Intercropping Salt-Sensitive Lactuca sativa L. and Salt-Tolerant Salsola soda L. in a Saline Hydroponic Medium: An Agronomic and Physiological Assessment
Abstract
1. Introduction
2. Results and Discussion
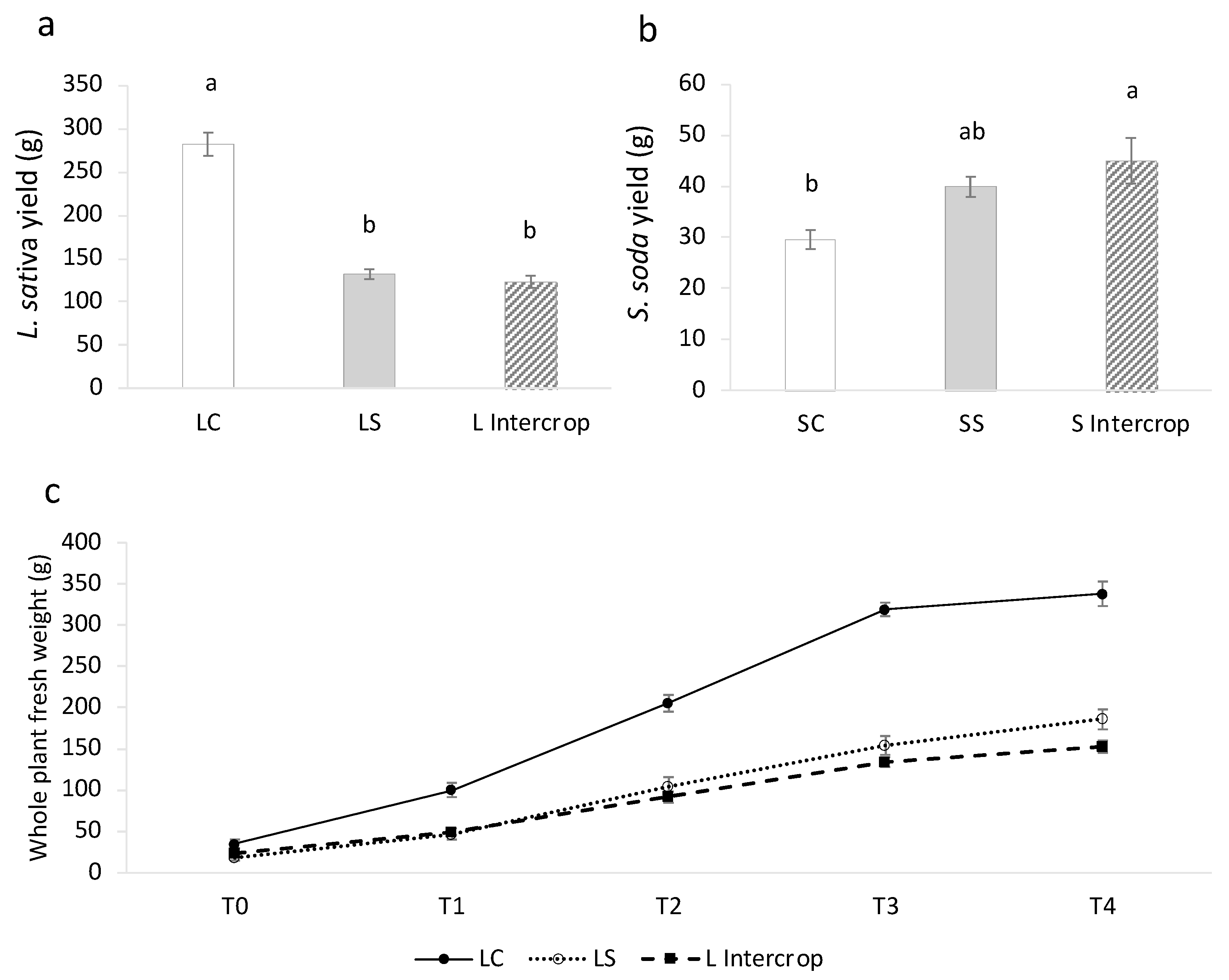
2.1. Different Salinity-Induced Response among the Tested Crops
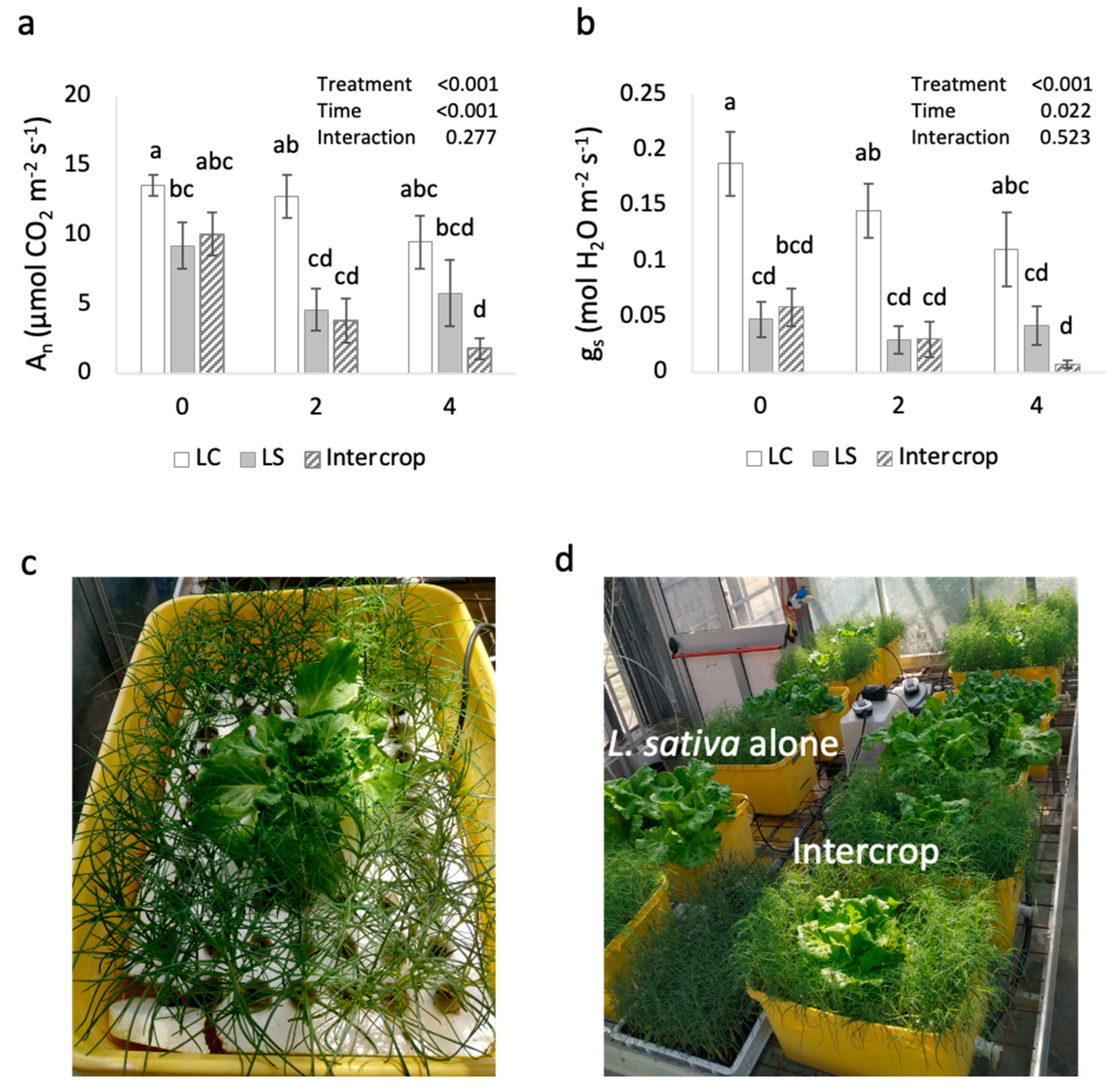
2.2. Physiological Response Suggests No Benefit Due to Intercropping under Saline Conditions
2.3. Nutritional Profile of L. sativa and S. soda
3. Materials and Methods
3.1. Plant Material, Growth Conditions and Experimental Design
- LC: L. sativa plants grown on modified Hoagland solution (2 plastic containers with 3 plants each, n = 6);
- LS: L. sativa plants grown on modified Hoagland solution with a 15% share of seawater, final EC of 12 dS m−1 (2 plastic containers with 3 plants each, n = 6);
- SC: S. soda plants grown on modified Hoagland solution (1 plastic container with 56 plants, n = 56);
- SS: S. soda plants grown on modified Hoagland solution with a 15% share of seawater, final EC of 12 dS m−1 (1 plastic container with 56 plants, n = 56);
- Intercrop: L. sativa and S. soda plants intercropped (1 L. sativa: 50 S. soda; ratio chosen based on the results of a previous publication assessing successful desalination by intercropping 1 pepper plant: 60 S. soda in a soil-based media; [18]) on modified Hoagland solution with a 15% share of seawater, final EC of 12 dS m−1 (6 plastic containers with 1 L. sativa and 50 S. soda plants each, n = 6 for lettuce and n = 300 for S. soda).
3.2. Growth and Biomass Yield Assessment
3.3. Physiological Parameters: Photosynthesis, Fluorescence, and Pigments
3.4. Concentration of Mineral Elements in Plant Tissues
3.5. Concentration of Carbohydrates, Proteins, Polyphenols, Proline and Nitrates in Plants Edible Leaves
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization (FAO): Rome, Italy. The State of the World’s Land and Water Resources for Food and Agriculture (SOLAW), Managing Systems at Risk; Earthscan: London, UK, 2011. [Google Scholar]
