89 enhances ultraviolet B tolerance and disease resistance in rice plants. Plant Mol. Biol. 2007, 65, 799–815. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell. 2004, 16, 319–331. [Google Scholar] [CrossRef]
- Liu, X.Q.; Bai, X.Q.; Qian, Q.; Wang, X.J.; Chen, M.S.; Chu, C.C. OsWRKY03, a rice transcriptional activator that functions in defense signaling pathway upstream of OsNPR1. Cell Res. 2005, 15, 593–603. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, Z.; Wang, W.; Jiang, X.; Li, D.; Pan, J.; Li, X. CsWRKY2, a novel WRKY gene from Camellia sinensis, is involved in cold and drought stress responses. Biol. Plant. 2016, 60, 443–451. [Google Scholar] [CrossRef]
- Su, L.; Wang, P.F.; Yang, Y.; Ren, F.S.; Wang, Y.M.; Chen, W.J. Identification and analysis of grape whole genome WRKY transcription factors. Heilongjiang Agric. Sci. 2019, 295, 21–30. [Google Scholar]
- Ullah, A.; Sun, H.; Hakim; Yang, X.Y.; Zhang, X.L. A novel cotton WRKY gene, GhWRKY6-like, improves salt tolerance by activating the ABA signaling pathway and scavenging of reactive oxygen species. Physiol. Plant. 2018, 162, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jiang, Y.N.; Guo, Y.; Huang, J.B.; Zhou, M.H.; Tang, Y.Y.; Sui, J.M.; Wang, J.S.; Qiao, L.X. A novel salt inducible WRKY transcription factor gene, AhWRKY75, confers salt tolerance in transgenic peanut. Plant Physiol. Biochem. 2021, 160, 175–183. [Google Scholar] [CrossRef]
- Yang, F.S.; Nie, S.; Liu, H.; Shi, T.L.; Tian, X.C.; Zhou, S.S.; Bao, Y.T.; Jia, K.H.; Guo, J.F.; Zhao, W.; et al. Chromosome-level genome assembly of a parent species of widely cultivated azaleas. Nat. Commun. 2020, 11, 5269. [Google Scholar] [CrossRef]
- Liu, N.Y.W.; Zhang, L.; Zhou, Y.L.; Tu, M.L.; Wu, Z.Z.; Gui, D.P.; Ma, Y.P.; Wang, J.H.; Zhang, C.J. The Rhododendron Plant Genome Database (RPGD): A comprehensive online omics database for Rhododendron. BMC Genom. 2021, 22, 376. [Google Scholar] [CrossRef]
- Marcher-bauer, A.; Bo, Y.; Han, L.Y.; He, J. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Elgebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A.M. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evolution. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Firth, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Y.F.; Zhang, M.C.; Du, G.H.; Wang, J.H. Selection and Evaluation of Candidate Reference Genes for Quantitative Real-Time PCR in Aboveground Tissues and Drought Conditions in Rhododendron Delavayi. Front. Genet. 2022, 13, 876482. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).