Broccoli Sprouts and Their Influence on Thyroid Function in Different In Vitro and In Vivo Models
Abstract
1. Introduction
2. Results and Discussion
2.1. Cytotoxic Acivity of Broccoli Sprouts on Thyroid Cells
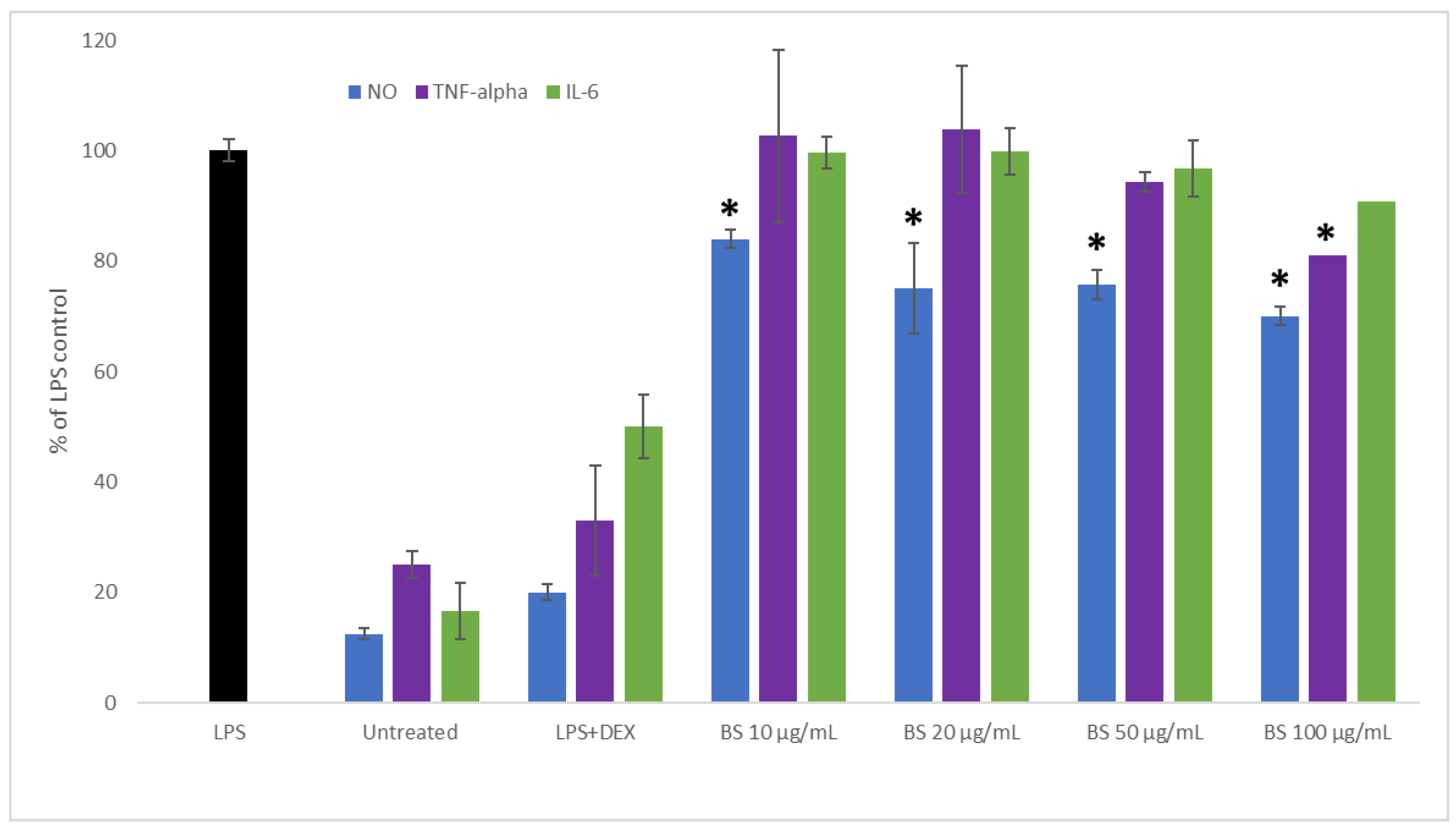
2.2. Influence of Broccoli Sprout Extract on the Inflammation Process
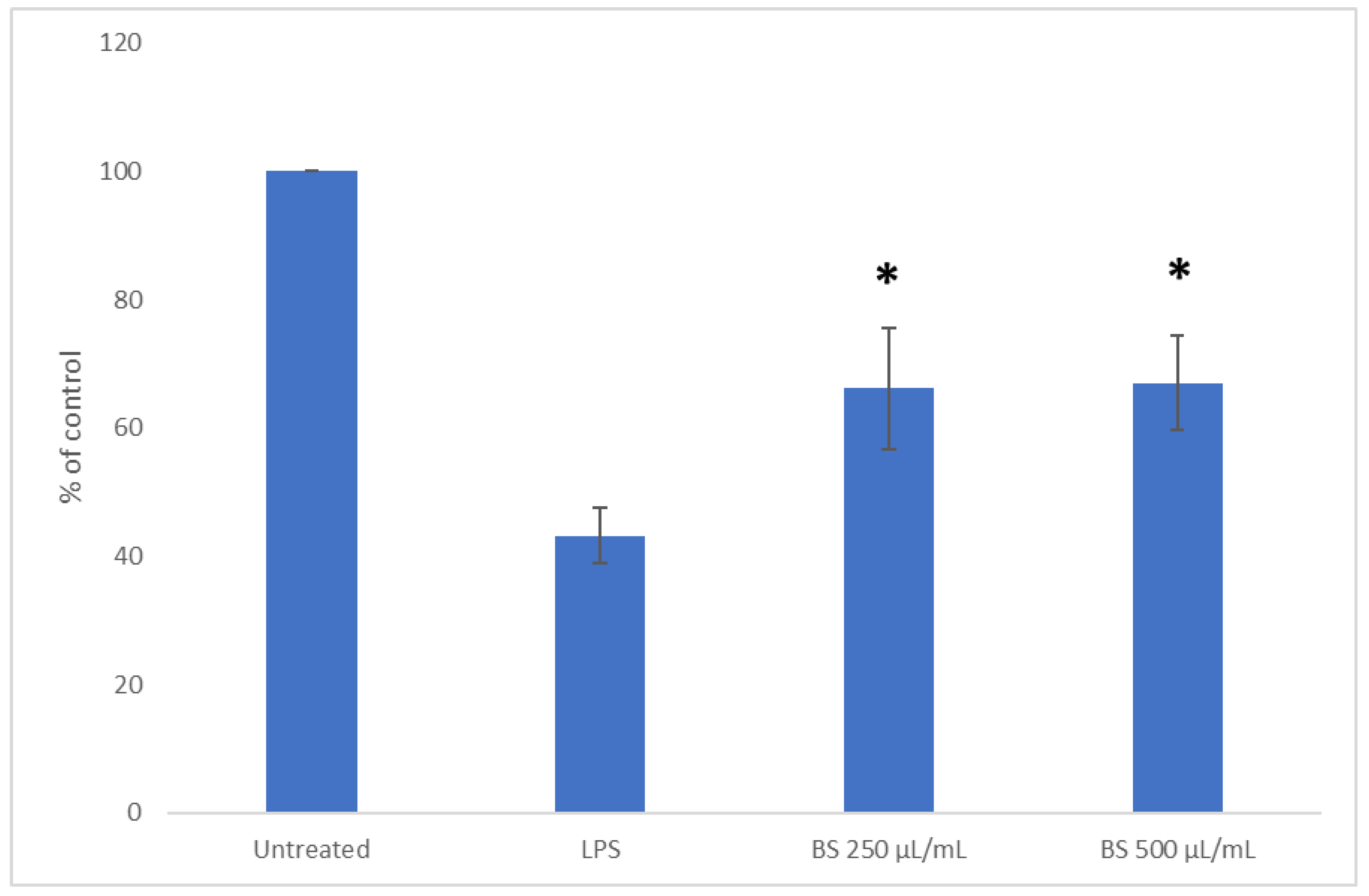
2.3. Influence of Broccoli Sprout Extract on SOD1 Activity
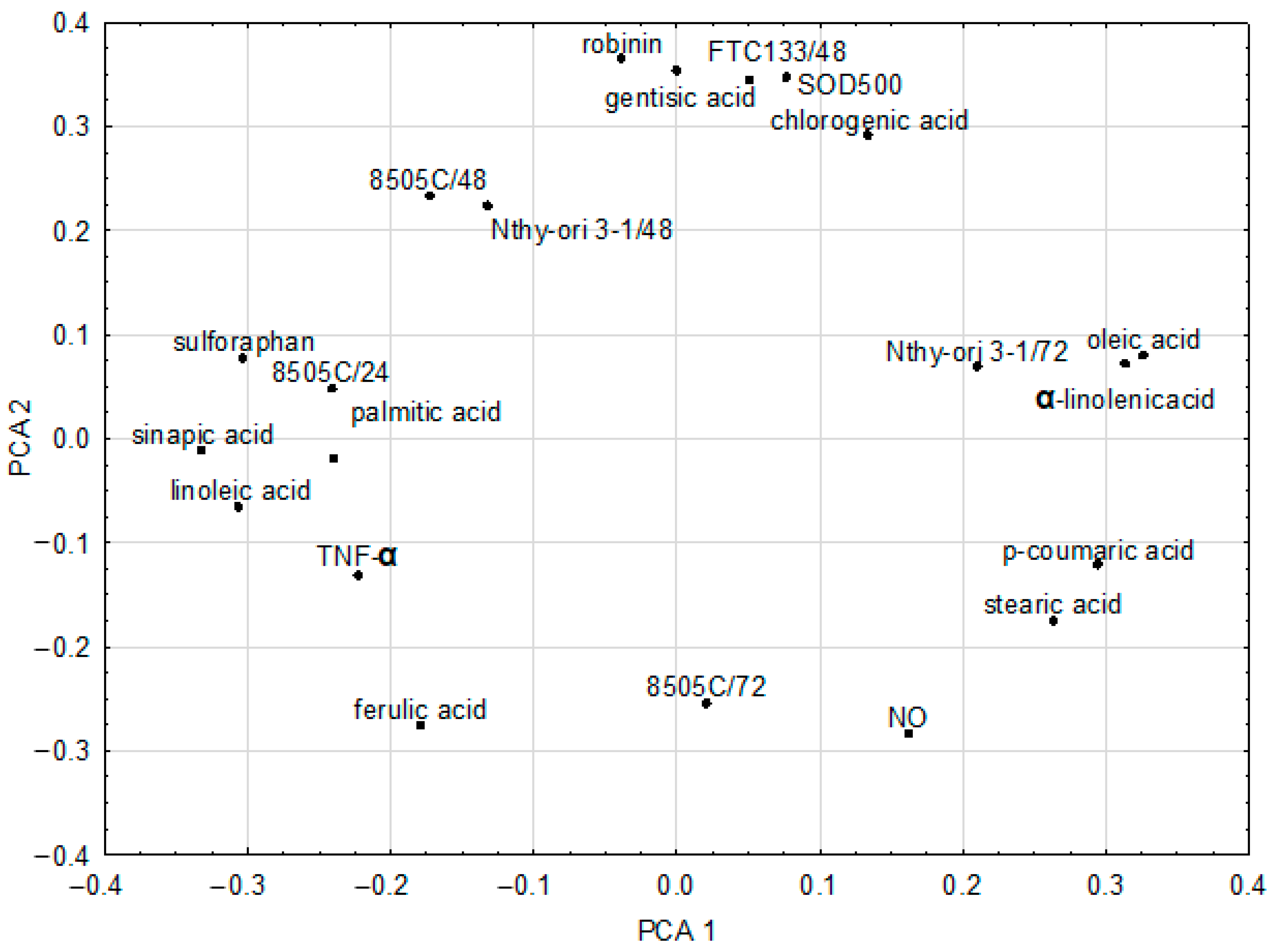
2.4. Chemometric Analysis of In Vitro Parameters
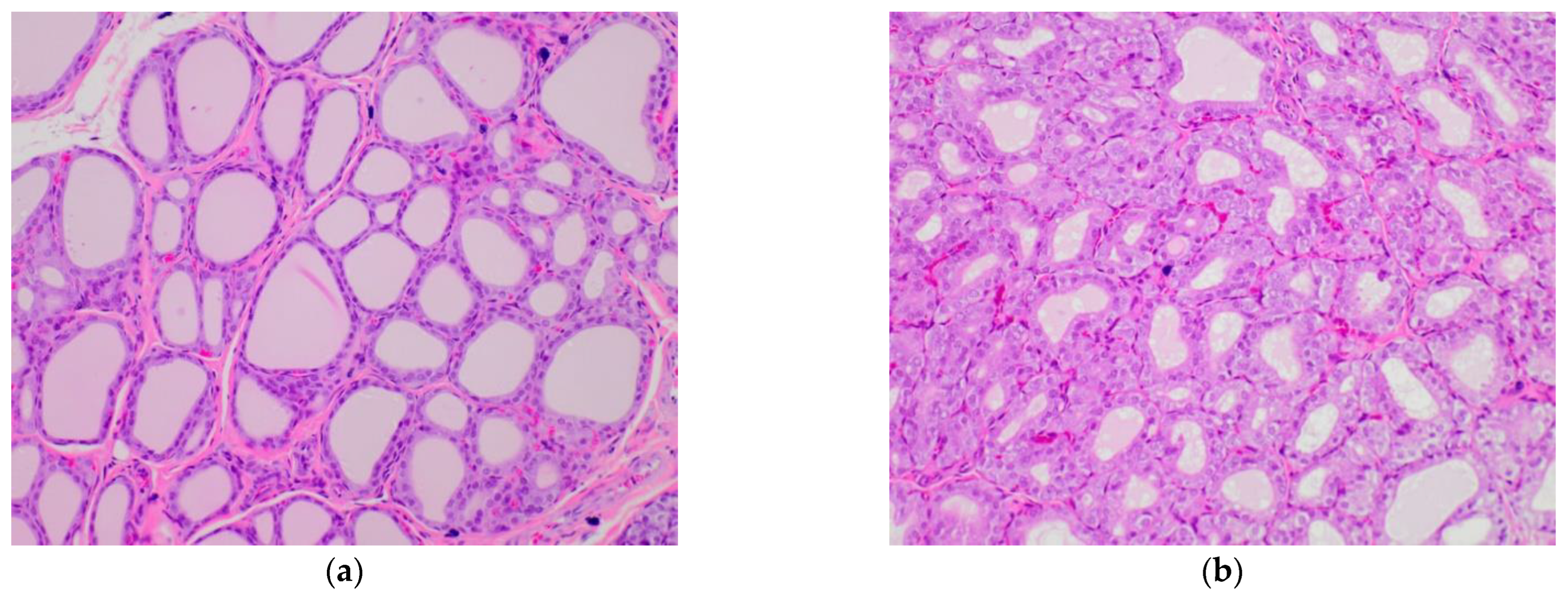
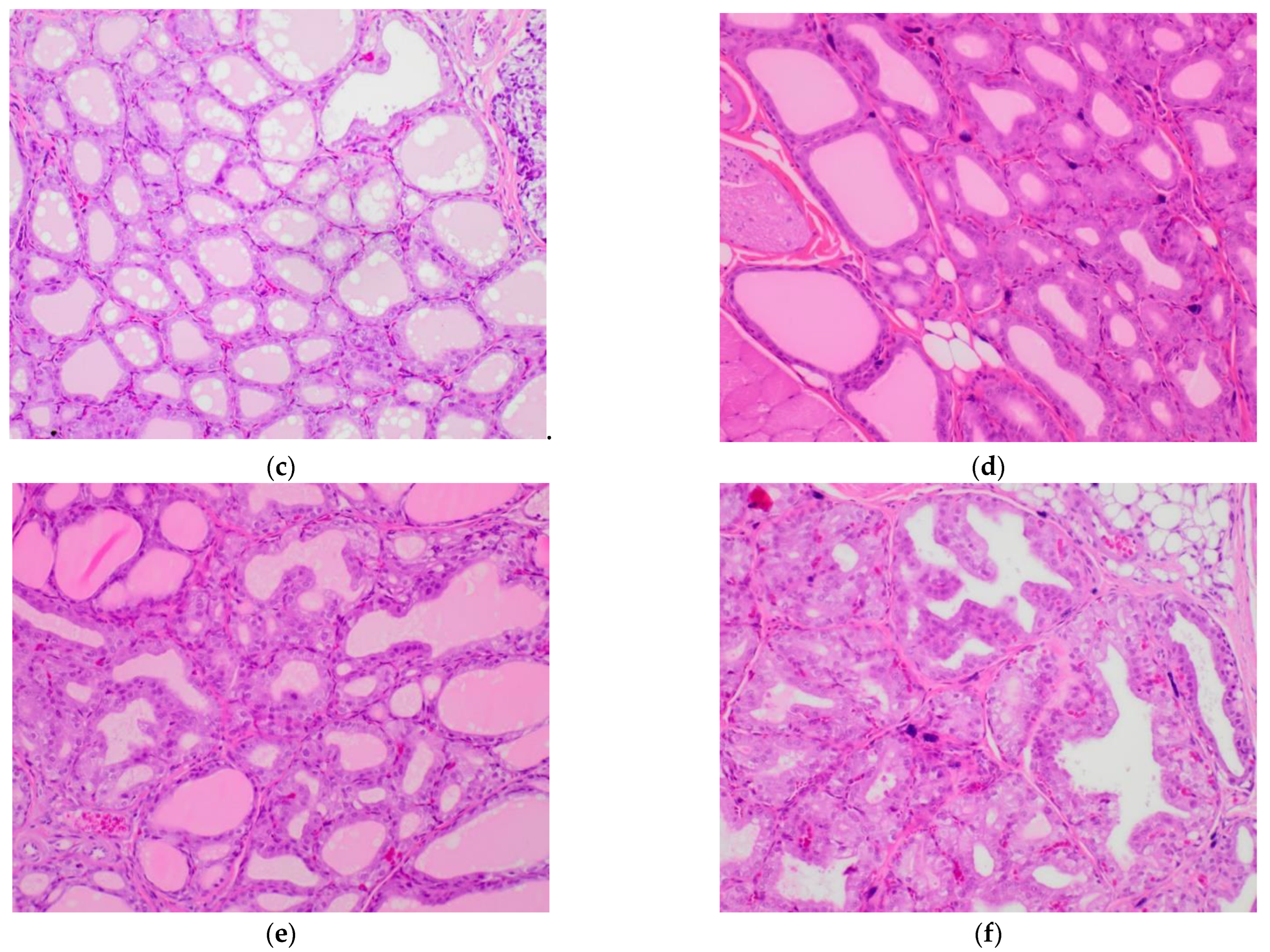
2.5. Influence of Broccoli Sprouts on Rat Thyroid Histopathology
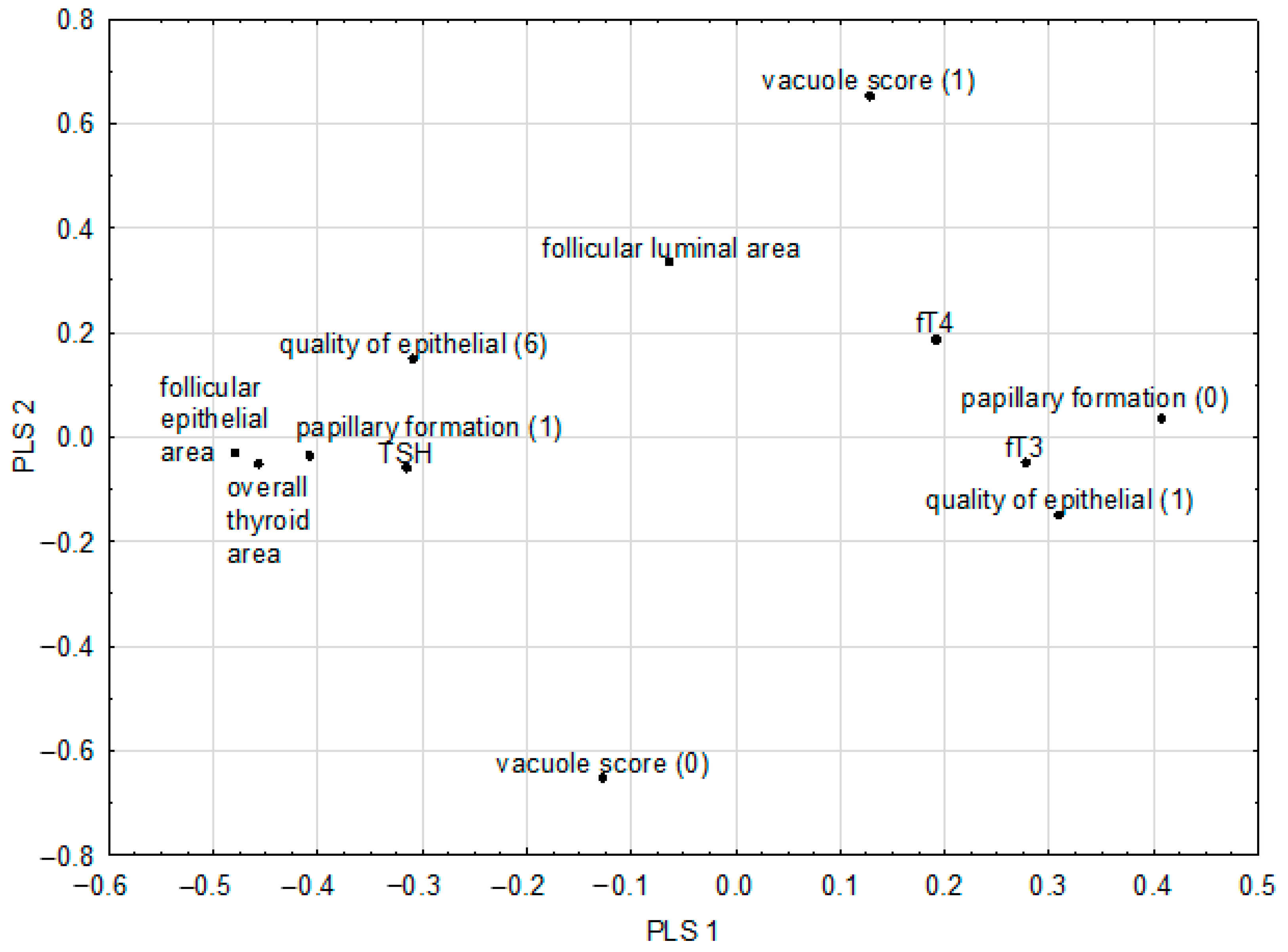
2.6. Chemometric Analysis of In Vivo Parameters
3. Materials and Methods
3.1. Plant Material
3.2. Extract Preparation and Quantitative Analysis
3.3. Broccoli Sprouts
3.3.1. Cytotoxic Activity of Broccoli Sprouts
3.3.2. Inflammation Model
3.3.3. Nitric Oxide Determination
3.3.4. TNF-Alpha and IL-6 Analysis
3.3.5. Human Superoxide Dismutase 1 Activity
3.4. Broccoli Sprouts—Experiment In Vivo
3.4.1. Animals
3.4.2. Histopathology of Rat Thyroids
3.5. Statistical Analysis
Chemometric Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahey, J.W.; Kensler, T.W. The challenges of designing and implementing clinical trials with broccoli sprouts… and turning evidence into public health action. Front. Nutr. 2021, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Tyszka-Czochara, M.; Galanty, A.; Gdula-Argasińska, J.; Żmudzki, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S. Comparative study of predominant phytochemical compounds