- Bouarfa, S.; Kuper, M. Groundwater in irrigation systems: From menace to mainstay. Irrig. Drain. 2012, 61 (Suppl. S1), 1–13. [Google Scholar] [CrossRef]
- Hoogesteger, J.; Wester, P. Intensive groundwater use and (in)equity: Processes and governance challenges. Environ. Sci. Policy 2015, 51, 117–124. [Google Scholar] [CrossRef]
- Atzori, G.; Mancuso, S.; Masi, E. Seawater potential use in soilless culture: A review. Sci. Hortic. 2019, 249, 199–207. [Google Scholar] [CrossRef]
- Zhu, J. Plant Salt Stress. In Encyclopedia of Life Sciences; O’Daly, A., Ed.; John Wiley & Sons: Chichester, UK, 2007; pp. 1–3. [Google Scholar] [CrossRef]
- Chérel, I.; Lefoulon, C.; Boeglin, M.; Sentenac, H. Molecular mechanisms involved in plant adaptation to low K+ availability. J. Exp. Bot. 2014, 65, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-Q.; Guo, H.; Wang, S.-M.; Zhao, B.; Zhang, J.-L.; Ma, Q.; Yin, H.-J.; Bao, A.-K. The photosynthesis, Na+/K+ homeostasis and osmotic adjustment of Atriplex canescens in response to salinity. Front. Plant Sci. 2016, 7, 848. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Ashraf, M.; Rehman, S.; Ahmad, M.; Shik Rha, E. Salinity induced changes in cell membrane stability, protein and RNA contents. Afr. J. Biotechnol. 2012, 24, 6476–6483. [Google Scholar]
- Chakraborty, K.; Bose, J.; Shabala, L.; Shabala, S. Difference in root K+ retention ability and reduced sensitivity of K+-permeable channels to reactive oxygen species confer differential salt tolerance in three Brassica species. J. Exp. Bot. 2016, 67, 4611–4625. [Google Scholar] [CrossRef]
- Cheeseman, J.M. The evolution of halophytes, glycophytes and crops, and its implications for food security under saline conditions. New Phytol. 2015, 206, 557–570. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Rozema, J.; Schat, H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ. Exp. Bot. 2013, 92, 83–95. [Google Scholar] [CrossRef]
