Effects of Chilling Treatment on Baicalin, Baicalein, and Wogonin Biosynthesis in Scutellaria baicalensis Plantlets
Abstract
1. Introduction
2. Results
2.1. HPLC Analysis of Baicalin, Baicalein, and Wogonin in Roots of S. baicalensis Plantlets after Chilling Treatment
2.2. HPLC Analysis of Baicalin, Baicalein, and Wogonin in Shoots of S. baicalensis Plantlets after Chilling Treatment
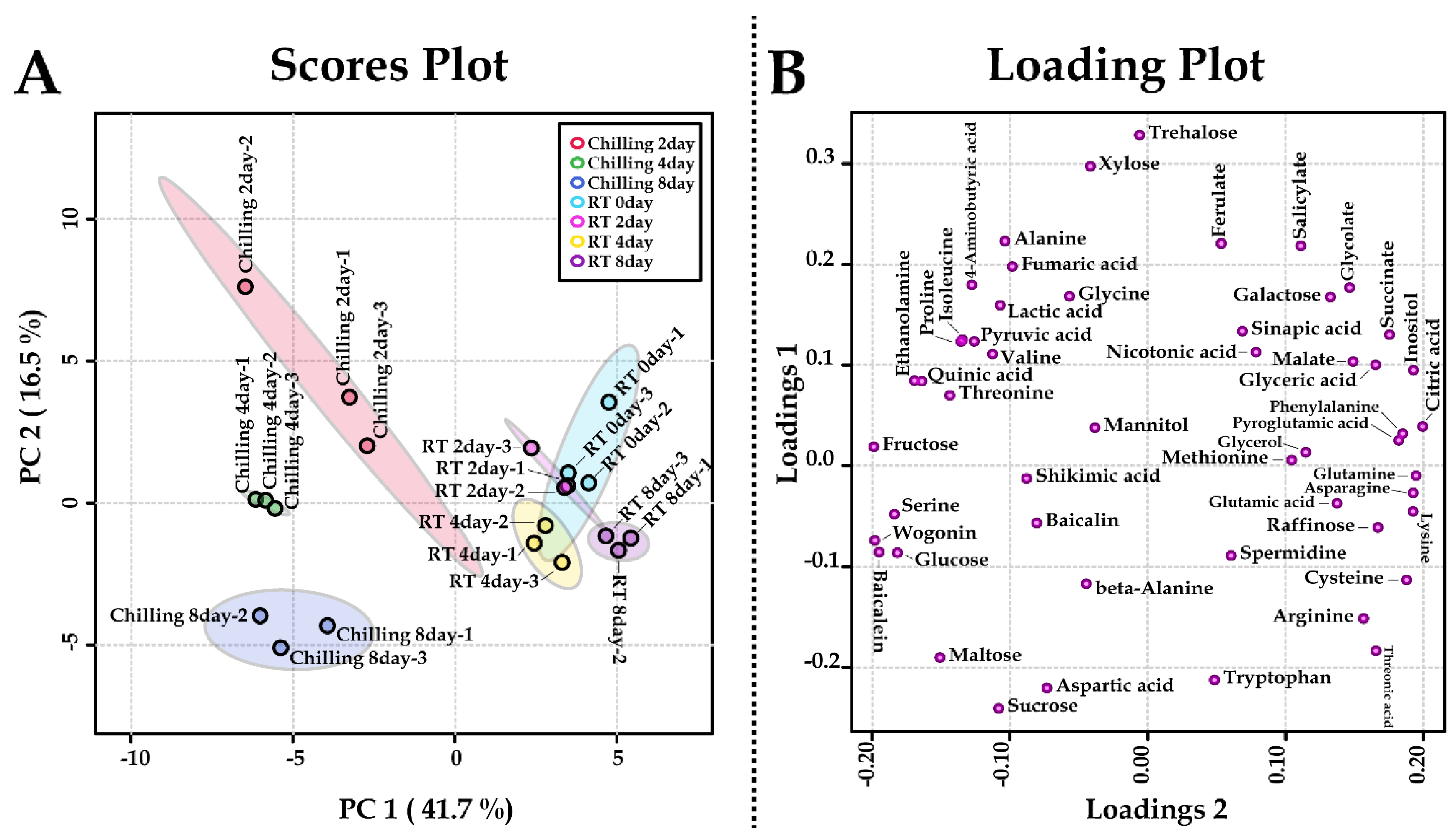
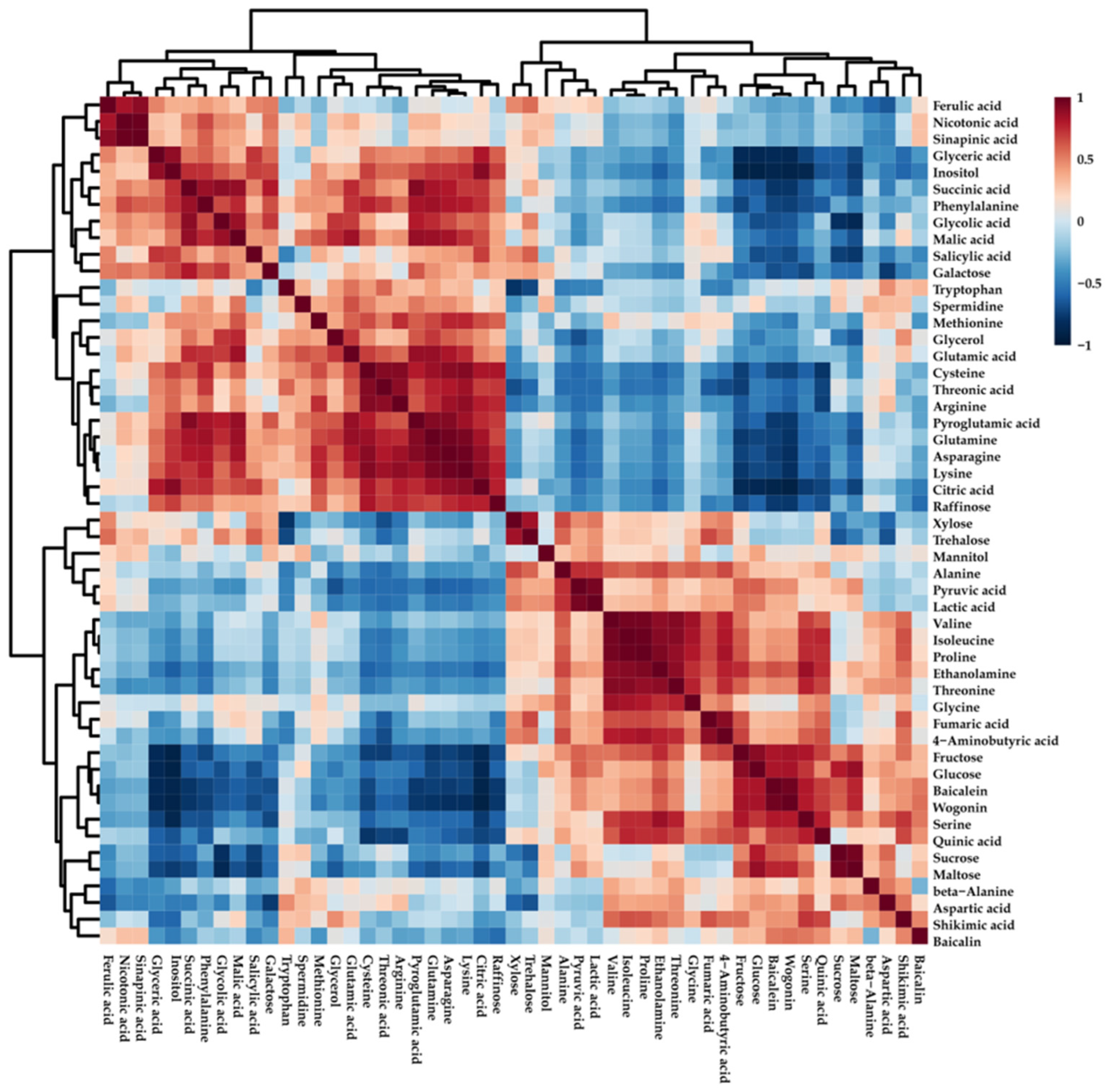
2.3. Metabolite Profiling of Roots of S. baicalensis after Chilling Treatment
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. HPLC Analysis of Baicalin, Baicalein, and Wogonin
4.3. GC-TOF-MS Analysis
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, M. Crop plants and abiotic stresses. J. Biomol. Res. Ther. 2013, 3, 1. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K.; Seki, M. Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 2003, 6, 410–417. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Liu, G.; Rajesh, N.; Wang, X.; Zhang, M.; Wu, Q.; Li, S.; Chen, B.; Yao, S. Identification of flavonoids in the stems and leaves of Scutellaria baicalensis Georgi. J. Chromatogr. B 2011, 879, 1023–1028. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, R.; Pu, X.; Xu, R.; Wang, J.; Zheng, S.; Zeng, Y.; Chen, J.; He, C.; Song, J. Comparative genome analysis of Scutellaria baicalensis and Scutellaria barbata reveals the evolution of active flavonoid biosynthesis. Genom. Proteom. Bioinform. 2020, 18, 230–240. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, X.-Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Y.; Wang, G.; Hill, L.; Weng, J.-K.; Chen, X.-Y.; Xue, H.; Martin, C. A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci. Adv. 2016, 2, e1501780. [Google Scholar] [CrossRef]
- Hummel, I.; El Amrani, A.; Gouesbet, G.; Hennion, F.; Couée, I. Involvement of polyamines in the interacting effects of low temperature and mineral supply on Pringlea antiscorbutica (Kerguelen cabbage) seedlings. J. Exp. Bot. 2004, 55, 1125–1134. [Google Scholar] [CrossRef]