and proapoptotic potential of broccoli sprouts and florets. Plant Foods Hum. Nutr. 2018, 73, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, L.T.; Bishayee, A. Sulforaphane: A broccoli bioactive phytocompound with cancer preventive potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Okoń, K.; Prochownik, E.; Krośniak, M.; Francik, R.; Kryczyk-Kozioł, J.; Grudzińska, M.; Tyszka-Czochara, M.; Malinowski, M.; Sikora., J.; et al. The Impact of Kohlrabi Sprouts on Various Thyroid Parameters in Iodine Deficiency-and Sulfadimethoxine-Induced Hypothyroid Rats. Nutrients 2022, 14, 2802. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Giuliani, C. Plant constituents and thyroid: A revision of the main phytochemicals that interfere with thyroid function. Food Chem. Toxicol. 2021, 152, 112158. [Google Scholar] [CrossRef] [PubMed]
- Langer, P.; Michajlovskij, N.; Sedlak, J.; Kutka, M. Studies on the antithyroid activity of naturally occurring L-5-vinyl-2-thiooxazolidone in man. Endokrinologie 1971, 57, 225–229. [Google Scholar]
- Paśko, P.; Krośniak, M.; Prochownik, E.; Tyszka-Czochara, M.; Fołta, M.; Francik, R.; Sikora, J.; Malinowski, M.; Zagrodzki, P. Effect of broccoli sprouts on thyroid function, haematological, biochemical, and immunological parameters in rats with thyroid imbalance. Biomed. Pharmacother. 2018, 97, 82–90. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Zagrodzki, P.; Prochownik, E.; Jarkiewicz, A.; Paśko, P. Influence of brassica sprouts on short chain fatty acids concentration in stools of rats with thyroid dysfunction. Acta Pol. Pharm. 2019, 76, 1005–1014. [Google Scholar] [CrossRef]