- Atzori, G. The potential of edible halophytes as new crops in saline agriculture—The ice plant (Mesembryanthemum crystallinum L.) case study. In Future of Sustainable Agriculture in Saline Environments; Negacz, K., Vellinga, P., Barrett-Lennard, E., Choukr-Allah, R., Elzenga, T., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 443–460. [Google Scholar] [CrossRef]
- Jesus, J.M.; Danko, A.S.; Fiúza, A.; Borges, M.T. Phytoremediation of salt-affected soils: A review of processes, applicability, and the impact of climate change. Environ. Sci. Pollut. Res. 2015, 22, 6511–6525. [Google Scholar] [CrossRef] [PubMed]
- Rabhi, M.; Hafsi, C.; Lakhdar, A.; Hajji, S.; Barhoumi, Z.; Hamrouni, M.H.; Abdelly, C.; Smaoui, A. Evaluation of the capacity of three halophytes to desalinize their rhizosphere as grown on saline soils under nonleaching conditions. Afr. J. Ecol. 2009, 47, 463–468. [Google Scholar] [CrossRef]
- Karakaş, S.; Çullu, M.A.; Dikilitaş, M. Comparison of two halophyte species (Salsola soda and Portulaca oleracea) for salt removal potential under different soil salinity conditions. Turk. J. Agric. For. 2017, 41, 183–190. [Google Scholar] [CrossRef]
- Sabzalian, M.R.; Dayani, S.; Torkian, M.; Leake, J.E. Comparison of Distichlis spicata and Suaeda aegyptiaca in response to water salinity: Candidate halophytic species for saline soils remediation. Int. J. Phytoremed. 2018, 20, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y.; Fallovo, C.; Cardarelli, M.; Graifenberg, A. Use of Salsola soda as a companion plant to improve greenhouse pepper (Capsicum annuum) performance under saline conditions. N. Z. J. Crop Hortic. Sci. 2006, 34, 283–290. [Google Scholar] [CrossRef]
- Zuccarini, P. Ion uptake by halophytic plants to mitigate saline stress in Solarium lycopersicon L., and different effect of soil and water salinity. Soil Water Res. 2008, 3, 62–73. [Google Scholar] [CrossRef]
- Rabhi, M.; Ferchichi, S.; Jouini, J.; Hamrouni, M.H.; Koyro, H.W.; Ranieri, A.; Abdelly, C.; Smaoui, A. Phytodesalination of a salt-affected soil with the halophyte Sesuvium portulacastrum L. to arrange in advance the requirements for the successful growth of a glycophytic crop. Bioresour. Technol. 2010, 101, 6822–6828. [Google Scholar]