- Kovács, Z.; Simon-Sarkadi, L.; Szűcs, A.; Kocsy, G. Differential effects of cold, osmotic stress and abscisic acid on polyamine accumulation in wheat. Amino Acids 2010, 38, 623–631. [Google Scholar] [CrossRef]
- Lei, X.Y.; Zhu, R.Y.; Zhang, G.Y.; Dai, Y.R. Attenuation of cold-induced apoptosis by exogenous melatonin in carrot suspension cells: The possible involvement of polyamines. J. Pineal Res. 2004, 36, 126–131. [Google Scholar] [CrossRef]
- Pérez-Ilzarbe, J.; Hernández, T.; Estrella, I.; Vendrell, M. Cold storage of apples (cv. Granny Smith) and changes in phenolic compounds. Z. Lebensm. Unters. Forsch. 1997, 204, 52–55. [Google Scholar] [CrossRef]
- Christie, P.J.; Alfenito, M.R.; Walbot, V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 1994, 194, 541–549. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Amarowicz, R.; Weidner, S. The effects of cold stress on the phenolic compounds and antioxidant capacity of grapevine (Vitis vinifera L.) leaves. J. Plant Physiol. 2015, 189, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, R.; Abdollahi Mandoulakani, B.; Fattahi, M. Cold stress changes antioxidant defense system, phenylpropanoid contents and expression of genes involved in their biosynthesis in Ocimum basilicum L. Sci. Rep. 2020, 10, 5290. [Google Scholar] [CrossRef] [PubMed]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Bryant, J.P.; Chapin, F.S., III; Klein, D.R. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Flavonoids are determinants of freezing tolerance and cold acclimation in Arabidopsis thaliana. Sci. Rep. 2016, 6, 34027. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J.K.; Wu, Q.; Park, S.U. Effects of cold stress on transcripts and metabolites in tartary buckwheat (Fagopyrum tataricum). Environ. Exp. Bot. 2018, 155, 488–496. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef] [PubMed]
- Kogan, M.J.; Kristoff, G.; Benavides, M.P.; Tomaro, M.L. Effect of pre-treatment with ethanolamine on the response of Helianthus annuus L. to salt stress. Plant Growth Regul. 2000, 30, 87–94. [Google Scholar] [CrossRef]