- Shapiro, T.A.; Fahey, J.W.; Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Stephenson, K.K.; Wade, K.L.; Ye, L.; Talalay, P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase I study. Nutr. Cancer 2006, 55, 53–62. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Ziros, P.G.; Chen, J.G.; Groopman, J.D.; Kensler, T.W.; Sykiotis, G.P. Broccoli sprout beverage is safe for thyroid hormonal and autoimmune status: Results of a 12-week randomized trial. Food Chem. Toxicol. 2019, 126, 1–6. [Google Scholar] [CrossRef]
- Galanty, A.; Zagrodzki, P.; Gdula-Argasińska, J.; Grabowska, K.; Koczurkiewicz-Adamczyk, P.; Wróbel-Biedrawa, D.; Podolak, I.; Pękala, E.; Paśko, P. A Comparative survey of anti-melanoma and anti-inflammatory potential of usnic acid enantiomers—A Comprehensive in vitro approach. Pharmaceuticals 2021, 14, 945. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Żmudzki, P.; Bieniek, U.; Prochownik, E.; Domínguez-Álvarez, E.; Bierła, K.; Łobiński, R.; Szpunar, J.; et al. Varied effect of fortification of kale sprouts with novel organic selenium compounds on the synthesis of sulphur and phenolic compounds in relation to cytotoxic, antioxidant and anti-inflammatory activity. Microchem. J. 2022, 179, 107509. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Z.; Yang, Q.; Li, H.; Guan, H.; Shi, B.; Hou, P.; Ji, M. Sulforaphane inhibits thyroid cancer cell growth and invasiveness through the reactive oxygen species-dependent pathway. Oncotarget 2015, 6, 25917. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Rhee, Y.; Chung, P.S.; Ge, R.F.; Ahn, J.C. Sulforaphene enhances the efficacy of photodynamic therapy in anaplastic thyroid cancer through Ras/RAF/MEK/ERK pathway suppression. J. Photochem. Photobiol. B. 2018, 179, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.M.; Akter, S.; Lin, C.N.; Nazzal, S. Sulforaphane as an anticancer molecule: Mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch. Pharmacal Res. 2020, 43, 371–384. [Google Scholar] [CrossRef]
- Shen, Y.; Velu, P.; Huang, X.; Dang, T. Evaluation of apoptotic and cytotoxic effect of robinin in TPC-1 and SW1736 human thyroid cancer cells. Phcog. Mag. 2022, 18, 360–365. [Google Scholar]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother. Res. 2020, 34, 729–741. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rajabi, S.; Martorell, M.; López, M.D.; Toro, M.T.; Barollo, S.; Pezzani, R. Plant natural products with anti-thyroid cancer activity. Fitoterapia 2020, 146, 104640. [Google Scholar] [CrossRef]
- Varnamkhasti, K.; Tavakoli, P.; Rouhi, L.; Raisi, S. Cytotoxicity, Apoptosis induction and change of p53, PARP, p21 and Bcl-2 genes expression in the human anaplastic thyroid carcinoma cells line (SW-1736) with curcumin. Genet. Appl. 2021, 5, 10–17. [Google Scholar]
- Tilg, H. Cruciferous vegetables: Prototypic anti-inflammatory food components. Clin. Phytosci. 2015, 1, 10. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Granica, S.; Kolodziejczyk-Czepas, J.; Magiera, A.; Czerwińska, M.E.; Nowak, P.; Rutkowska, M.; Wasiński, P.; Owczarek, A. Variability of sinapic acid derivatives during germination and their contribution to antioxidant and anti-inflammatory effects of broccoli sprouts on human plasma and human peripheral blood mononuclear cells. Food Funct. 