- Simpson, C.R.; Franco, J.G.; King, S.R.; Volder, A. Intercropping halophytes to mitigate salinity stress in watermelon. Sustainability 2018, 10, 681. [Google Scholar] [CrossRef]
- Atzori, G.; Guidi Nissim, W.; Caparrotta, S.; Santantoni, F.; Masi, E. Seawater and water footprint in different cropping systems: A chicory (Cichorium intybus L.) case study. Agric. Water Manag. 2019, 211, 172–177. [Google Scholar] [CrossRef]
- Guidi Nissim, W.; Masi, E.; Pandolfi, C.; Mancuso, S.; Atzori, G. The response of halophyte (Tetragonia tetragonioides (pallas) kuntz.) and glycophyte (Lactuca sativa L.) crops to diluted seawater and NaCl solutions: A comparison between two salinity stress types. Appl. Sci. 2021, 11, 6336. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.; Dong, F.; Li, J.; Zeng, L.; Tang, J.; Gu, D. Mechanism underlying the shading-induced chlorophyll accumulation in tea leaves. Front. Plant Sci. 2021, 12, 779819. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Waskiewicz, A.; Muzolf-Panek, M.; Golinski, P. Phenolic content changes in plants under salt stress. In Ecophysiology and Responses of Plants under Salt Stress; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2013; pp. 283–314. [Google Scholar]
- Atzori, G.; Nissim, W.G.; Macchiavelli, T.; Vita, F.; Azzarello, E.; Pandolfi, C.; Masi, E.; Mancuso, S. Tetragonia tetragonioides (Pallas) Kuntz. as promising salt-tolerant crop in a saline agriculture context. Agric. Water Manag. 2020, 240, 106261. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolutions. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Dubois, M.K. Use of phenol reagent for the determination of total sugar. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. Protocol: Extraction and Determination of Proline. 2011. Available online: https://prometheuswiki.publish.csiro.au/tikiindex.php?page=PROTOCOL%3A+Extraction+and+determination+of+proline (accessed on 11 August 2021).