- Haider, S.; Raza, A.; Iqbal, J.; Shaukat, M.; Mahmood, T. Analyzing the regulatory role of heat shock transcription factors in plant heat stress tolerance: A brief appraisal. Mol. Biol. Rep. 2022, 49, 5771–5785. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Iqbal, J.; Naseer, S.; Yaseen, T.; Shaukat, M.; Bibi, H.; Ahmad, Y.; Daud, H.; Abbasi, N.L.; Mahmood, T. Molecular mechanisms of plant tolerance to heat stress: Current landscape and future perspectives. Plant Cell Rep. 2021, 40, 2247–2271. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Iqbal, J.; Shaukat, M.; Naseer, S.; Mahmood, T. The epigenetic chromatin-based regulation of somatic heat stress memory in plants. Plant Gene 2021, 27, 100318. [Google Scholar] [CrossRef]
- Lloyd, J.C.; Zakhleniuk, O.V. Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. J. Exp. Bot. 2004, 55, 1221–1230. [Google Scholar] [CrossRef]
- Park, C.H.; Xu, H.; Yeo, H.J.; Park, Y.E.; Hwang, G.-S.; Park, N.I.; Park, S.U. Enhancement of the flavone contents of Scutellaria baicalensis hairy roots via metabolic engineering using maize Lc and Arabidopsis PAP1 transcription factors. Metab. Eng. 2021, 64, 64–73. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Park, Y.E.; Kim, Y.J.; Park, C.; Kim, J.K.; Park, S.U. Integrated analysis of transcriptome and metabolome and evaluation of antioxidant activities in Lavandula pubescens. Antioxidants 2021, 10, 1027. [Google Scholar] [CrossRef]
- Park, C.H.; Park, Y.E.; Yeo, H.J.; Yoon, J.S.; Park, S.-Y.; Kim, J.K.; Park, S.U. Comparative analysis of secondary metabolites and metabolic profiling between diploid and tetraploid Morus alba L. J. Agric. Food Chem. 2021, 69, 1300–1307. [Google Scholar] [CrossRef]
- Zhao, S.; Park, C.H.; Yang, J.; Yeo, H.J.; Kim, T.J.; Kim, J.K.; Park, S.U. Molecular characterization of anthocyanin and betulinic acid biosynthesis in red and white mulberry fruits using high-throughput sequencing. Food Chem. 2019, 279, 364–372. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Kim, Y.J.; Nguyen, B.V.; Park, Y.E.; Sathasivam, R.; Kim, J.K.; Park, S.U. Profiles of secondary metabolites (phenolic acids, carotenoids, anthocyanins, and galantamine) and primary metabolites (carbohydrates, amino acids, and organic acids) during flower development in Lycoris radiata. Biomolecules 2021, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yeo, H.J.; Park, Y.E.; Baek, S.-A.; Kim, J.K.; Park, S.U. Transcriptome analysis and metabolic profiling of Lycoris radiata. Biology 2019, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kim, Y.S.; Li, X.; Kim, H.H.; Arasu, M.V.; Al-Dhabi, N.A.; Lee, S.Y.; Park, S.U. Influence of different carbohydrates on flavonoid accumulation in hairy root cultures of Scutellaria baicalensis. Nat. Prod. Commun. 2016, 11, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Role of salicylic acid in induction of plant defense system in chickpea (Cicer arietinum L.). Plant Signal. Behav. 2011, 6, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.-J.; Park, C.-H.; Park, S.-Y.; Chung, S.-O.; Kim, J.-K.; Park, S.-U. Metabolic analysis of root, stem, and leaf of Scutellaria baicalensis plantlets treated with different LED lights. Plants 2021, 10, 940. [Google Scholar] [CrossRef]
- Park, C.H.; Park, S.-Y.; Park, Y.J.; Kim, J.K.; Park, S.U. Metabolite profiling and comparative analysis of secondary metabolites in Chinese cabbage, radish, and hybrid xBrassicoraphanus. J. Agric. Food Chem. 2020, 68, 13711–13719. [Google Scholar] [CrossRef]
- Breitel, D.; Brett, P.; Alseekh, S.; Fernie, A.R.; Butelli, E.; Martin, C. Metabolic engineering of tomato fruit enriched in L-DOPA. Metab. Eng. 2021, 65, 185–196. [Google Scholar] [CrossRef]
- Hoffmann, S.L.; Kohlstedt, M.; Jungmann, L.; Hutter, M.; Poblete-Castro, I.; Becker, J.; Wittmann, C. Cascaded valorization of brown seaweed to produce L-lysine and value-added products using Corynebacterium glutamicum streamlined by systems metabolic engineering. Metab. Eng. 2021, 67, 293–307. [Google Scholar] [CrossRef]
- Lawson, C.E.; Christopher, E.; Martí, J.M.; Radivojevic, T.; Jonnalagadda, S.V.R.; Gentz, R.; Hillson, N.J.; Peisert, S.; Kim, J.; Simmons, B.A.; et al. Machine learning for metabolic engineering: A review. Metab. Eng. 2021, 63, 34–60. [Google Scholar] [CrossRef]