2020, 11, 7231–7244. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.; Chiu, C.H.; Hsieh, P.C. Bioactive compounds and bioactivities of Brassica oleracea L. var. italica sprouts and microgreens: An updated overview from a nutraceutical perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef]
- Paśko, P.; Prochownik, E.; Galanty, A.; Bartoń, H.; Tyszka-Czochara, M.; Zagrodzki, P.; Zachwieja, Z. Comparative study of antioxidant capacity and total polyphenols content of broccoli sprouts and florets. Bromat. Chem. Toksykol. 2018, 2, 120–123. [Google Scholar]
- Chou, S.T.; Peng, H.Y.; Hsu, J.C.; Lin, C.C.; Shih, Y. Achillea millefolium L. essential oil inhibits LPS-induced oxidative stress and nitric oxide production in RAW 264.7 macrophages. Int. J. Mol. Sci. 2013, 14, 12978–12993. [Google Scholar] [CrossRef]
- Paśko, P.; Okoń, K.; Krośniak, M.; Prochownik, E.; Żmudzki, P.; Kryczyk-Kozioł, J.; Zagrodzki, P. Interaction between iodine and glucosinolates in rutabaga sprouts and selected biomarkers of thyroid function in male rats. J. Trace Elem. Med. Biol. 2018, 46, 110–116. [Google Scholar] [CrossRef]
- Bell, J.M.; Benjamin, B.R.; Giovannetti, P.M. Histopathology of thyroids and livers of rats and mice fed diets containing Brassica glucosinolates. Can. J. Anim. Sci. 1972, 52, 395–406. [Google Scholar] [CrossRef][Green Version]
- Rajab, N.M.A.; Ukropina, M.; Cakic-Milosevic, M. Histological and ultrastructural alterations of rat thyroid gland after short-term treatment with high doses of thyroid hormones. Saudi J. Biol. Sci. 2017, 24, 1117–1125. [Google Scholar] [CrossRef]