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]

| Change in pH | Change in EC (dS m−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Starting pH | End of First Half | Starting pH | End of Second Half | Starting EC | End of First Half | Starting EC | End of Second Half | |
| LC | 6.2 | 6.1 ± 0.20 | 6.2 | 6.4 ± 0.10 | 2.3 | 3.5 ± 0.17 | 2.6 | 3.3 ± 0.09 |
| LS | 6.4 | 6.1 ± 0.00 | 6.4 | 6.2 ± 0.00 | 12.0 | 15 ± 0.19 | 11.6 | 15.4 ± 1.01 |
| Intercrop | 6.3 | 6.3 ± 0.04 | 6.3 | 6.1 ± 0.03 | 12.0 | 16.3 ± 0.33 | 11.8 | 16.3 ± 0.25 |
| SC | 6.1 | 6.1 ± 0.00 | 6.1 | 6.1 ± 0.00 | 2.3 | 4.2 ± 0.00 | 2.7 | 3.1 ± 0.00 |
| SS | 6.3 | 6.2 ± 0.00 | 6.3 | 5.9 ± 0.00 | 12.0 | 15.4 ± 0.00 | 11.1 | 14.3 ± 0.00 |
| Treatments | Total Plant FW | Total Plant DW | Root FW | Root DW | Root:Shoot Ratio |
|---|---|---|---|---|---|
| g | g | g | g | ||
| LC | 323.4 ± 15.1 a | 11.9 ± 0.7 a | 41.2 ± 2.5 | 2.2 ± 0.3 a | 0.22 ± 0.02 |
| LS | 173.8 ± 7.99 b | 8.998 ± 0.4 b | 41.8 ± 0.6 | 1.7 ± 0.1 ab | 0.24 ± 0.01 |
| L intercrop | 155.2 ± 8.7 b | 8.6 ± 0.4 b | 31.98 ± 2.1 | 1.4 ± 0.04 b | 0.19 ± 0.01 |
| SC | 31.3 ± 2.2 | 2.4 ± 0.1 | 1.7 ± 0.5 b | 0.1 ± 0.03 b | 0.1 ± 0.01 |
| SS | 44.1 ± 1.5 | 3.1 ± 0.2 | 4.2 ± 1.1 a | 0.2 ± 0.02 a | 0.08 ± 0.02 |
| S intercrop | 47.5 ± 4.5 | 3.3 ± 0.3 | 2.4 ± 0.2 ab | 0.1 ± 0.01 b | 0.07 ± 0.01 |
| Parameters | Treatment Group | Time | Two-Way ANOVA Results | |||
|---|---|---|---|---|---|---|
| 0 Weeks | 2 Weeks | 4 Weeks | ||||
| Fv/Fm | LC | 0.82 ± 0.002 a | 0.82 ± 0.001 a | 0.80 ± 0.005 ab | Treatment | 0.473 |
| LS | 0.82 ± 0.001 ab | 0.82 ± 0.003 a | 0.81 ± 0.003 ab | Time | <0.001 | |
| L Intercrop | 0.82 ± 0.003 a | 0.82 ± 0.004 ab | 0.79 ± 0.017 b | Interaction | 0.273 | |
| F’v/F’m | LC | 0.45 ± 0.008 ab | 0.49 ± 0.011 a | 0.46 ± 0.013 ab | Treatment | <0.001 |
| LS | 0.41 ± 0.028 abc | 0.37 ± 0.021 c | 0.41 ± 0.011 abc | Time | 0.454 | |
| L Intercrop | 0.42 ± 0.025 abc | 0.37 ± 0.018 c | 0.39 ± 0.011 bc | Interaction | 0.095 | |
| ΦPSII | LC | 0.21 ± 0.010 ab | 0.24 ± 0.019 a | 0.20 ± 0.018 ab | Treatment | 0.008 |
| LS | 0.20 ± 0.024 ab | 0.17 ± 0.016 ab | 0.18 ± 0.030 ab | Time | 0.146 | |