| Duration (Day) | Control | Chilling Treatment | |
|---|---|---|---|
| Baicalin | 0 | 63.43 ± 7.99 ab | |
| 2 | 57.71 ± 4.02 b | 66.48 ± 3.27 b | |
| 4 | 70.18 ± 4.36 a | 86.08 ± 4.21 a | |
| 8 | 65.63 ± 3.98 ab | 78.44 ± 14.86 a | |
| Baicalein | 0 | 12.55 ± 4.96 c | |
| 2 | 9.09 ± 3.91 c | 22.28 ± 4.26 b | |
| 4 | 12.08 ± 5.20 c | 32.54 ± 4.20 a | |
| 8 | 9.08 ± 3.17 c | 33.63 ± 6.55 a | |
| Wogonin | 0 | 1.60 ± 0.61 d | |
| 2 | 0.98 ± 0.12 d | 3.06 ± 0.45 c | |
| 4 | 1.26 ± 0.37 d | 4.48 ± 0.43 a | |
| 8 | 0.73 ± 0.21 d | 4.57 ± 1.13 b |
| Duration (Day) | Control | Chilling Treatment | |
|---|---|---|---|
| Baicalin | 0 | 0.77 ± 0.05 c | |
| 2 | 1.15 ± 0.18 b | 1.23 ± 0.08 b | |
| 4 | 0.83 ± 0.25 c | 0.51 ± 0.02 d | |
| 8 | 0.55 ± 0.37 d | 2.01 ± 0.09 a | |
| Baicalein | 0 | 0.04 ± 0.02 c | |
| 2 | 0.12 ± 0.05 b | 0.12 ± 0.02 b | |
| 4 | 0.17 ± 0.03 a | 0.06 ± 0.02 c | |
| 8 | 0.04 ± 0.00 c | 0.04 ± 0.00 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, H.J.; Park, C.H.; Kim, J.K.; Sathasivam, R.; Jeong, J.C.; Kim, C.Y.; Park, S.U. Effects of Chilling Treatment on Baicalin, Baicalein, and Wogonin Biosynthesis in Scutellaria baicalensis Plantlets. Plants 2022, 11, 2958. https://doi.org/10.3390/plants11212958
Yeo HJ, Park CH, Kim JK, Sathasivam R, Jeong JC, Kim CY, Park SU. Effects of Chilling Treatment on Baicalin, Baicalein, and Wogonin Biosynthesis in Scutellaria baicalensis Plantlets. Plants. 2022; 11(21):2958. https://doi.org/10.3390/plants11212958
Chicago/Turabian StyleYeo, Hyeon Ji, Chang Ha Park, Jae Kwang Kim, Ramaraj Sathasivam, Jae Cheol Jeong, Cha Young Kim, and Sang Un Park. 2022. "Effects of Chilling Treatment on Baicalin, Baicalein, and Wogonin Biosynthesis in Scutellaria baicalensis Plantlets" Plants 11, no. 21: 2958. https://doi.org/10.3390/plants11212958
APA StyleYeo, H. J., Park, C. H., Kim, J. K., Sathasivam, R., Jeong, J. C., Kim, C. Y., & Park, S. U. (2022). Effects of Chilling Treatment on Baicalin, Baicalein, and Wogonin Biosynthesis in Scutellaria baicalensis Plantlets. Plants, 11(21), 2958. https://doi.org/10.3390/plants11212958