- De Groot, A.P.; Willems, M.I.; De Vos, R.H. Effects of high levels of Brussels sprouts in the diet of rats. Food Chem. Toxicol. 1991, 29, 829–837. [Google Scholar] [CrossRef]
- Galanty, A.; Popiół, J.; Paczkowska-Walendowska, M.; Studzińska-Sroka, E.; Paśko, P.; Cielecka-Piontek, J.; Pękala, E.; Podolak, I. (+)-Usnic acid as a promising candidate for a safe and stable topical photoprotective agent. Molecules 2021, 26, 5224. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Luksirikul, P.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Dragon fruits as a reservoir of natural polyphenolics with chemopreventive properties. Molecules 2021, 26, 2158. [Google Scholar] [CrossRef]
- Paśko, P.; Prochownik, E.; Krośniak, M.; Tyszka-Czochara, M.; Francik, R.; Marcinkowska, M.; Sikora, J.; Malinowski, M.; Zagrodzki, P. Animals in iodine deficiency or sulfadimethoxine models of thyroid damage are differently affected by the consumption of Brassica sprouts. Biol. Trace Elem. Res. 2020, 193, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Pasko, P.; Gdula-Argasinska, J.; Podporska-Carroll, J.; Quilty, B.; Wietecha-Posluszny, R.; Tyszka-Czochara, M.; Zagrodzki, P. Influence of selenium supplementation on fatty acids profile and biological activity of four edible amaranth sprouts as new kind of functional food. J. Food Sci. Technol. 2015, 52, 4724–4736. [Google Scholar] [CrossRef] [PubMed]
- Zagrodzki, P.; Paśko, P.; Galanty, A.; Tyszka-Czochara, M.; Wietecha-Posłuszny, R.; Rubió, P.S.; Bartoń, H.; Prochownik, E.; Muszyńska, B.; Sułkowska-Ziaja, K.; et al. Does selenium fortification of kale and kohlrabi sprouts change significantly their biochemical and cytotoxic properties? J. Trace Elem. Med. Biol. 2020, 59, 126466. [Google Scholar] [CrossRef] [PubMed]