| L Intercrop | 0.20 ± 0.025 ab | 0.15 ± 0.17 ab | 0.13 ± 0.013 b | Interaction | 0.182 | |
| NPQ | LC | 3.01 ± 0.083 a | 2.26 ± 0.134 c | 2.24 ± 0.113 c | Treatment | 0.010 |
| LS | 2.96 ± 0.167 a | 2.78 ± 0.079 ab | 2.43 ± 0.094 bc | Time | <0.001 | |
| L Intercrop | 3.00 ± 0.96 a | 2.79 ± 0.077 ab | 2.45 ± 0.036 bc | Interaction | 0.07 | |
| Chl a | LC | 210.0 ± 9.9 a | 238.3 ± 21.1 a | 204.7 ± 18.4 a | Treatment | 0.110 |
| µg g−1 FW | LS | 235.9 ± 16.1 a | 266.3 ± 8.1 a | 220.0 ± 25.3 a | Time | 0.007 |
| L Intercrop | 221.1 ± 10.7 a | 286.1 ± 17.3 a | 235.9 ± 24.8 a | Interaction | 0.817 | |
| SC | 276.5 ± 20.0 | |||||
| SS | 252.2 ± 15.7 | |||||
| Chl b | LC | 60.2 ± 4.8 a | 110.4 ± 40.4 a | 58.1 ± 2.2 a | Treatment | 0.531 |
| µg g−1 FW | LS | 57.1 ± 2.8 a | 67.5 ± 3.6 a | 64.8 ± 5.8 a | Time | 0.134 |
| L Intercrop | 69.1 ± 7.2 a | 71.9 ± 4.9 a | 67.4 ± 4.4 a | Interaction | 0.315 | |
| SC | 55.7 ± 1.7 | |||||
| SS | 55.5 ± 1.7 | |||||
| Carotenoids | LC | 65.1 ± 3.6 ab | 64.7 ± 8.4 ab | 60.7 ± 6.5 b | Treatment | 0.004 |
| µg g−1 FW | LS | 80.0 ± 6.1 ab | 85.4 ± 2.9 ab | 66.3 ± 8.7 ab | Time | 0.048 |
| L Intercrop | 71.8 ± 3.7 ab | 91.4 ± 5.9 a | 76.8 ± 6.7 ab | Interaction | 0.358 | |
| SC | 81.6 ± 5.8 | |||||
| SS | 70.4 ± 4.7 | |||||
| Treatments | P | K | Ca | Fe | Mg |
| ppm | ppm | ppm | ppm | ppm | |
| LC | 6841.6 ± 252.8 b | 54,420.8 ± 3141.8 a | 8028.3 ± 155.81 a | 98.6 ± 5.4 a | 2401.8 ± 119.8 b |
| LS | 7176.5 ± 38.9 b | 42,996 ± 454.8 b | 4851.1 ± 87.2 c | 65.3 ± 2.8 b | 2606.5 ± 78.8 ab |
| L Intercrop | 7773.5 ± 74.7 a | 41,608.3 ± 1265.1 b | 5537.8 ± 235.2 b | 107.9 ± 23.2 a | 3063.5 ± 276.1 a |
| SC | 12334.7 ± 430.6 a | 60,710.7 ± 1584.7 a | 7541.3 ± 351.4 a | 64.8 ± 1.8 a | 2785.3 ± 162.4 a |
| SS | 8438.3 ± 211 b | 43,029 ± 565.5 b | 3891 ± 195.1 b | 70.3 ± 2.9 a | 2856.5 ± 167 a |
| S Intercrop | 8705.4 ± 567.6 b | 44,459.5 ± 688.3 b | 3558.8 ± 253.1 b | 69.7 ± 2.3 a | 2469.4 ± 331.2 a |
| Treatments | Mn | Cu | Zn | Na | Mo |
| ppm | ppm | ppm | ppm | ppm | |
| LC | 79.9 ± 2.3 b | 12.6 ± 0.4 a | 57.4 ± 3.2 b | 1010.9 ± 82.7 b | 0.9 ± 0.05 a |
| LS | 155.1 ± 7.7 a | 12.7 ± 0.1 a | 90.5 ± 4.5 a | 20,739.3 ± 529.7 a | 0.5 ± 0.1 b |