| IC50 µg/mL | 24 h | 48 h | 72 h |
|---|---|---|---|
| Nthy-ori 3-1 | >Cmax | >Cmax | >Cmax |
| FTC-133 | 35.2 | 24.7 | 16.9 |
| 8505C | 114.1 | 57.5 | 29.5 |
| Pairs of Correlated Parameters | Correlation Weights | |
|---|---|---|
| SOD500 | robinin | 0.126 |
| FTC133/48 | robinin | 0.126 |
| FTC133/48 | gentisic acid | 0.120 |
| FTC133/48 | SOD500 | 0.119 |
| Parameters | C | BS | DI | BS/DI | S | BS/S |
|---|---|---|---|---|---|---|
| Papillary formation (%) | 0% | 25% | 0% | 40% | 60% | 60% |
| Aggregates of lymphocytes (%) | 0% | 0% | 0% | 0% | 0% | 0% |
| Vacuolization of the colloid (%) | 0% | 0% | 40% | 0% | 0% | 0% |
| Shape of epithelial cells (flattened vs. cuboidal) (%) | 100% Flattened | 80% Cuboidal | 60% Cuboidal | 40% Cuboidal | 100% Cuboidal | 80% Cuboidal |
| Follicular epithelial area (mm2) | 1.57 ± 0.14 ab | 1.62 ± 0.82 c | 3.06 ± 0.71 | 2.36 ± 0.55 de | 4.37 ± 1.03 bcd | 3.96 ± 0.65 ae |
| Follicular luminal area (mm2) | 0.32 ± 0.06 a | 0.43 ± 0.13 | 0.69 ± 0.24 abc | 0.31 ± 0.04 c | 0.41 ± 018 | 0.45 ± 0.06 b |
| Overall thyroid area (mm2) | 1.79 ± 0.25 a | 1.61 ± 0.56 | 3.50 ± 1.13 | 2.67 ± 0.60 b | 4.64 ± 1.40 ab | 4.42 ± 0.63 |
| Pairs of Correlated Parameters | Correlation Weights | |
|---|---|---|
| Overall thyroid area | Follicular epithelial area | 0.219 |
| Follicular luminal area | Vacuolization of the colloid | 0.218 |
| Follicular epithelial area | Papillary formation | 0.196 |
| Overall thyroid area | Papillary formation | 0.186 |
| Follicular epithelial area | TSH | 0.150 |
| Overall thyroid area | TSH | 0.144 |
| Cuboidal epithelial shape | Follicular luminal area | 0.129 |
| Papillary formation | TSH | 0.128 |
| Overall thyroid area | Flattened epithelial shape | −0.133 |
| Follicular epithelial area | Flattened epithelial shape | −0.137 |
| Overall thyroid area | Lack of papillary formation | −0.183 |
| Follicular epithelial area | Lack of papillary formation | −0.194 |
| Follicular luminal area | Lack of vacuolization of the colloid | −0.202 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paśko, P.; Zagrodzki, P.; Okoń, K.; Prochownik, E.; Krośniak, M.; Galanty, A. Broccoli Sprouts and Their Influence on Thyroid Function in Different In Vitro and In Vivo Models. Plants 2022, 11, 2750. https://doi.org/10.3390/plants11202750
Paśko P, Zagrodzki P, Okoń K, Prochownik E, Krośniak M, Galanty A. Broccoli Sprouts and Their Influence on Thyroid Function in Different In Vitro and In Vivo Models. Plants. 2022; 11(20):2750. https://doi.org/10.3390/plants11202750
Chicago/Turabian StylePaśko, Paweł, Paweł Zagrodzki, Krzysztof Okoń, Ewelina Prochownik, Mirosław Krośniak, and Agnieszka Galanty. 2022. "Broccoli Sprouts and Their Influence on Thyroid Function in Different In Vitro and In Vivo Models" Plants 11, no. 20: 2750. https://doi.org/10.3390/plants11202750
APA StylePaśko, P., Zagrodzki, P., Okoń, K., Prochownik, E., Krośniak, M., & Galanty, A. (2022). Broccoli Sprouts and Their Influence on Thyroid Function in Different In Vitro and In Vivo Models. Plants, 11(20), 2750. https://doi.org/10.3390/plants11202750