| L Intercrop | 88.7 ± 3.7 b | 14.1 ± 0.7 a | 84.8 ± 8.4 a | 24,925 ± 1686.6 a | 0.6 ± 0.02 b |
| SC | 55.1 ± 3.6 a | 14.6 ± 0.3 a | 47.9 ± 2.2 b | 13,820.6 ± 481.7 b | 1.8 ± 0.1 b |
| SS | 61 ± 4.7 a | 14.9 ± 0.7 a | 58.6 ± 3.3 ab | 44,936 ± 804.7 a | 2.3 ± 0.1 a |
| S Intercrop | 73 ± 6.9 a | 18.7 ± 2.4 a | 69.8 ± 6 a | 44,567.5 ± 1.77.7 a | 2.5 ± 0.1 a |
| Treatments | Carbohydrates | Proteins | Polyphenols | Nitrates | Proline |
|---|---|---|---|---|---|
| % | mg g−1 (DW) | mg g−1 (FW) | mg g−1 (FW) | µmol g−1 (FW) | |
| LC | 71.4 ± 5.9 | 0.5 ± 0.03 | 0.3 ± 0.02 b | 1670 ± 331 b | 2.73 ± 0.51 |
| LS | 54.1 ± 4.6 | 0.7 ± 0.1 | 0.3 ± 0.02 ab | 3659 ± 186 a | 5.06 ± 0.66 |
| L Intercrop | 56.5 ± 4.2 | 0.6 ± 0.02 | 0.4 ± 0.03 a | 4693 ± 452 a | 7.32 ± 2.31 |
| SC | 31.6 ± 1.2 b | 0.4 ± 0.04 b | 0.7 ± 0.05 a | 6859 ± 742 a | 2.21 ± 0.14 |
| SS | 39.4 ± 2.7 a | 0.5 ± 0.02 a | 0.6 ± 0.04 b | 5121 ± 421 ab | 2.79 ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atzori, G.; Guidi Nissim, W.; Mancuso, S.; Palm, E. Intercropping Salt-Sensitive Lactuca sativa L. and Salt-Tolerant Salsola soda L. in a Saline Hydroponic Medium: An Agronomic and Physiological Assessment. Plants 2022, 11, 2924. https://doi.org/10.3390/plants11212924
Atzori G, Guidi Nissim W, Mancuso S, Palm E. Intercropping Salt-Sensitive Lactuca sativa L. and Salt-Tolerant Salsola soda L. in a Saline Hydroponic Medium: An Agronomic and Physiological Assessment. Plants. 2022; 11(21):2924. https://doi.org/10.3390/plants11212924
Chicago/Turabian StyleAtzori, Giulia, Werther Guidi Nissim, Stefano Mancuso, and Emily Palm. 2022. "Intercropping Salt-Sensitive Lactuca sativa L. and Salt-Tolerant Salsola soda L. in a Saline Hydroponic Medium: An Agronomic and Physiological Assessment" Plants 11, no. 21: 2924. https://doi.org/10.3390/plants11212924
APA StyleAtzori, G., Guidi Nissim, W., Mancuso, S., & Palm, E. (2022). Intercropping Salt-Sensitive Lactuca sativa L. and Salt-Tolerant Salsola soda L. in a Saline Hydroponic Medium: An Agronomic and Physiological Assessment. Plants, 11(21), 2924. https://doi.org/10.3390/plants11212924






